Production process of human immune globulin for intravenous injection
An immunoglobulin and production process technology, applied in the field of production process from protein stock solution to finished product, can solve the problems of difficult control of finished product quality, product contamination, impact on safety, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
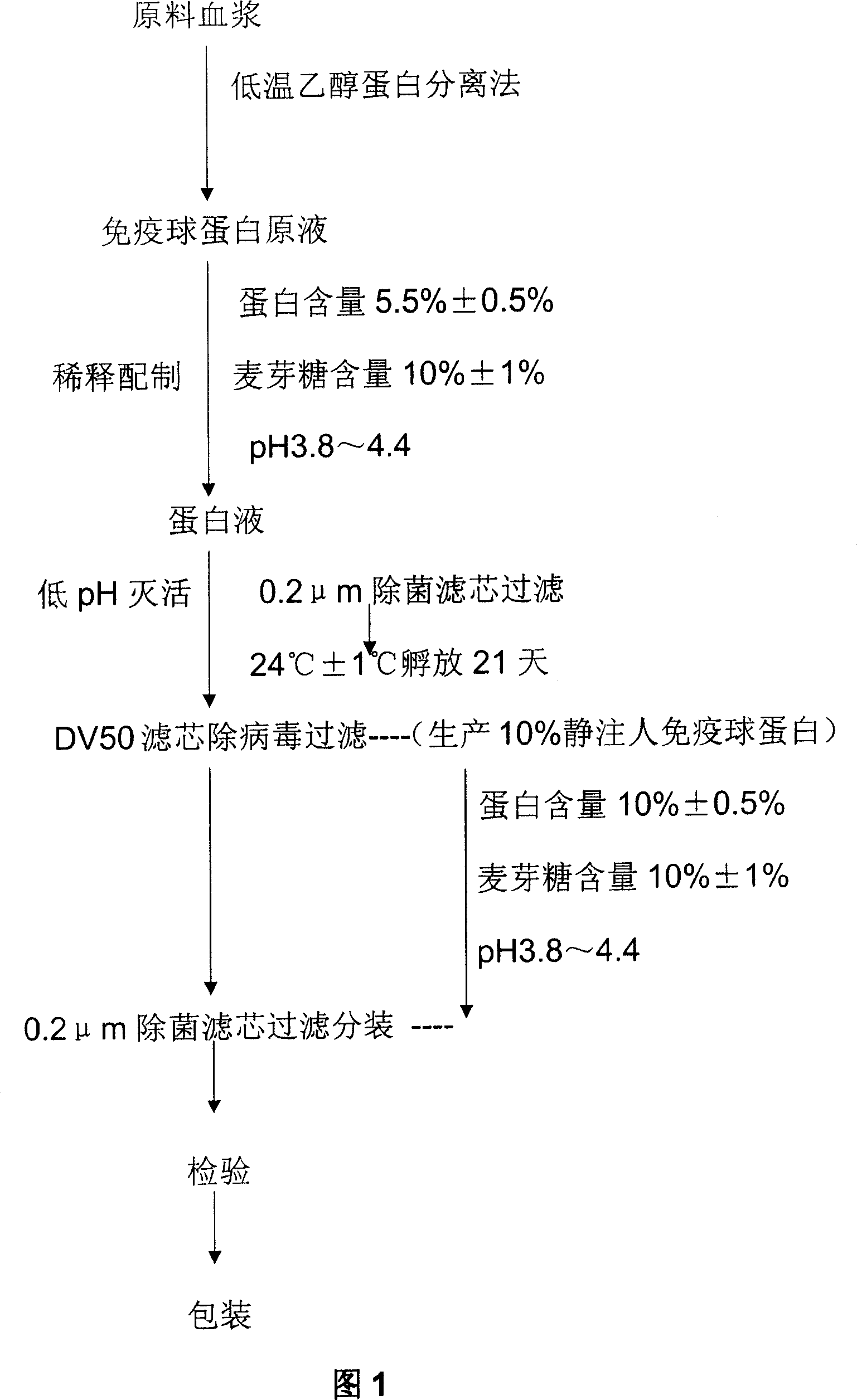
[0024] A production process of human immunoglobulin for intravenous injection according to the present invention, as shown in Figure 1, includes collecting healthy human plasma as a raw material according to the requirements of Pharmacopoeia, adopting low-temperature ethanol protein separation method to prepare human immunoglobulin stock solution, and pressing the finished product Specification preparation, verification, the production process from protein stock solution to finished products with protein concentration of 5% and 10% includes the following steps:
[0025] A) Concentrate the protein stock solution by ultrafiltration to a concentration of 5.5%±0.5%, add maltose to a content of 10%±1%, and adjust the pH value of the protein solution to 3.8-4.4 with 1M / L HCl;
[0026] B) Filter the protein solution through a 0.2 μm sterile filter element, and put it into a sealed serum bottle;
[0027] C) placed in a low pH incubation room, and incubated at a low pH of 24°C ± 1°C fo...
Embodiment 2
[0032] A production process of human immunoglobulin for intravenous injection according to the present invention, as shown in Figure 2, includes collecting healthy human plasma as a raw material according to the requirements of the Pharmacopoeia, and preparing human immunoglobulin stock solution by using low-temperature ethanol protein separation method, according to the specifications of the finished product Preparation, verification, and the production process from protein stock solution to finished products with protein concentration of 5% and 10% include the following steps:
[0033] A) Concentrate the protein stock solution by ultrafiltration to a concentration of 10.5±0.5% or 5.5±0.5%, add maltose to a content of 10%±1%, and adjust the pH value of the protein solution to 3.8-4.4 with 1M / L HCl;
[0034] B) Filter the protein solution through a 0.2 μm sterile filter element, and put it into a sealed serum bottle;
[0035] C) Place it in a low pH incubation room, and incuba...
Embodiment 3
[0039] Example 3: Detection of 5% and 10% intravenous human immunoglobulin products
[0040] In Example 1 and Example 2, the quality standards of 5% and 10% intravenous human immunoglobulin are the same, see Table 1 and Table 2 below.
[0041] Table 1: List of Quality Standards for 5% Intravenous Human Immunoglobulin Finished Products
[0042] Test items
[0043] Bovine, anti-pig, anti-goat sera do not produce precipitation lines. Immunoelectrophoresis, Master
[0044] Table 2: List of Quality Standards for 10% Intravenous Human Immunoglobulin Finished Products
[0045] Test items
[0046] anti-HBs
[0047] The following three batches of 5% intravenous human immunoglobulin products were selected for quality inspection, and these products were prepared by the production process described in Example 1 or Example 2 respectively.
[0048] Table 3: Quality Inspection Report of 5% Intravenous Human Immunoglobulin
[0049] (batch number ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com