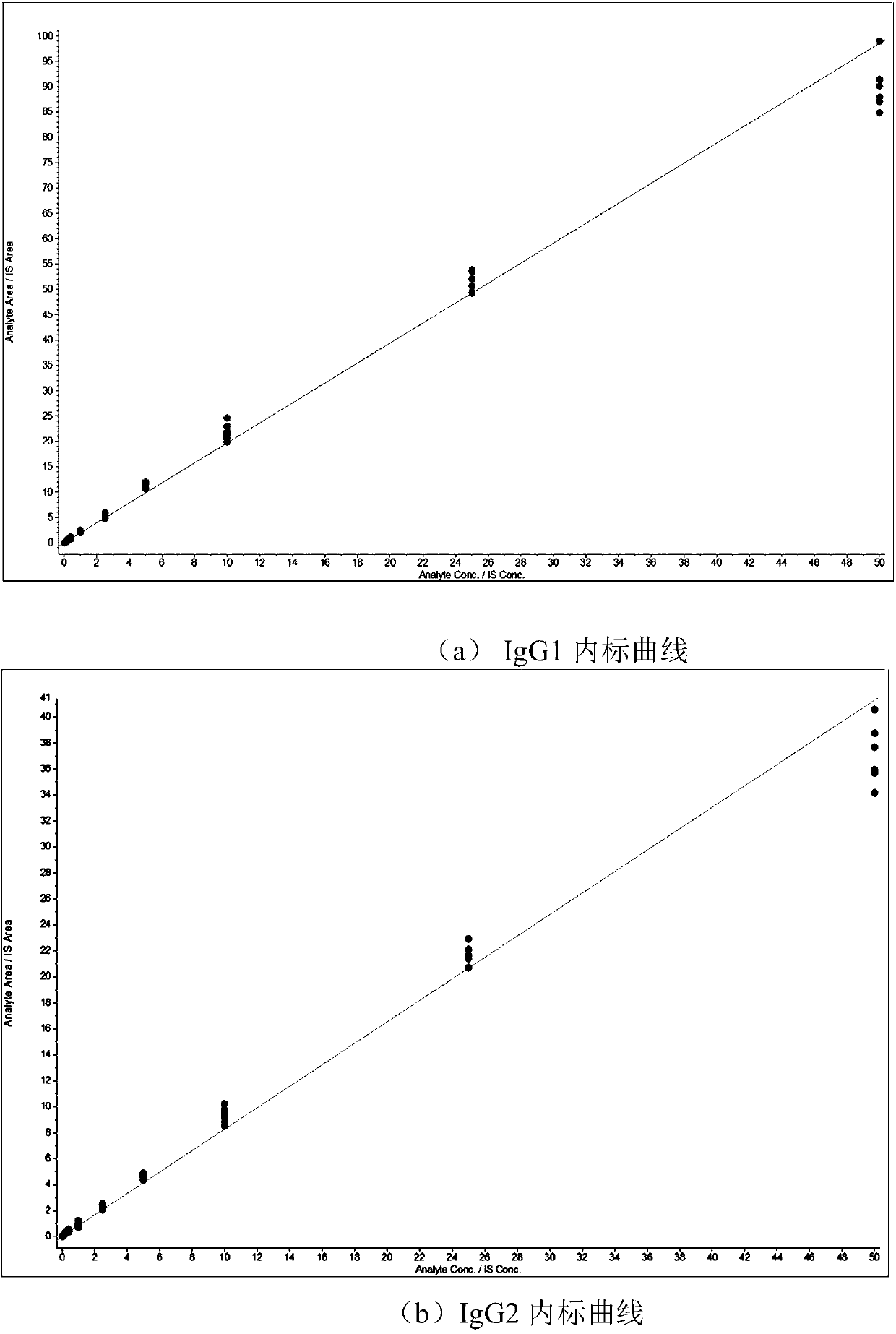
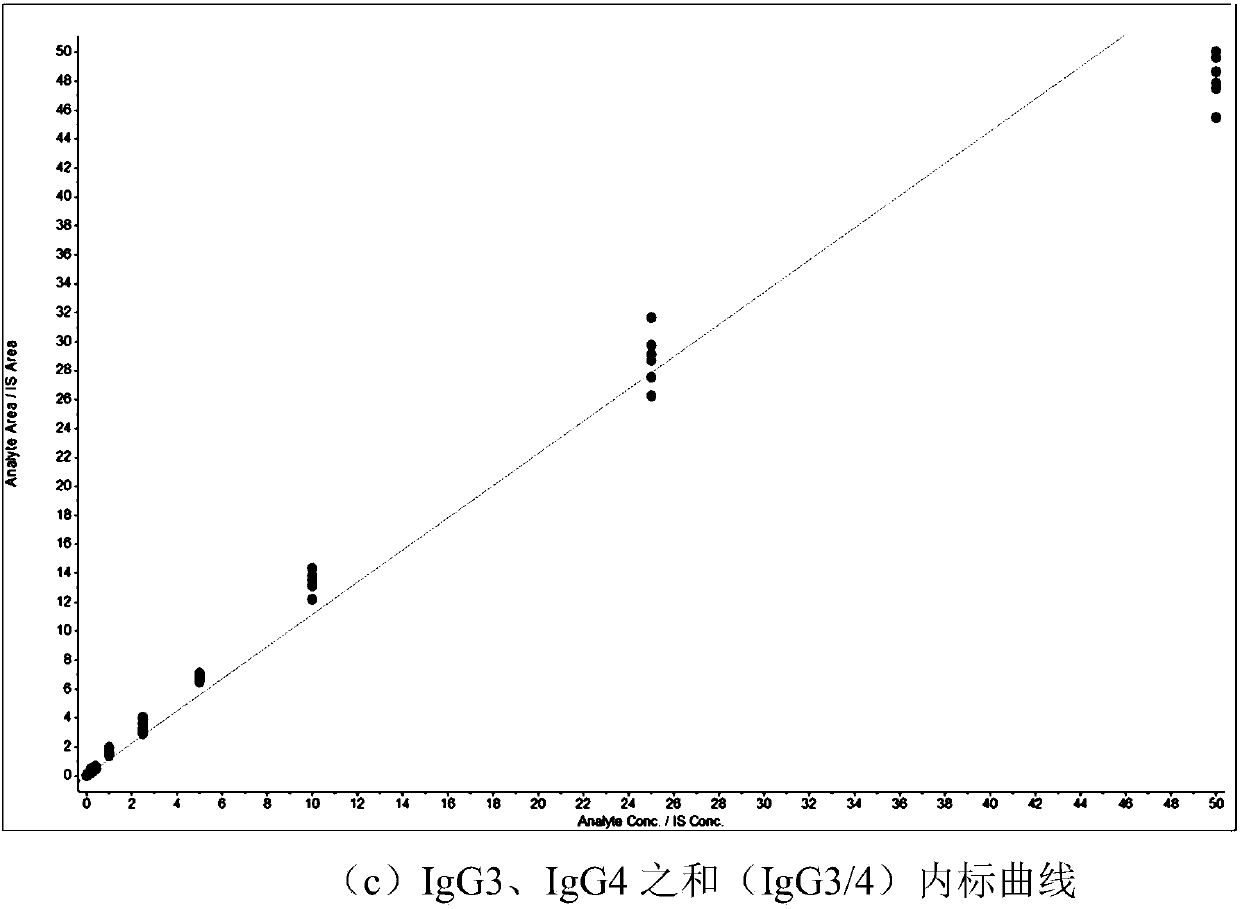
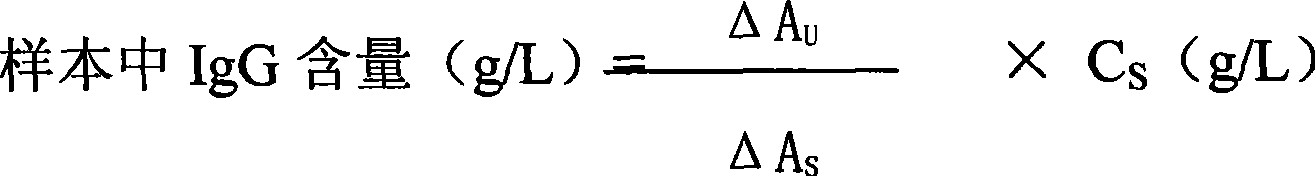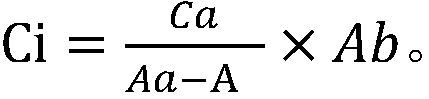Patents
Literature
60 results about "Globulin G" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Compound probiotic agent for improving immunity of dogs and reducing incidence of diarrhea as well as preparation method and application thereof
ActiveCN107058158AImprove immunityControl white blood cell countBacteriaDigestive systemInterleukin 6Feces
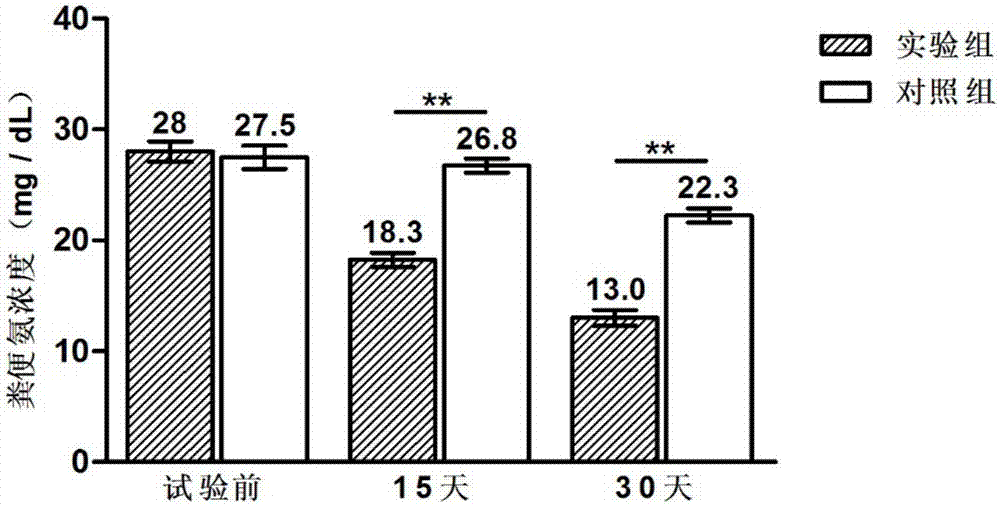
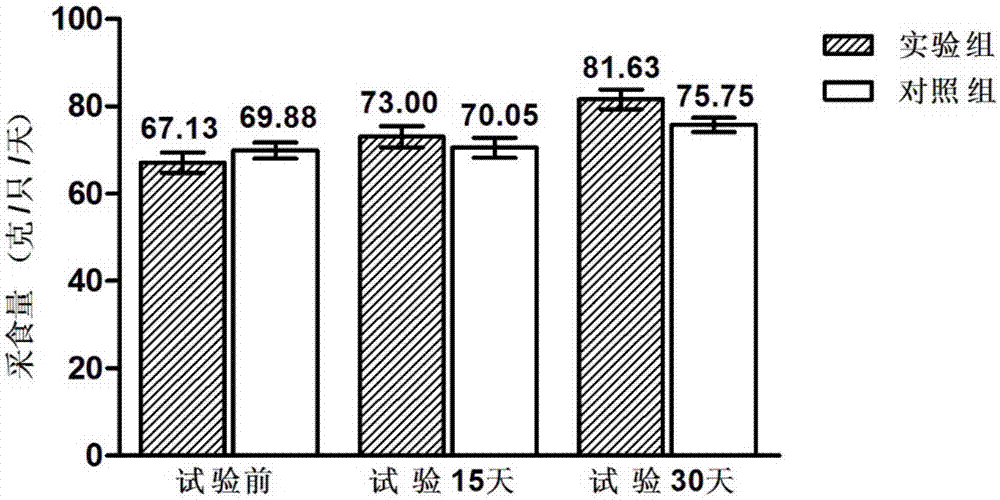
The invention discloses a compound probiotic agent for improving immunity of dogs and reducing incidence of diarrhea as well as a preparation method and application thereof, and belongs to the field of the compound probiotic agent. The compound probiotic agent is prepared by mixing and compounding a lactobacillus plantarum KT-Lp9 microbial agent, a lactobacillus casei zhang microbial agent, a lactobacillus plantarum P-8 microbial agent, a bifidobacterium animalis subsp. lactis V9 microbial agent and a dilution carrier, wherein the quantity of viable bacteria of the four kinds of microbial agents is more than or equal to 2*10<11>CFU / g. The compound probiotic agent with different contents of viable bacteria only can be applied to 96 dogs at different ages for 60 days, so that the immunity of the test dogs can be improved, and the incidence of diarrhea and the sickness degree are reduced. Main representations are that the feed intake and growth rate of the dogs are increased, leukocyte counts, lymphocytes counts and neutrophil counts in blood are controlled, and the contents of four immunologic factors including immune globulin G, interleukin 6, interferon alpha and tumor necrosis factor alpha in the blood can be increased; and meanwhile, the content of secretory immunoglobulin A (SigA) in excrement is increased.
Owner:INNER MONGOLIA SCI PLUS BIOTECH
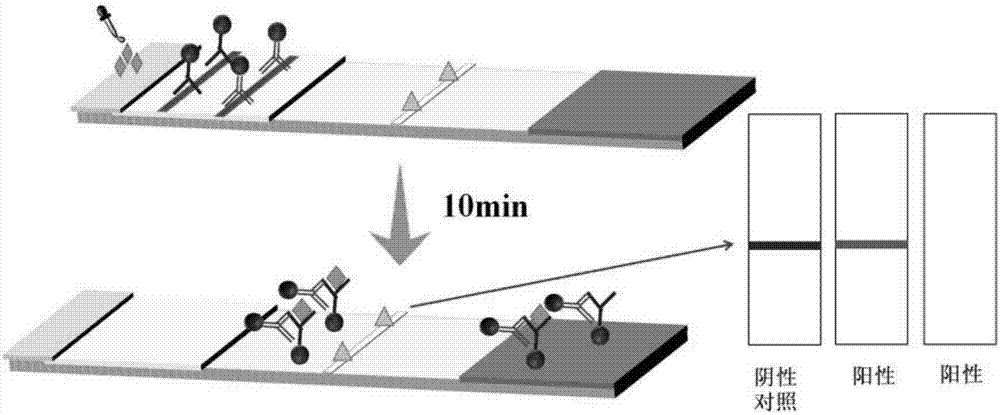
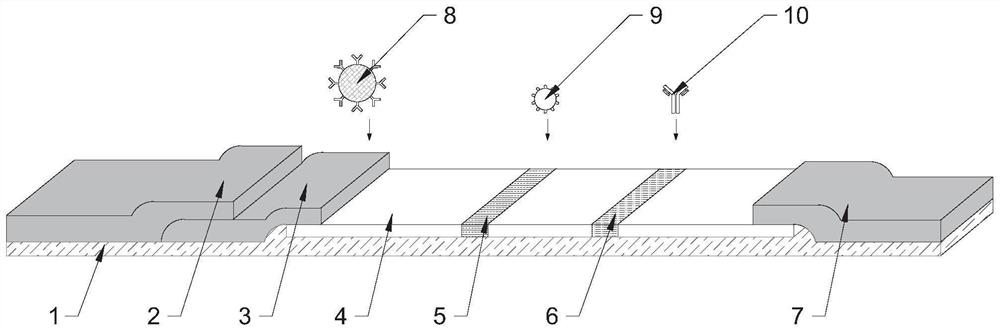
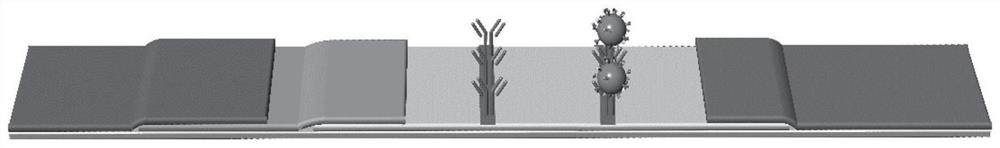
Premature rupture of membrane fast examination tool using ICAM-1 as examination index and examination kit
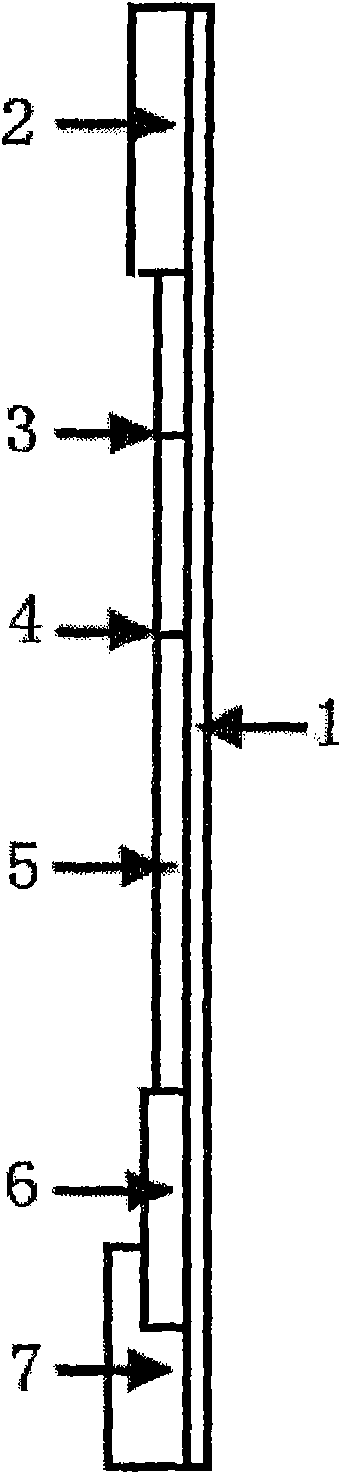
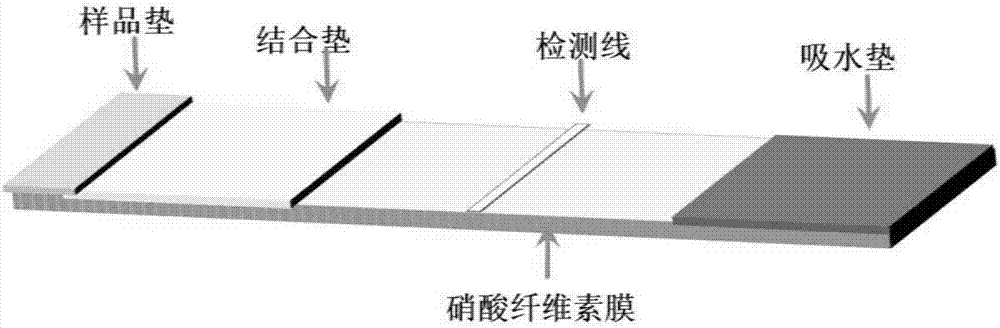
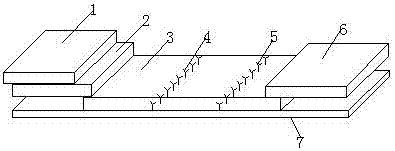
The invention discloses a premature rupture of membrane (PROM) fast examination tool using an intercellular adhesion molecular (ICAM)-1 as an examination index. The tool comprises a gasket, an absorbent pad, a nitrocellulose membrane, a conjugate pad and a sample pad, wherein the absorbent pad, the nitrocellulose membrane, the conjugate pad and the sample pad are connected from top to bottom and adhered to the gasket; the conjugate pad is covered by the sample pad part; the nitrocellulose membrane is provided with a goat-anti-human ICAM-1 polyclonal antibody coated examination line and a goat-anti-mouse immunoglobulin G polyclonal antibody coated control line; the examination line is below and away from the control line at intervals; and the conjugate pad is a water absorbing fibber coated with conjugated mouse-anti-human ICAM-1 monoclonal antibody conjugate. A PROM fast examination kit using the ICAM-1 as the examination index consists of a kit body and the examination tool arranged in the kit body.
Owner:ORIGISSAY BIOLOGICS TECH
Immunoglobulin G detection reagent
The invention discloses a detecting reagent for immunoglobulin G, which comprises an IgG reactant, an anti-IgG antibody reagent and a liquid serological type constant-value calibrating agent, wherein, the IgG reactant can cause IgG antigen sites in a sample to be fully exposed so as to facilitate the full combination between the IgG antigens and the anti-IgG antibody reagent; the anti-IgG antibody reactant has high idiosyncrasy with IgG antigens in human serum; and the liquid serological type constant-value calibrating agent is used for sample comparison and result calculation. The anti-IgG antibody is from mammals of sheep, horses, mice, rabbits, or the like. The IgG reactant and the anti-IgG antibody reagent can be used as two independent reagents to form a double-reagent form of a product and also can be mixed according to certain proportions to form a single-reagent form of the product.
Owner:王贤理
Enzyme-linked immunosorbent kit for inspecting porcine immunoglobulin G and application thereof
InactiveCN101571540AImprove accuracySimple structureColor/spectral properties measurementsElisa kitGlobulin G
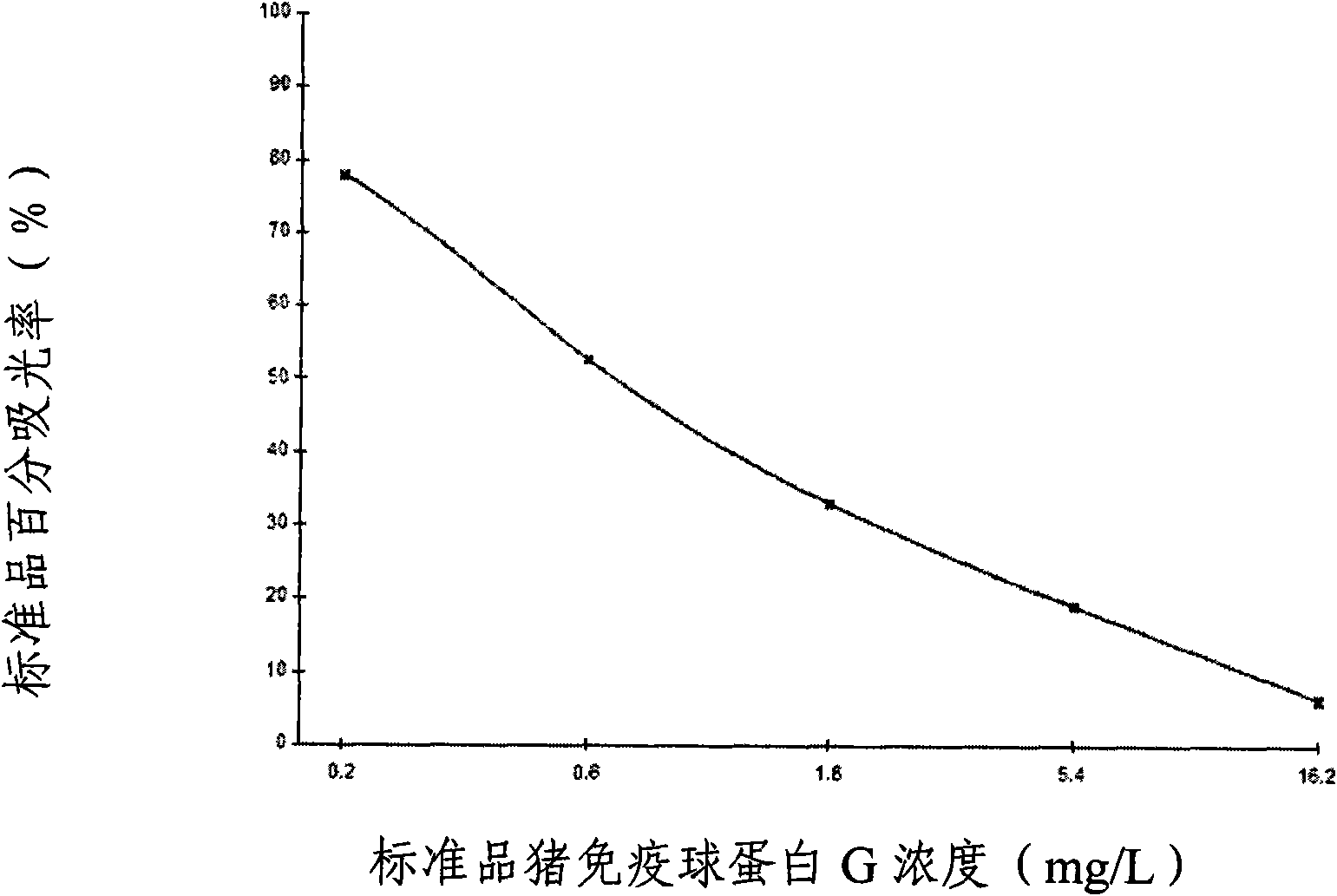
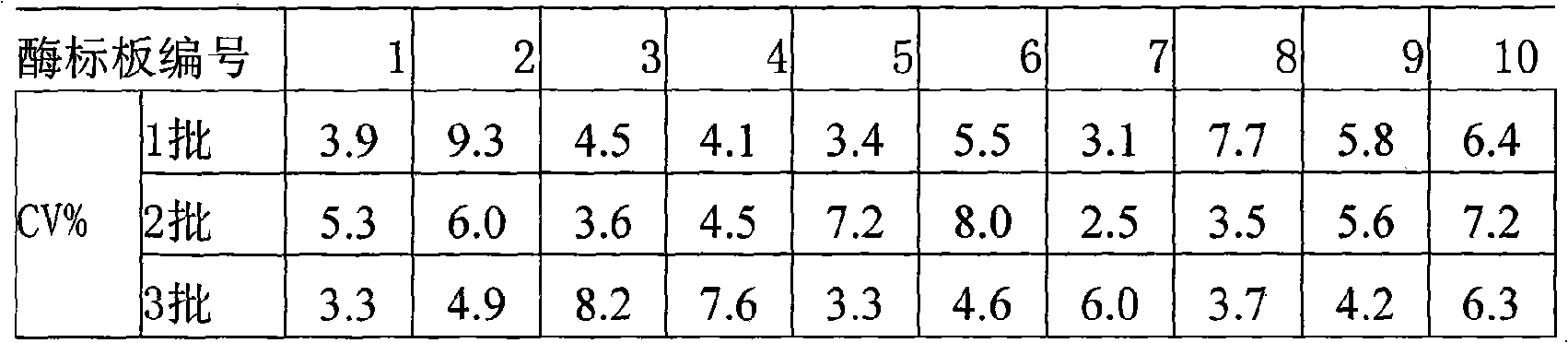
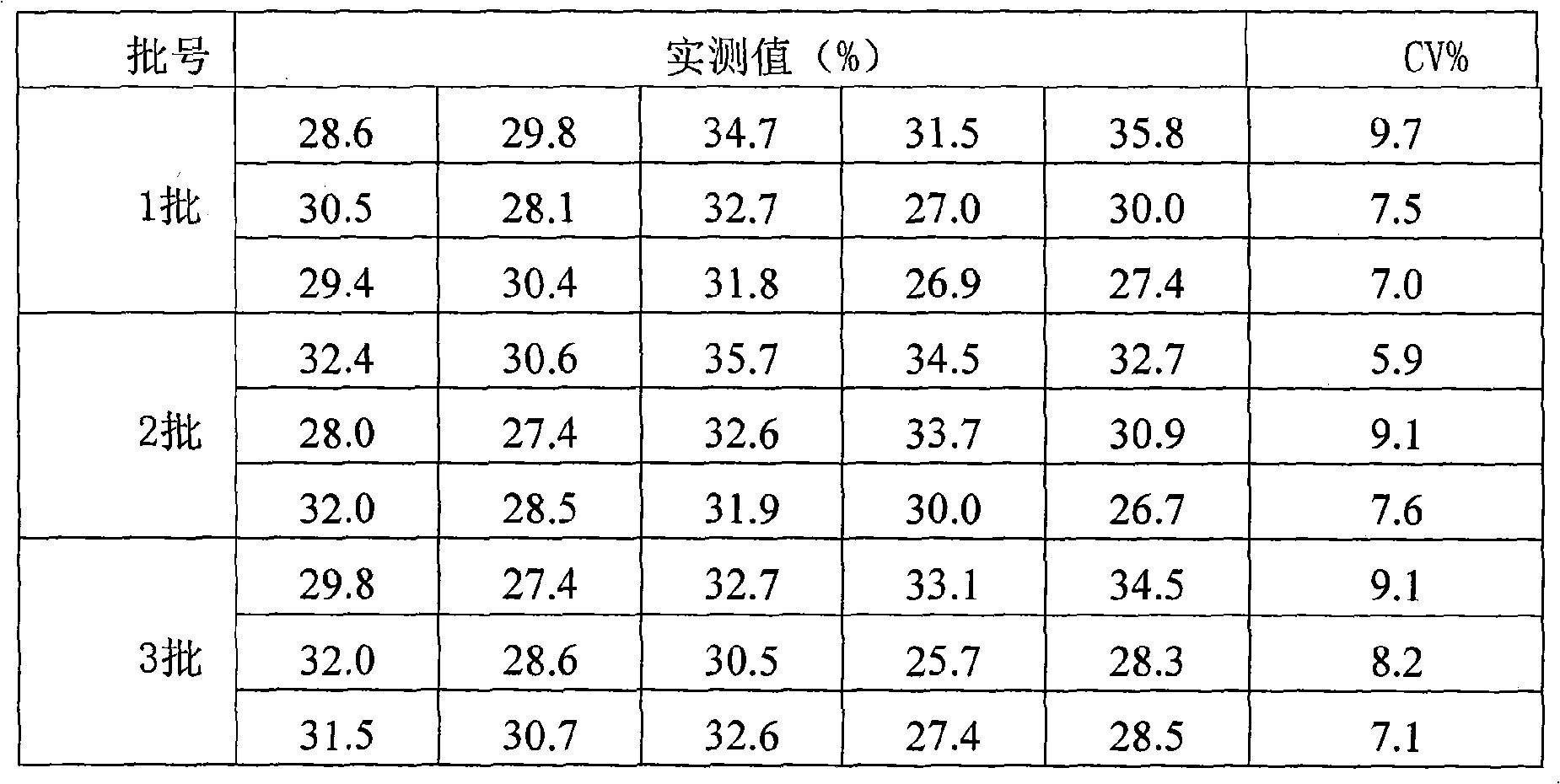
The invention provides an enzyme-linked immunosorbent kit for inspecting porcine immunoglobulin G, comprising an ELISA plate which is coated with coating antigen, an enzyme label, porcine immunoglobulin G specific antibody working liquid (being contained when the antigen is coated on the ELISA plate and the enzyme label is enzyme labeling antibody or antibody is coated on the ELISA plate and the enzyme label is enzyme labeling antigen), porcine immunoglobulin G standard product solution, substrate color development solution, stop solution, concentrated washing liquid and concentrated complex solution. The invention further discloses a method which applies the enzyme-linked immunosorbent kit for inspecting the porcine immunoglobulin G, and the method comprises the steps of firstly carrying out the pre-treatment on a sample, then using the kit for inspecting and finally analyzing the inspection result. The provided enzyme-linked immunosorbent kit can be used for inspecting the content ofthe porcine immunoglobulin G in porcine plasma protein powder and other samples, the operation is simple, the cost is low, and the enzyme-linked immunosorbent kit can be monitored on-site and is appl icable in screening mass samples.
Owner:贵州谱尼测试技术有限公司
Compound lactobacillus micro-ecological preparation and preparation method and application thereof
ActiveCN107964520AStrong stress resistanceHigh survival rate of live bacteriaBacteriaAnimal feeding stuffFecesBiology
The invention provides a compound lactobacillus micro-ecological preparation and a preparation method and application thereof. The compound lactobacillus micro-ecological preparation is prepared by mixed compounding of a lactobacillus plantarum LP6 bacterial agent, a Lactobacillus casei zhang bacterial agent, a lactobacillus plantarum P-8 bacterial agent, a bifidobacterium animalis V9 bacterial agent and a dilute carrier, wherein the viable count of each of the four bacterial agents is larger than or equal to 2*10<11>CFU / g. By application of the compound lactobacillus micro-ecological preparation with the viable count of 1*10<9>CFU / g to 30 different kinds of pet cats for 30 days on trail, the health level of the pet cats can be raised, for instance, the diarrhea incidence rate of the pet cats can be controlled effectively, mental conditions and fur conditions are optimized, hair loss is reduced, the ammonia concentration content of excrement is reduced, and cat litter odor is alleviated; in addition, the feed intake and growth rate are increased evidently, and the content of immune globulins G in blood of the pet cats and the content of secretory immunoglobulins A in excrement of the pet cats are remarkably increased.
Owner:INNER MONGOLIA SCI PLUS BIOTECH
Micro-fluidic chip immunoassay kit and detection method thereof
InactiveCN111077319AReduce consumptionRelieve testing pressureLaboratory glasswaresBiological testingAntigenFluoProbes
The invention discloses a micro-fluidic chip immunoassay kit and a detection method thereof. The kit comprises a micro-fluidic chip, a wafer cover plate and a wafer bottom plate, wherein the wafer cover plate and the wafer bottom plate are bonded up and down. One or more detection units are arranged in the wafer bottom plate, and each detection unit comprises a liquid storage tank, a mixed reaction tank, a detection tank, a waste liquid tank and a first vent hole which are connected in sequence; the mixed reaction tank is fixedly provided with a fluorescent probe corresponding to a detection drug; goat anti-mouse immune globulin G and corresponding drug antigens are fixed in the detection tank. The invention also discloses a detection method using the kit. The method has the characteristics of high flux, low sample consumption, high detection speed, simplicity and convenience in operation and the like; the method has the advantages of no need of an electrode, a voltage or an external magnetic field, no need of electroosmosis driving, hot air pump driving or optical capture micropump driving, simultaneous detection of various veterinary drug residues in various matrixes, suitableness for on-site rapid screening analysis, and great alleviation of the detection pressure of related detection personnel.
Owner:SOUTH CHINA AGRI UNIV
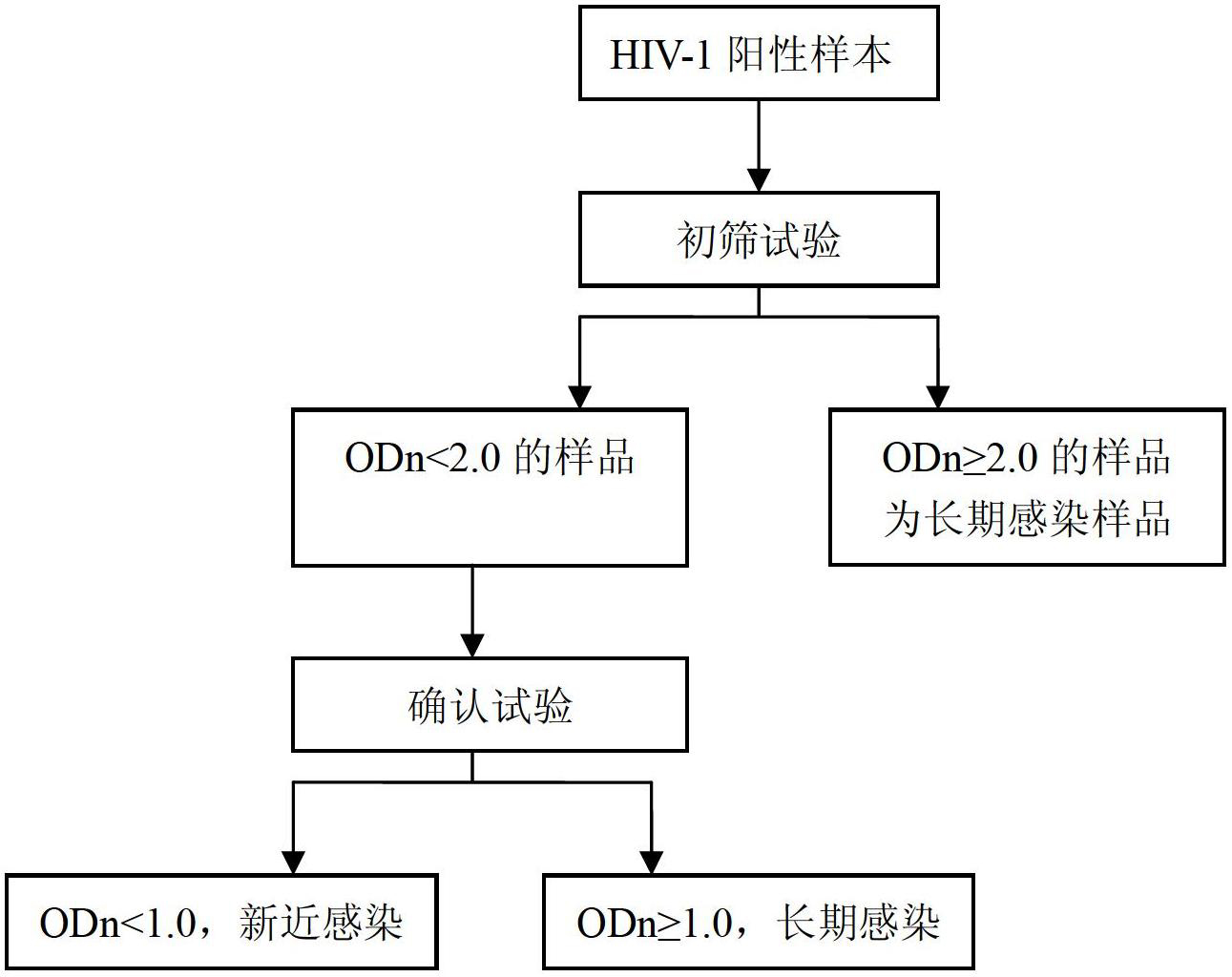
Novel I-type human immunodeficiency virus (HIV-1) infection enzyme test-free reagent kit
The invention provides a novel I-type human immunodeficiency virus (HIV-1) infection enzyme test-free reagent kit. The reagent kit comprises A, HIV-1gp41 protein, B, a blank enzyme linked plate, C, acid eluent, D, goat anti-human immunoglobulin G (IgG) marked with horseradish peroxidase, E, tetramethyl benzidine (TMB) color development solutions and F, stop solutions.
Owner:BEIJING KINGHAWK PHARMA
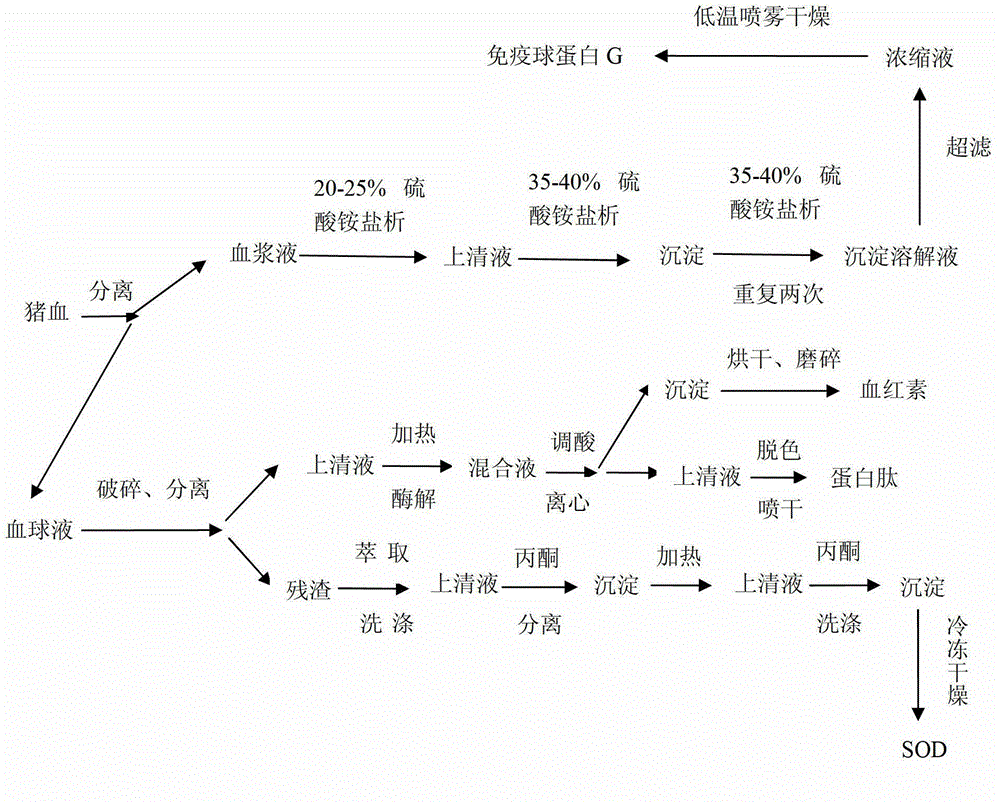
Co-production method for extracting bioactive substances from pig blood
InactiveCN102942628AIncrease profitEasy to operateSerum immunoglobulinsEnzymesFiltrationFreeze-drying
The invention discloses a co-production method for extracting bioactive substances from pig blood. The co-production method comprises immune globulin G extraction, heme iron and protein peptide extraction and superoxide dismutase (SOD) extraction. The immune globulin G extraction orderly comprises carrying out salting-out to remove a fibrous protein and to obtain blood serum and repeating salting-out extraction three times to obtain immune globulin G crude extract. The heme iron and protein peptide extraction comprises breaking blood cells in a blood cell liquid, carrying out press filtration, respectively collecting a supernatant and precipitates, drying the precipitates at a low temperature to obtain heme iron powder, carrying out decoloration of the supernatant by activated carbon, carrying out spray drying to obtain a protein peptide finished product. The SOD extraction comprises collecting residues obtained by the blood cell breaking, carrying out extraction and washing, carrying out press filtration, collecting a supernatant which is a crude enzyme liquid, carrying out separation by acetone, collecting precipitates, heating, washing by acetone, carrying out press filtration, collecting precipitates, washing the precipitates by ethanol, and carrying out freeze drying to obtain SOD. The co-production method fully utilizes raw materials, realizes extraction of four high-additional value products from pig blood, has a high equipment utilization rate and simple extraction processes, and is suitable for industrial production.
Owner:HUAIBEI ENBI FEED
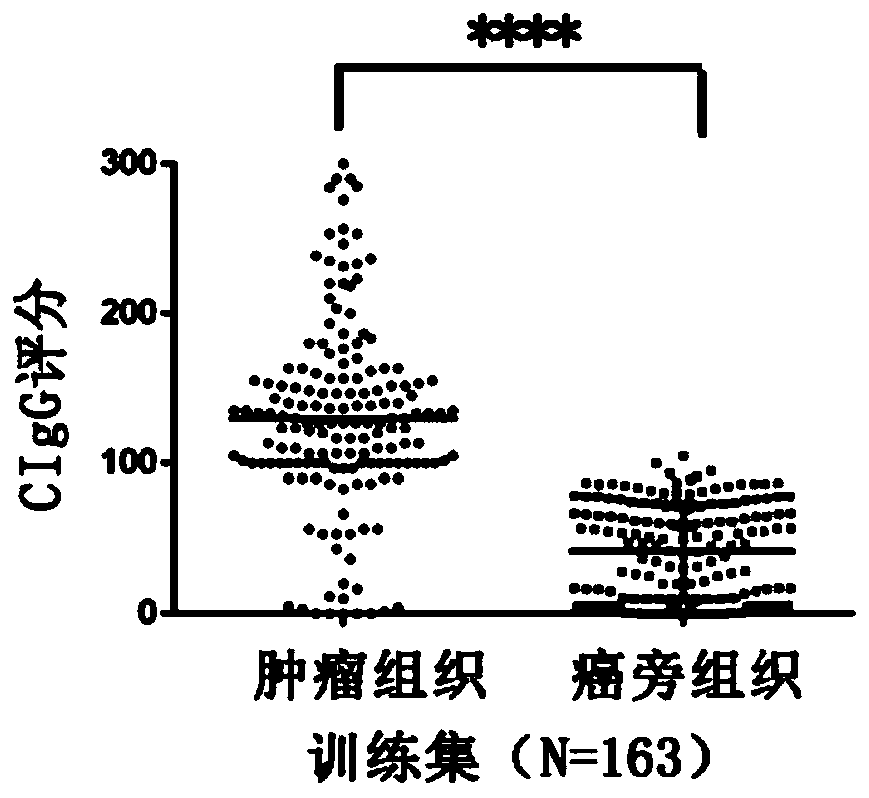
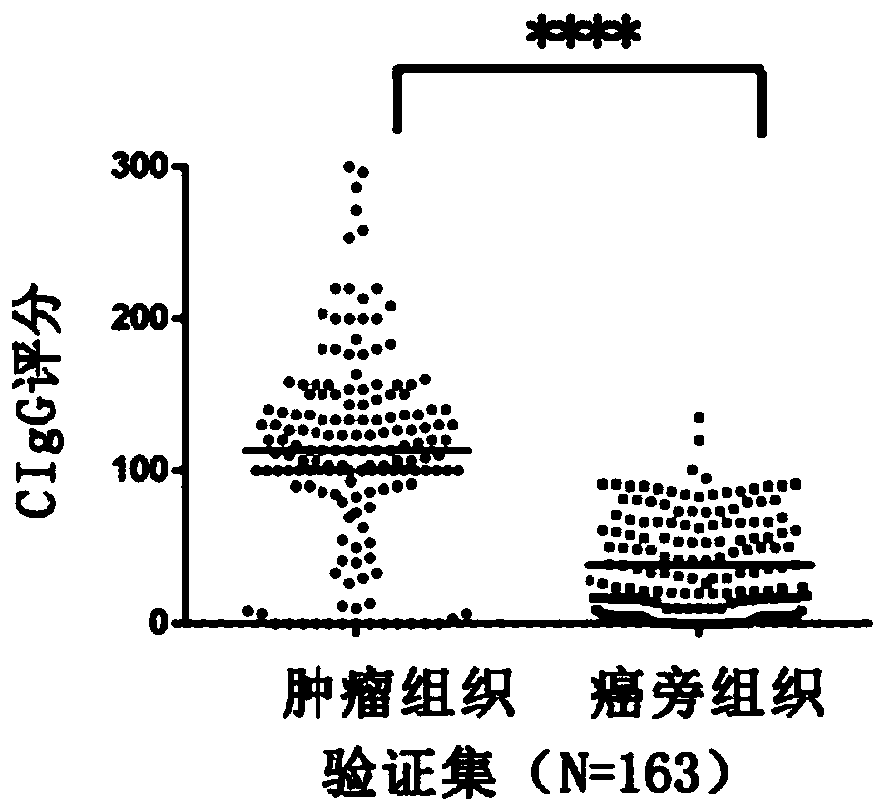
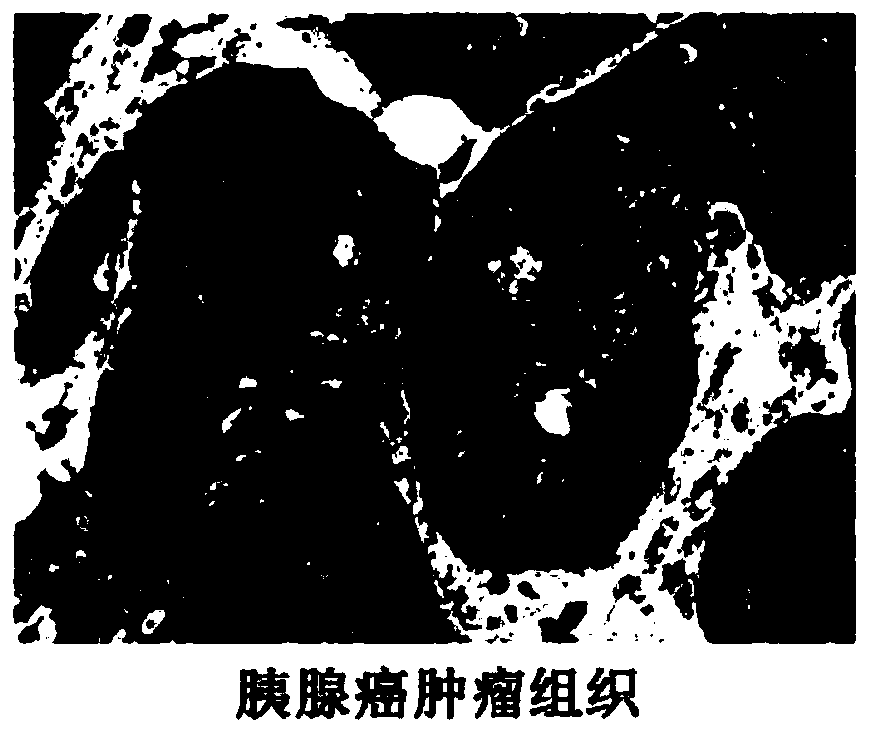
Application of tumor-derived IgG in diagnosis or prognosis of pancreatic cancer
The invention relates to an application of tumor-derived IgG in diagnosis or prognosis of pancreatic cancer. The invention discloses the application of monoclonal antibodies that specifically recognize a human tumor-derived immune globulin G(CIgG) constant heavy-chain region. The application is characterized by utilizing an immunohistochemical or immunofluorescence technique, determining a pancreatic cancer pathological differentiated degree and patient prognosis according to antibody staining pathological scores, and being used for preparing an immunohistochemistry kit for detecting pancreatic cancer differentiated degree and prognosis evaluation. The application provided by the invention has an important application value for improving the accuracy of pancreatic cancer prognosis evaluation in clinical and scientific research, and provides a basis for individualized diagnosis and treatment scheme of tumor.
Owner:PEKING UNIV +1
Absolute quantitative analysis of IgG glycopeptide in serum
ActiveCN109900815AHigh sensitivityHigh repetition rateComponent separationReversed-Phase Liquid ChromatographyIntravenous gammaglobulin
The invention provides an absolute quantitative analysis of immune globulin G (IgG) glycopeptide in serum. The technical process comprises two parts: on the one hand, performing two-dimensional hydrophilic chromatography purification preparation of an IgG glycopeptide standard product and quantitative analysis thereof; on the other hand, performing the IgG glycopeptide absolute quantitative analysis in a serum sample by adopting a standard adding way. The preparation of the glycopeptide standard is performed by adopting the standard IgG, the preparation comprises: releasing of the glycopeptide, enriching of IgG glycopeptide through a reversed-phase chromatography, and separation purification and mass spectrum representation of the two-dimensional hydrophilic chromatography; the quantitative analysis performs PNGase F carbohydrate chain releasing on the glycopeptide standard, performing MRM quantitative analysis on the deglycosylated IgG glycopeptide fragment by adopting an internal standard curve, thereby acquiring molar concentration of each purified IgG glycopeptide; the IgG glycopeptide content determination in the serum is realized through a standard adding method, that is, onepart is added with the IgG glycopeptide standard with the known concentration, and the other part is free from adding the IgG glycopeptide standard, and then the MRM analysis is performed, the content of the IgG corresponding glycoform glycopeptide in the serum is computed according to the peak area difference and the adding standard glycopeptide concentration. On this basis, the glycopeptide absolute corresponding factor can be computed.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of high-immune plasma protein powder
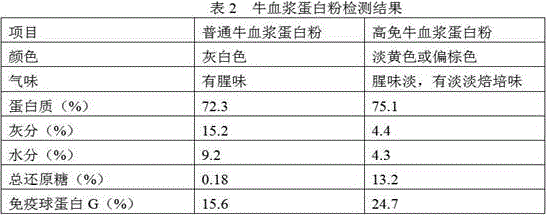
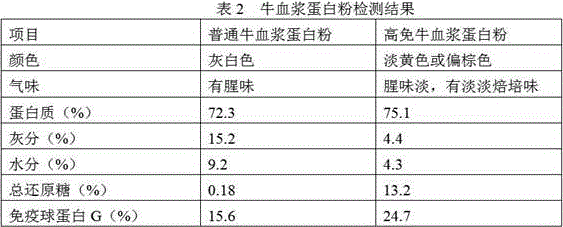
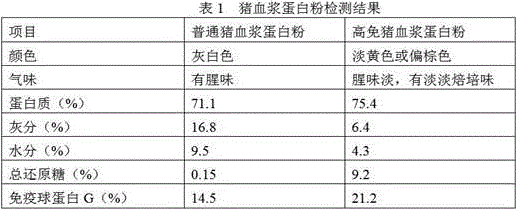
InactiveCN105851455AImprove stabilityIncrease G contentProtein composition from bloodAccessory food factorsGlobulin GHigh pressure
The invention relates to a preparation method of high-immune plasma protein powder. According to the method, fresh plasma liquid of healthy slaughtered livestock and poultry as a raw material, a process method including the steps of inorganic membrane filtering, organic membrane concentrating, reducing sugar adding, high-pressure homogenizing and spray drying is adopted, the prepared high-immune plasma protein powder basically has no blood smell, the protein content of the high-immune plasma protein powder reaches 75% or above, and the immune globulin G content of the high-immune plasma protein powder is increased by 40% or above compared with common plasma protein powder; reducing sugar and plasma immune globulin particles are prepared through reducing sugar adding, homogenizing and centrifugal atomization treating, damage of the high temperature to immune globulin in the spray drying process is reduced through the affinity effect of rich hydrophilic hydroxyl groups in reducing sugar to water, therefore, the activity of the immune globulin is guaranteed, and preparation of the high-immune plasma protein powder is achieved.
Owner:HUAIBEI ENBI FEED
Detection method for measuring titer of antigens of enterovirus71 inactivated vaccine
InactiveCN101881774AHigh sensitivityStrong specificityColor/spectral properties measurementsVaccine antigenImmuno detection
The invention relates to the technical field of biology, in particular to an enzyme-linked immunological detection method for measuring the titer of antigens of an enterovirus71 inactivated vaccine. The method adopts goat-anti-mouse immunoglobulin G antibody labeled with horse radish peroxidase as an antibody so as to be beneficial to amplifying developing signals and improving sensitivity of detection; generally, the detected titer of the virus is 3.7-4.3 LogTCID50 / ml virus antigen, and when detection is carried out on the EV71 inactivated vaccine, a series of complex and time-consuming work, such as cell culture and the like, do not need to be carried out, thus shortening the detection time to 4 hours from the general days; and when being adopted for enzyme-linked immunological reaction, the method has the advantages of low background, and the A value of a negative control hole is generally less than 0.08. The detection method established by the invention is an enzyme-linked immunological detection method for measuring the titer of antigens of an enterovirus71 inactivated vaccine, which has good specificity, high sensitivity, good repeatability, simpleness and convenience.
Owner:ZHEJIANG PUKANG BIOTECH
High-cost-performance human blood immune globulin G detection reagent kit
InactiveCN105572384AImprove detection efficiencySmall sample sizeBiological testingLatex particleGlobulin G
Owner:BYRON DIAGNOSTICS SHANGHAI
Hypericin albumin nanoparticle-immune globulin G antibody conjugate and preparation method thereof
ActiveCN102579354AImprove efficacyImprove targetingOrganic active ingredientsPowder deliveryHypericinAntibody conjugate
The invention discloses a hypericin albumin nanoparticle-immune globulin G antibody conjugate, which comprises the following preparing steps: step (1), dissolving the hypericin albumin nanoparticle and the immune globulin G antibody conjugate into phosphate buffer solution (PBF), step (2), adding glutaraldehyde into the solution obtained by the step (1), and obtaining the hypericin albumin nanoparticle-immune globulin G antibody conjugate. The hypericin albumin nanoparticle-immune globulin G antibody conjugate has the advantages of high effective utilization; simultaneously, the preparation method of the hypericin albumin nanoparticle-immune globulin G antibody conjugate has the advantages of being simple in process, easy to operate and specially suitable for industrial production.
Owner:GUANGDONG HINAPHARM PHARMA CO LTD
Preparation of thiophilic magnetic compound microballoons and method for separating immune globulin G using the compound microballoons
InactiveCN101279246BOther chemical processesPeptide preparation methodsPolymer scienceDivinylbenzene
The invention provides a preparation method for a thiophilic composite microsphere and a novel method for separating and purifying serum immunoglobulin G (IgG) with high purity and high activity in batch, which has simple and convenient operation. In the invention, ferroferric oxide particles with superparamagnetism are prepared by redox reaction, magnetic microspheres are prepared by miniemulsion polymerization technology, the full and complete coating of magnetic particles with divinylbenzene monomers is realized making use of the concentration difference of moisture repel and vinyl ester is added to carry out a second coating, and the magnetic microspheres are prepared by free radical polymerization. After full alcoholysis reaction, large quantity of active functional groups appear on the surface of the magnetic microspheres, which the thiophilic magnetic composite microsphere is formed by Michael addition reaction and grafting thiophilic petunidin such as sulfhydryl-nicotinic acid, etc., thus realizing specific recognition and separation for human immunoglobulin.
Owner:HUAQIAO UNIVERSITY
Four causative agent differential immunoglobulin G antibody and affinity index testing kit
A reagent kit of specificity IgG and its affine index detection is prepared by planting giant cell virus AD169 plant, herpes simplex virus I type SM-44 plant, herpes simplex II type G pland on diploid cell of human embryo lungs, planting nettle rash virus Gos plant, bowworm RH plant on Vero cell, obtaining antigen Via developing, encapsulating polystrene ELISA reaction plate after tiration and purification to be certain concentration as well as preparing specimen dilution liquid developer solution, concentration cleaning liquid and so on.
Owner:国家人口和计划生育委员会出生缺陷干预工程技术中心 +1
Urine special protein composite quality control substance and preparation method thereof
PendingCN110333356AAchieve quality controlReduce the influence of matrix effectsDisease diagnosisBiological testingCreatinine riseProtein detection
The invention relates to the technical field of urine special protein detection, in particular to a urine special protein composite quality control substance and a preparation method thereof, the quality control substance comprises a low-value positive quality control substance and a high-value positive quality control substance, and the low-value positive quality control substance and the high-value positive quality control substance respectively comprise a buffer agent, urea, salts, a preservative, creatinine, PH, a protein protective agent, a stabilizer, human albumin, transferrin, retinolconjugated protein, alpha1-microglobulin, beta2-microglobulin, immune globulin G, neutrophil gelatinase associated lipocalin. The urine special protein composite quality control substance and the preparation method thereof provided by the invention have the advantages of simple operation, instant use after decapping, good stability, low production cost and easiness to achieve the quality control of a urine special protein detection system.
Owner:URIT MEDICAL ELECTRONICS CO LTD
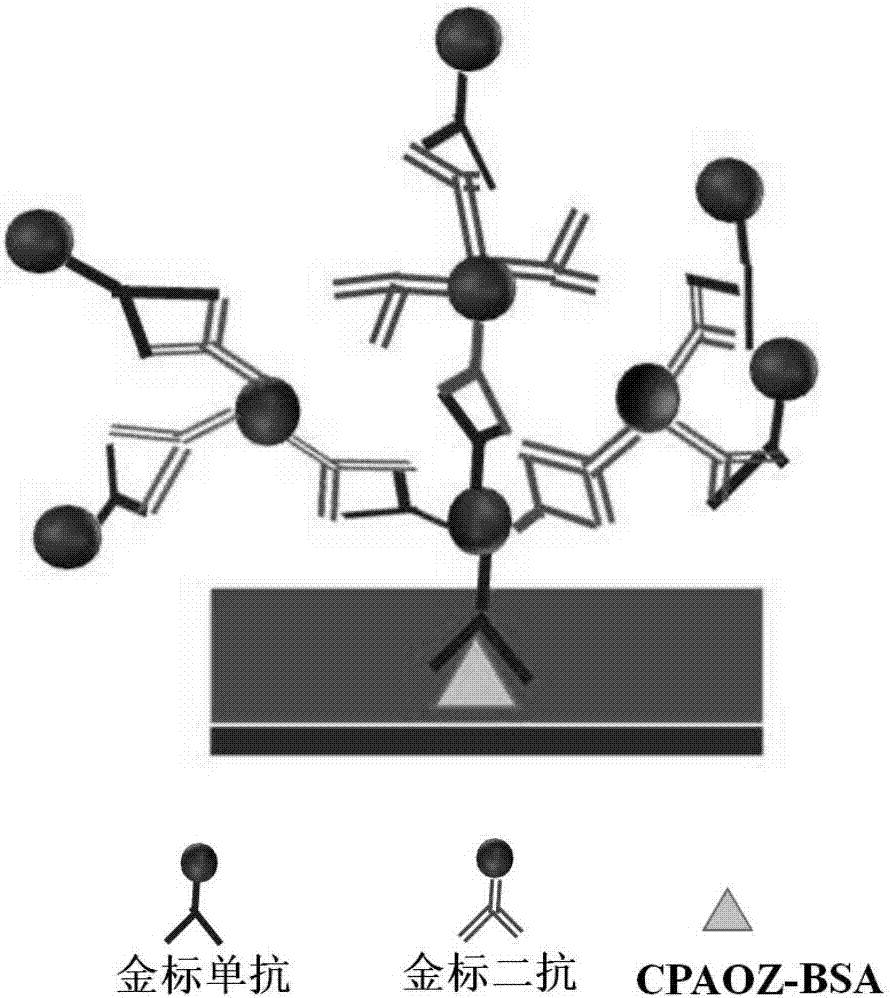
Biological probe, test strip for detecting furazolidone and applications thereof
InactiveCN107515299AHigh sensitivityTo amplify the signalBiological testingTesting medicinal preparationsNetwork structureGlobulin G
The invention discloses a biological probe, a test strip for detecting furazolidone and applications thereof. A nano gold labeled anti-furazolidone antibody is taken as the detection probe. A nano gold labeled goat-anti-mouse immune globulin G is taken as the enhancing probe. Two probes are sprayed on a hydrophobic pad. When a sample solution flows, two probes are eluted from the hydrophobic pad; the gold of two probes is aggregated through the combination of antibody and second antibody, a complicated network structure is formed, the gold flows forward under the capillary action, a clear red strip is formed in the T line, a signal amplifying effect is achieved, at the same time, the using amount of antibody is obviously reduced, more fierce competing reactions are triggered, and the sensitivity is improved.
Owner:NORTHWEST A & F UNIV
Ice-cream contg. immune globulin-G biological factor
InactiveCN1785031AStrong bifidus proliferationFrozen sweetsAntibody ingredientsDiseaseBifidobacterium
An immunoglobulin-G biologic factor ice cream for preventing and treating diseases and delaying sanility features that its raw materials includes immunoglobulin-G, the bifidobacterium reproduction factor composition including bifidobacterium, erythrol, lactitol and oligose, beta-carrotene, VE, VC, etc.
Owner:刘利 +1
Aggregation-induced emission microsphere based on N-hydroxyethyl-1, 8-naphthalimide tetraphenyl ethylene derivative and application of aggregation-induced emission microsphere
PendingCN114774106AHigh sensitivitySimple and fast operationBiological material analysisBiological testingCelluloseMicrosphere
The invention discloses an aggregation-induced emission microsphere based on an N-hydroxyethyl-1, 8-naphthalimide tetraphenyl ethylene derivative and application of the aggregation-induced emission microsphere, and belongs to the field of analysis and detection. The aggregation-induced emission microspheres are prepared by taking N-hydroxyethyl-1, 8-naphthalimide tetraphenylethylene derivatives as AIE molecules through a swelling method, then the aggregation-induced emission microspheres are used for preparing the immunochromatography test strip for quantitatively detecting amino-terminal B-brain natriuretic peptide, and the test strip structurally comprises a PVC (polyvinyl chloride) bottom plate, a nitrocellulose membrane, a binding pad, a sample pad and a water absorption pad. An aggregation-induced emission microsphere-labeled anti-amino-terminal B brain natriuretic peptide labeled antibody is sprayed on a combination pad of the immunochromatography test strip, the nitrocellulose membrane is provided with a detection area (T line) and a control area (C line), and a specific labeled antibody (coated antibody) and an anti-immune globulin G antibody (secondary antibody) are respectively sprayed on the detection area (T line) and the control area (C line). Compared with a traditional FITC fluorescent test strip, the sensitivity is improved by 14 times, and the fluorescent test strip has the advantages of being easy and convenient to operate, rapid in quantification, convenient to carry, small in sample dosage and the like.
Owner:NANCHANG UNIV
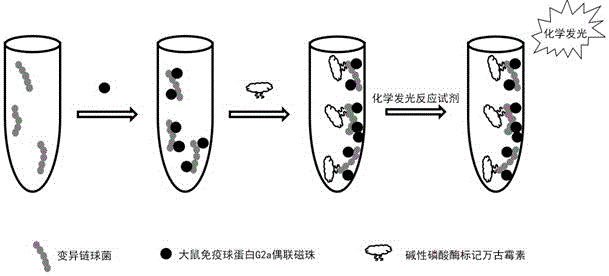
Chemiluminiscence detection kit of streptococcus mutans and application method of chemiluminiscence detection kit
InactiveCN106199001AStable detectionQuick checkChemiluminescene/bioluminescenceDisease diagnosisSphaerotrichia divaricataMagnetic bead
The invention discloses a chemiluminiscence detection kit of streptococcus mutans and an application method of the chemiluminiscence detection kit. The kit comprises immune globulin G coupling magnetic beads, enzyme-labeled vancomycin, an enzymatic chemiluminiscence substrate, a standard streptococcus mutans suspension, a buffer solution and washing liquid. The application method is characterized in that the vancomycin which is simple, cheap and stable is used as the molecular-recognition reagent, the vancomycin is used along with the specific molecular-recognition reagent immune globulin G to form a double-site molecular recognition mode, and the double-site molecular recognition mode is combined with the enrichment and separation technology and the enzymatic chemiluminiscence technology to perform whole-cell detection of the streptococcus mutans. The detection kit is fast in detection, convenient, accurate, specific, sensitive, stable and cheap, is hopefully applicable to the field detection and fast screening of the streptococcus mutans, and is capable of providing a powerful technical support platform for the streptococcus mutans detection in fields such as clinical diagnosis, food safety and environment detection.
Owner:SOUTHWEST UNIVERSITY
Artificial antigen, antibody of fenvalerate and uses thereof
The invention discloses a fenvalerate artificial antigen, with the molecular structural formula is as formulaó±, n=1-5, employing formula ó� as partial antigen, covalent coupling with protein for synthesis; the combination ratio between the partial antige and protein is 5:1-100:1. The invention also discloses the monoclonal or polyclonal immune globulin G which is got from immunizating mouse or rabbit by the above said fenvalerate artificial antigen and can occur specific immunological reaction with fenvalerate, the said fenvalerate specific antibody can be used to check the residual quantity of fenvalerate in sample. The invention also discloses a detecting agent box of direct and indirect competeing enzyme and immunoadsorption for fenvalerate residual analysis. The got antibody is used to detect fenvalerate by ELISA method, with the lowest detecting limit being 4.0í‚1.5 ug / l (0.004ppm), and the detecting sensitivity is high.
Owner:ZHEJIANG UNIV
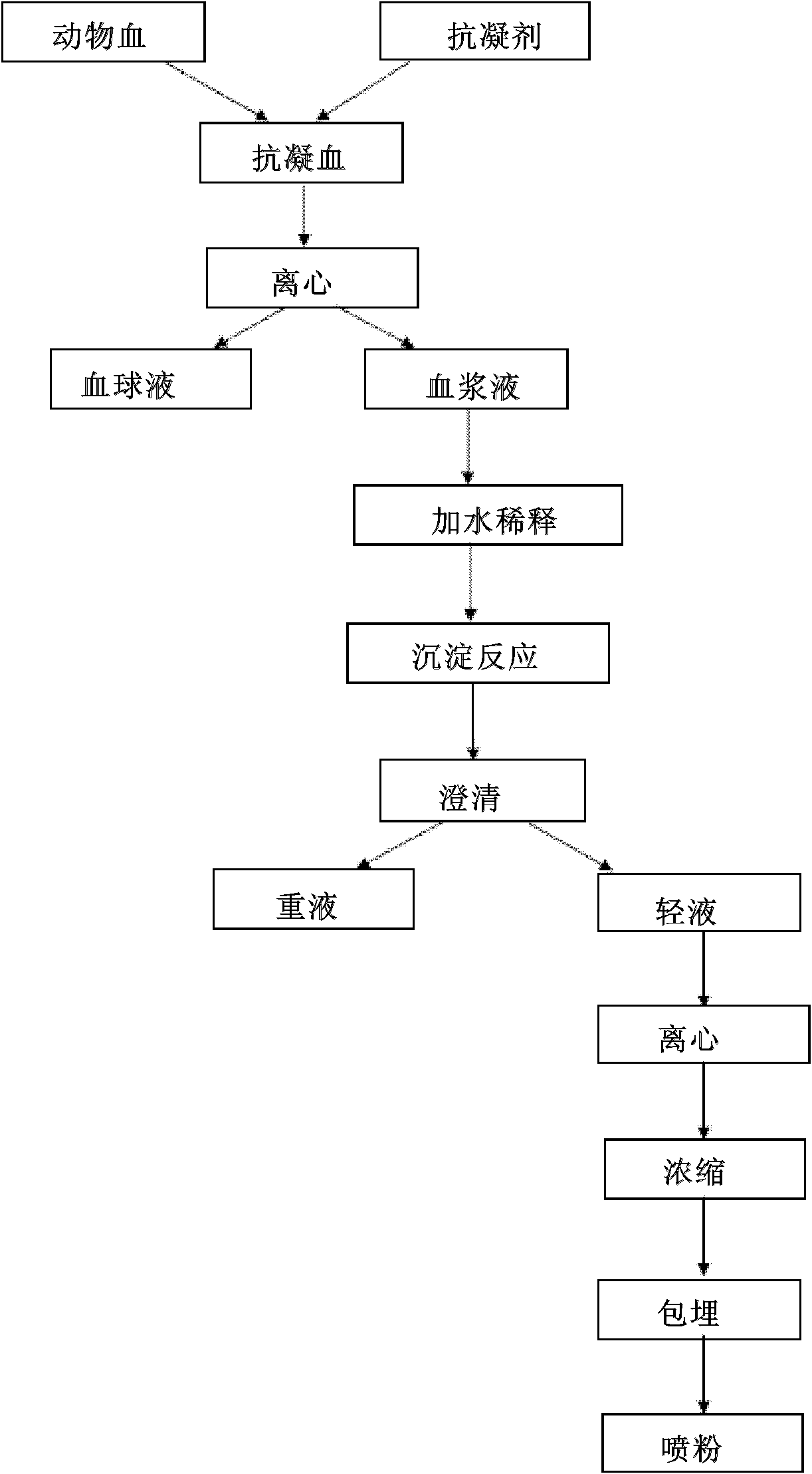
Production method and application of immune globulin G
InactiveCN103044543ASimple and safe processEasy to operateSerum immunoglobulinsDigestive systemBiotechnologyDisease
The invention relates to a production method and an application of an immune globulin G. Specifically, the production method comprises steps of: carrying out whole blood anticoagulating, centrifugal separation, diluting, FeCl3 precipitation reaction, clarifying, centrifugal separation, nano filtration and desalination, embedding, spray-drying, etc. The production method disclosed by the invention not only solves the problems of resource waste, environmental pollution and the like of animal byproduct pig blood, but also greatly improves the utilization value of the pig blood through continuous mass production processing process; the prepared immune globulin G, through an enzyme-linked immuno sorbent assay (ELISA) activity identification, is greater than 90% in activity, and has a special treating effect on intestinal diseases of small animals; and the immune globulin G can be used as an immuno potentiator for a weak animal group and as normal nutrition supplementary for the body.
Owner:SHANGHAI GENON BIOLOGICAL PROD
Method for polyclonal immunoglobulin G production by human B cells
This application relates to an in vitro method of producing a polyclonal IgG preparation. The method comprises (i) placing a polyclonal B-cell population enriched in IgG-secreting B cells in a culture medium; and (ii) culturing the polyclonal B-cell population under conditions enabling the production of the polyclonal IgG preparation from the polyclonal B-cell population. This improved method enables the production of antibodies (preferably IgG) and facilitates long-term culture of polyclonal B-cell populations.
Owner:HEMA QUEBEC +1
Medicine composition treating obesity and application of medicine composition
ActiveCN104524568ALose weightLow in fatHydroxy compound active ingredientsPeptide/protein ingredientsGlobulin GHigh fat
The invention discloses a medicine composition treating obesity and an application of the medicine composition and relates to immune globulin G and the application of the combined usage of the immune globulin G and a medicine capable of simulating food limiting in treating and / or preventing the obesity. By means of the weight and fat losing experiment through the immune globulin G under the food limiting condition, it is proved that the immune globulin G can promote the reduction of the weight and the fat content of normal mice and high-fat induction obesity mice. By means of the experiment of combining the medicine capable of simulating the food limiting and the immune globulin G to reduce the weight and fat, it is proved that the combination of the immune globulin G and the medicine capable of simulating the food limiting can promote the reduction of the weight and the fat content of the mice. A new application is provided for the immune globulin G, and a more effective treatment medicine is provided for treating the obesity.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Gold label kit using NCAM-1 (neural cell adhesion molecule-1) as detection index and preparation method and application thereof
The invention relates to a gold label kit using NCAM-1 (neural cell adhesion molecule-1) as a detection index, which aims at obtaining a kit for detecting activeness of lupus nephritis. The gold label kit comprises a substrate, a water absorbing pad, a nitrocellulose membrane, a gold label pad and a sample pad, wherein the sample pad, the gold label pad, the nitrocellulose membrane and the water absorbing pad are sequentially connected to and cover the substrate; one part of the gold label pad is covered by the sample pad; a detection line coated by any of mouse-anti-human / rabbit-anti-human NCAM-1 monoclonal antibody or rabbit-anti-human / sheep-anti-human NCAM-1 polyclonal antibody, and a detection line coated by any of sheep-anti-mouse immune globulin G and sheep-anti-rabbit immune globulin G polyclonal antibodies are arranged on the nitrocellulose membrane; the gold label pad is coated by a complex which is formed by coupling of any of colloidal gold and mouse-anti-human / rabbit-anti-human NCAM-1 monoclonal antibody. The kit is used for detecting the NCAM-1 concentration in a urea sample, so as to judge the activeness of lupus nephritis, thereby realizing quick and non-wound detection.
Owner:THE WEST CHINA SECOND UNIV HOSPITAL OF SICHUAN
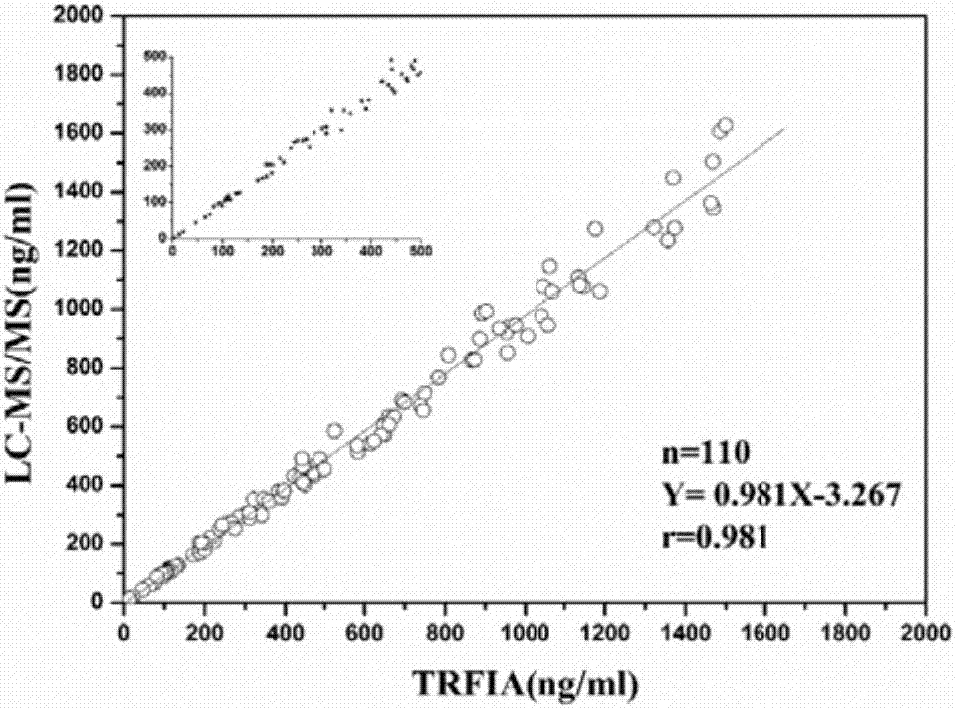
Time-resolved fluorescence detection kit for monitoring 5-fluorouracil plasma-drug concentration in real time
InactiveCN107132347AHigh sensitivityImprove stabilityFluorescence/phosphorescenceFluorescenceEuropium
The invention discloses a time-resolved fluorescence detection kit for monitoring 5-fluorouracil plasma-drug concentration in real time. The kit comprises calibrator 5-FU, calibrator dilution liquid, europium-standard 5-FUA-OVA antigens, anti-5FU murine polyclonal antibodies, analysis buffer solution, scrubbing solution, enhancement solution and a goat-anti-mouse IgG (immune globulin G) package reaction plate. The kit can quantitatively detect plasma-drug concentration of the 5-fluorouracil in peripheral blood of chemotherapy patients, the testing results show that the kit has the advantages that the kit is high in sensitivity, good in stability and reliability, high in efficiency, simple and convenient to operate, wide in detection range and free from radioactive pollution, time is saved and the like, the sensitivity is 2.1ng / ml, chemotherapy drugs are timely monitored, the kit is suitable for plasma-drug monitoring of clinical fluorouracil drugs, and clinical medication is guided.
Owner:SOUTHERN MEDICAL UNIVERSITY
Feed for sows in suckling period
InactiveCN106942494AIncrease feed intakeIncrease milk productionAnimal feeding stuffAccessory food factorsAnimal scienceGlucocorticoid
The present invention discloses a feed for sows in a suckling period. The feed comprises the following raw material components in parts by weight: 10-30 parts of bananas, 4-16 parts of valeriana officinalis tea, 5-11 parts of calcium hydrogen phosphate, 6-9 parts of calcium carbonate, 5-8 parts of methionine, 3-6 parts of aureomycin, 12-48 parts of fish meal, 14-54 parts of corn and 3-12 parts of protease. The valeriana officinalis tea can soothe disturbed mood of the sows, reduce stress behavior, can relatively better prevent produced glucocorticoids induced by the stress from penetrating placentas into fetus bodies, thereby prevents the lack of immune globulin G (IgG) in piglets, also reduces the risk of the sows in pressing the piglets dead, and increases milk of the sows.
Owner:重庆市正品农业发展有限公司
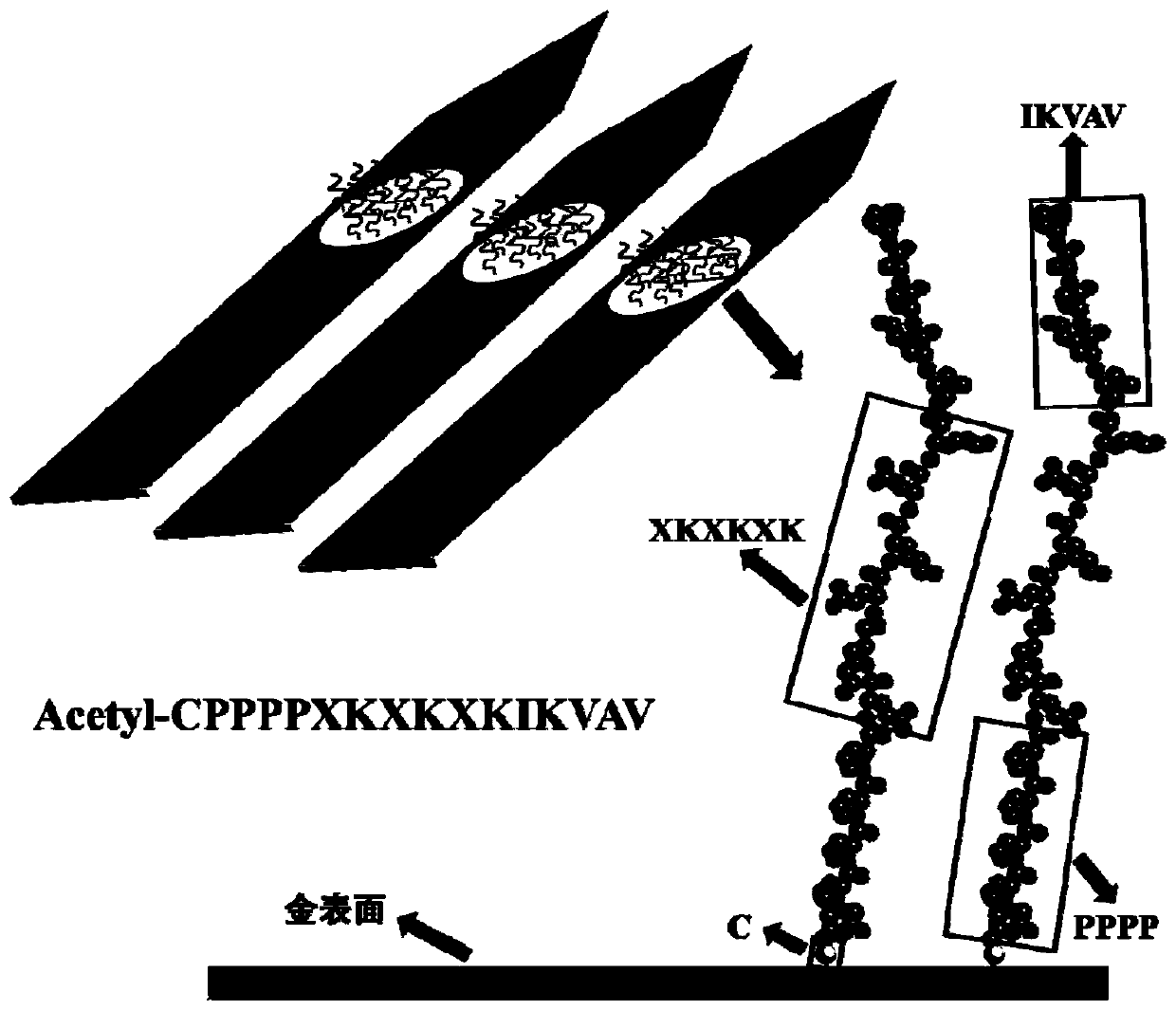
Anti-pollution polypeptide, nerve electrode modified by anti-pollution polypeptide, modification method of nerve electrode and application of anti-pollution polypeptide
PendingCN111440242AGuaranteed validityGuaranteed long-term effectivenessHead electrodesAntibody mimetics/scaffoldsNeuron adhesionProtein adsorption
The invention relates to an anti-pollution polypeptide, a nerve electrode modified by the anti-pollution polypeptide, a modification method of the nerve electrode and an application of the anti-pollution polypeptide. The anti-pollution polypeptide comprises a connecting section, a supporting section, an anti-protein adsorption section and a neuron adhesion section which are connected in sequence;and the anti-protein adsorption section comprises a zwitterionic polypeptide fragment. According to the anti-pollution polypeptide, through electrostatic interaction between charged side groups of thezwitterionic polypeptide fragment and water molecules, the adsorbability of the surface of the nerve electrode to common proteins such as lysozyme, fibrinogen, immune globulin G and human serum albumin is reduced; and meanwhile, the connecting section, the supporting section, the anti-protein adsorption section and the neuron adhesion section are matched with one another, so that the protein pollution resisting function of the polypeptide is guaranteed, the adhesion of the modified surface to neuronal cells is improved, and the long-term signal regulation and measurement stability of the neural electrode in a biological environment is effectively improved.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Application of magnetic functionalized magnetic nanomaterial and mixed microorganism identification method
PendingCN113155942ASimple and fast operationFewer beadsMaterial analysis by electric/magnetic meansMicroorganismMagnetic bead
The invention relates to an application of a magnetic functionalized magnetic nanomaterial and a mixed microorganism identification method. A capture material is formed by simultaneously connecting mannose binding lectin and immune globulin G to the surface of a magnetic bead; and the mannose binding lectin is recombinant protein, the immune globulin G is natural protein, and the magnetic functionalized magnetic nanomaterial is used for capturing or identifying mixed microorganisms in a sample. By adopting the application and the method provided by the invention, the specific variety of mixed bacterial infection in the sample can be quickly obtained without long-time culture.
Owner:CHUAN-MING (NINGBO) CHEM SCITECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com