Detection method for content of Tau protein antibodies in human immune globulin product
A human immunoglobulin and immunoglobulin technology, which is applied in measurement devices, biological tests, material inspection products, etc., can solve problems such as difficulty in detection, influence on the accuracy of detection methods, and influence on the accuracy of detection methods, and achieve accurate measurement. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of diluent: Measure bovine serum albumin and sodium phosphate-Tween buffer solution without immunoglobulin respectively, mix and prepare diluent. The mass content of bovine serum albumin without immunoglobulin G in the diluent is 0.5%.
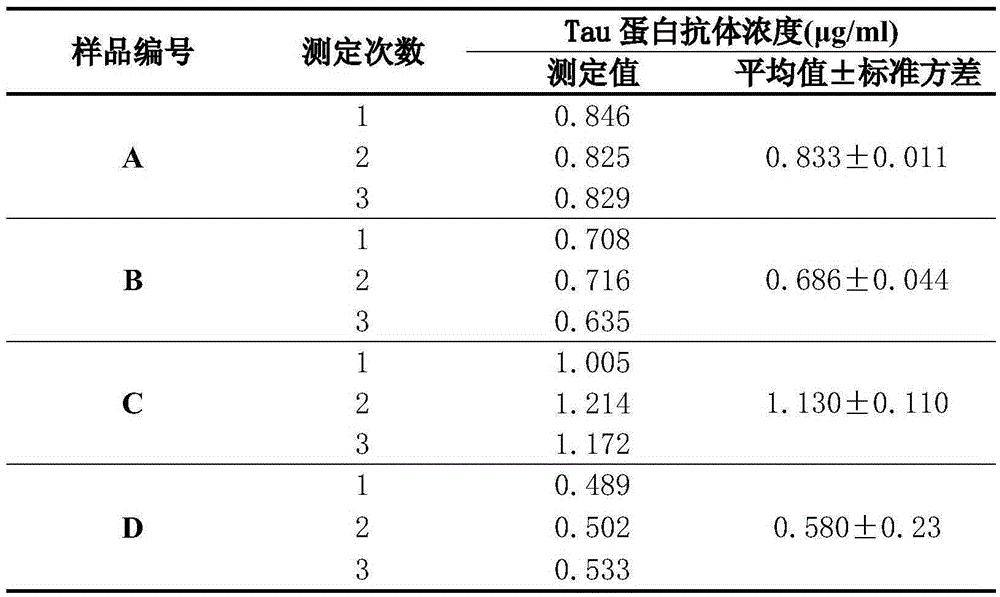
[0045] Preparation of samples to be tested: Human immunoglobulin (IVIG) products selected from four different manufacturers were respectively diluted with diluents to obtain samples to be tested, which were numbered A, B, C, and D accordingly.
[0046] Determination of the absorbance value of the sample to be tested
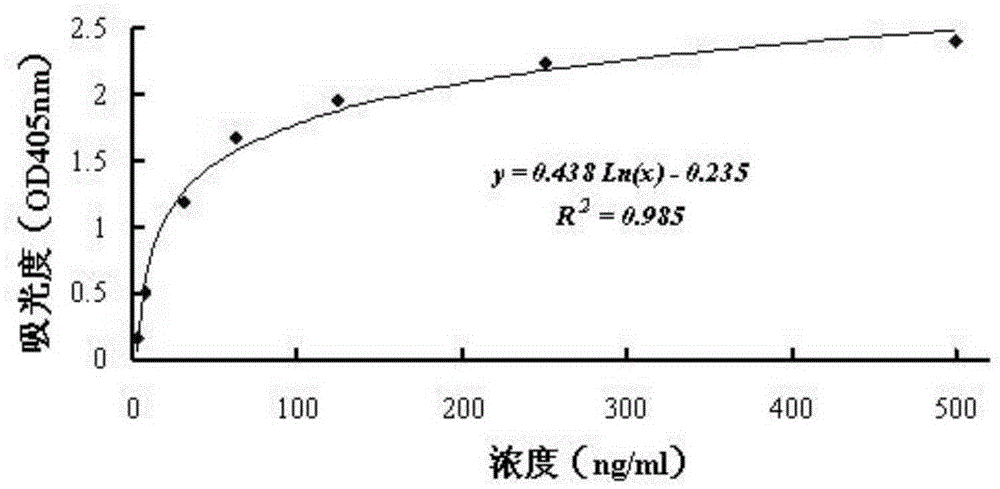
[0047] Add the Tau protein solution with a concentration of 5 μg / ml into each well of the microtiter plate, and then incubate at 4°C for 10-16 hours. After the Tau protein is fully adsorbed on the microtiter plate, wash it with phosphate buffer solution 3 times to remove free components on the microtiter plate.
[0048] After washing, add protein-free blocking solution to each well of the ELISA plate for bloc...
Embodiment 2
[0068] Preparation of diluent: Measure bovine serum albumin and sodium phosphate-Tween buffer solution without immunoglobulin respectively, mix and prepare diluent. The mass content of bovine serum albumin without immunoglobulin G in the diluent is 1.0%.
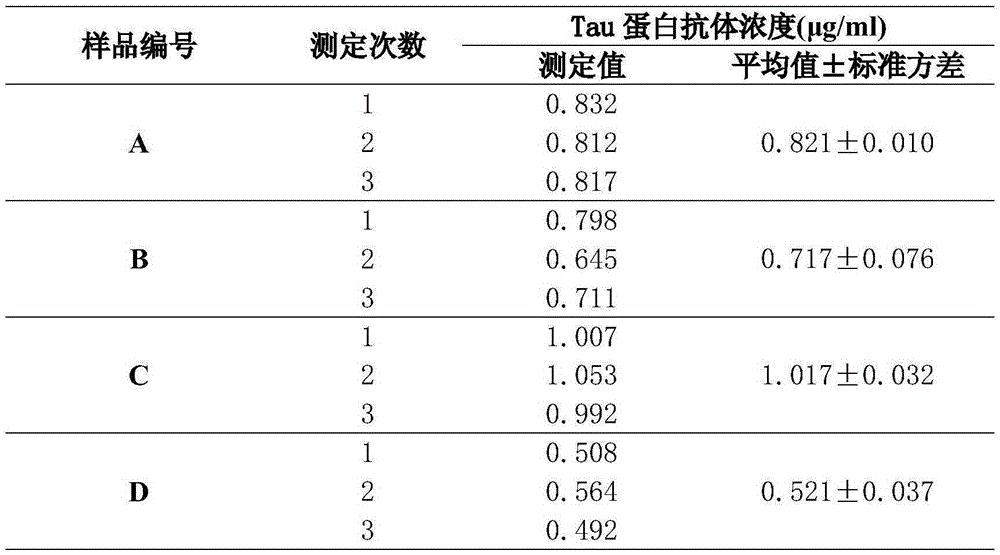
[0069] Preparation of samples to be tested: Human immunoglobulin (IVIG) products selected from four different manufacturers were respectively diluted with diluents to obtain samples to be tested, which were numbered A, B, C, and D accordingly.
[0070] Determination of the absorbance value of the sample to be tested
[0071] Add the Tau protein solution with a concentration of 15 μg / ml into each well of the microtiter plate, and then incubate at 37°C for 5min-2h. After the Tau protein is fully adsorbed on the microtiter plate, wash it with phosphate buffer solution 4 times to remove free components on the microtiter plate.
[0072] After washing, add protein-free blocking solution to each well of the ELISA plate for blocki...
Embodiment 3
[0091] Preparation of diluent: Measure bovine serum albumin and sodium phosphate-Tween buffer solution without immunoglobulin respectively, mix and prepare diluent. The mass content of bovine serum albumin without immunoglobulin G in the diluent is 1.5%.
[0092] Preparation of samples to be tested: Human immunoglobulin (IVIG) products selected from three different manufacturers were diluted with diluents respectively to obtain samples to be tested, which were numbered A, B, and C accordingly.
[0093] Determination of the absorbance value of the sample to be tested
[0094] Add the Tau protein solution with a concentration of 20μg / ml into each well of the microplate, and then incubate at 25°C for 1-4h. After the Tau protein is fully adsorbed on the microplate, wash it with phosphate buffer solution 5 times to remove free components on the microtiter plate.
[0095] After washing, add protein-free blocking solution to each well of the ELISA plate for blocking, and incubate a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com