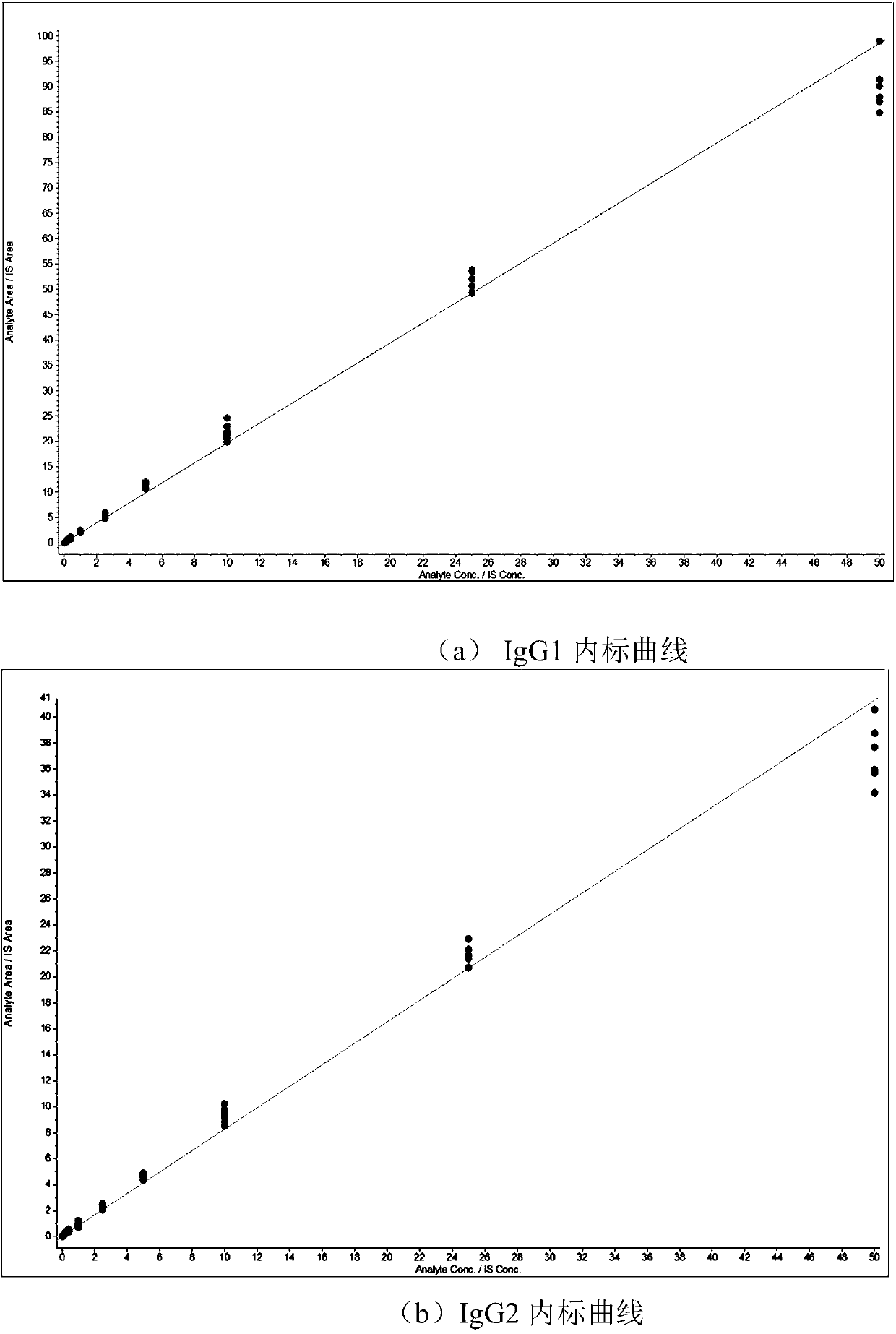
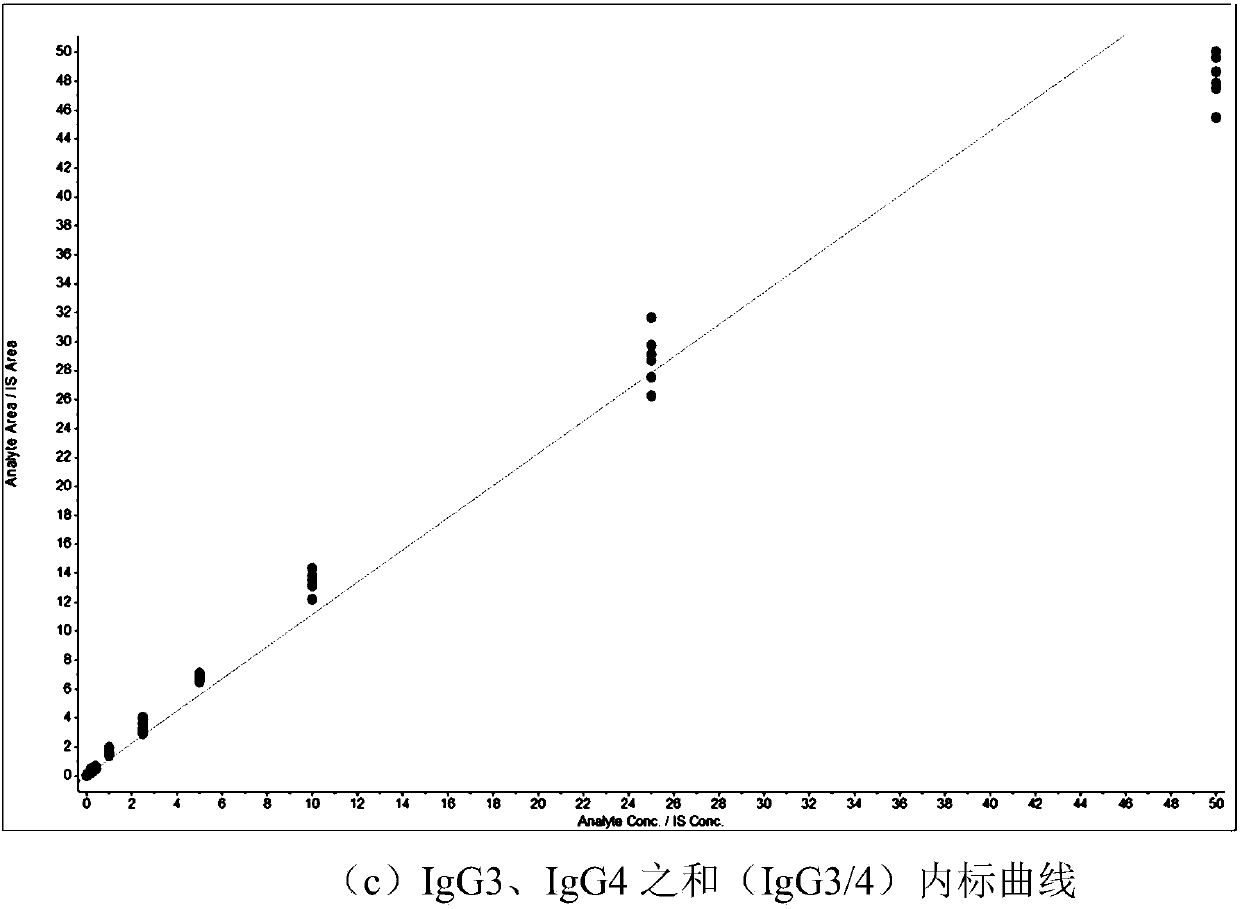
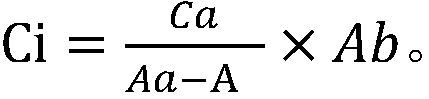Absolute quantitative analysis of IgG glycopeptide in serum
A quantitative analysis and glycopeptide technology, applied in the field of proteomics, can solve the problems of long cycle, complex chemical synthesis, expensive glycosyltransferase, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Simultaneous absolute quantification of glycopeptides in IgG1, IgG2, and the sum of IgG3 and IgG4 (IgG3 / 4).
[0059] (1) Trypsin hydrolysis of standard IgG and serum: Add 50 μL of 50 mM (pH=7.4) ammonium bicarbonate solution to 5 mg IgG / 50 μL serum each, then add 10 μL of 0.3M dithiothreitol, and reduce for 180 min at 37°C; Add 20 μL of 0.3M iodoacetic acid to the reaction product, and store in the dark at 20°C for 60 minutes; add 60 μg of trypsin to the reaction solution, and perform enzymatic hydrolysis reaction at 40°C for 72 hours. After the enzymatic hydrolysis, 1% (v / v) formic acid was added to terminate the enzymatic hydrolysis reaction.
[0060] (2) Enrichment of standard IgG glycopeptides: the enzymatic hydrolysis solution was passed through a reversed-phase XAqua C18 (4.6*150mm, 5μ) chromatographic column for glycopeptide enrichment. The mobile phase uses methanol and water, and the condition is 90% (v / v) water isocratic elution; the detection wavelength is 1...
Embodiment 2
[0068] Absolute quantification of glycopeptides of the sum of IgG1, 2 and 3, 4, respectively.
[0069] (1) Trypsin hydrolysis of serum: Add 100 μL of 100 mM (pH=7.8) ammonium bicarbonate solution to 5 mg IgG / 50 μL serum, then add 10 μL of 0.5M dithiothreitol, and reduce for 120 min at 37 ° C; Add 20 μL of 0.5M iodoacetic acid, and store in the dark at 25°C for 40 minutes; add 75 μg of trypsin to the reaction solution, and perform enzymatic hydrolysis reaction at 37°C for 36 hours. After the enzymatic hydrolysis, 2% (v / v) formic acid was added to terminate the enzymatic hydrolysis reaction.
[0070] (2) Enrichment of IgG glycopeptides: the enzymatic hydrolysis solution was passed through a reversed-phase XAqua C18 (4.6*150mm, 5μ) chromatographic column for glycopeptide enrichment. The mobile phase is acetonitrile and water, the condition is 80% (v / v) water isocratic elution; detection wavelength is 280nm; TOF mass spectrometry analysis, the 5-12min fraction is IgG1, the 13-15...
Embodiment 3
[0078] (1) Trypsin hydrolysis of serum: Add 150 μL of 100 mM (pH=7.8) ammonium bicarbonate solution to 5 mg IgG / 50 μL serum, then add 10 μL of 0.6M dithiothreitol, and reduce for 90 min at 37°C; Add 20 μL of 0.6M iodoacetic acid, and store in the dark at 30°C for 30 minutes; add 150 μg of trypsin to the reaction solution, and perform enzymatic hydrolysis reaction at 35°C for 18 hours. After the enzymatic hydrolysis, 5% (v / v) formic acid was added to terminate the enzymatic hydrolysis reaction.
[0079] (2) Enrichment of IgG glycopeptides: the enzymatic hydrolysis solution was passed through a reversed-phase XAqua C18 (4.6*150mm, 5μ) chromatographic column for glycopeptide enrichment. The mobile phase was acetonitrile and water, containing 0.1% (v / v) formic acid. The condition is that the water phase changes from 98% (v / v) to 70% (v / v) linear gradient for 5-60 minutes to elute; the detection wavelength is 254nm; the column temperature is 35°C, and the eluent flow rate is 1.2mL...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com