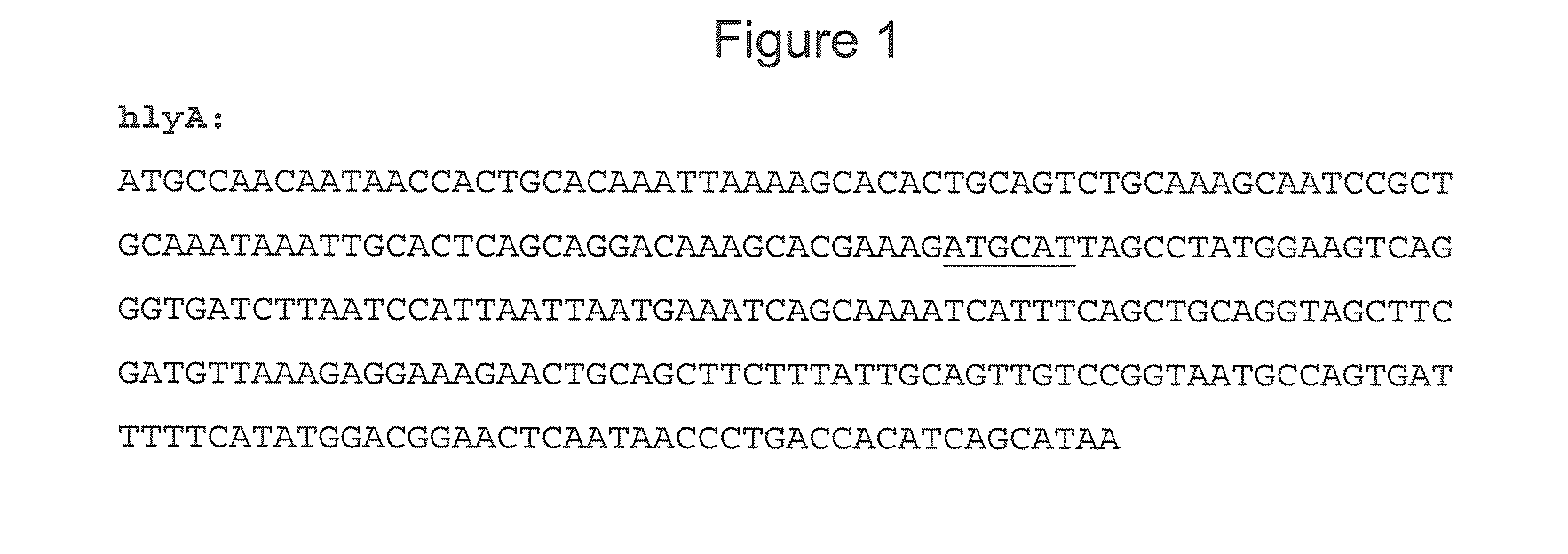
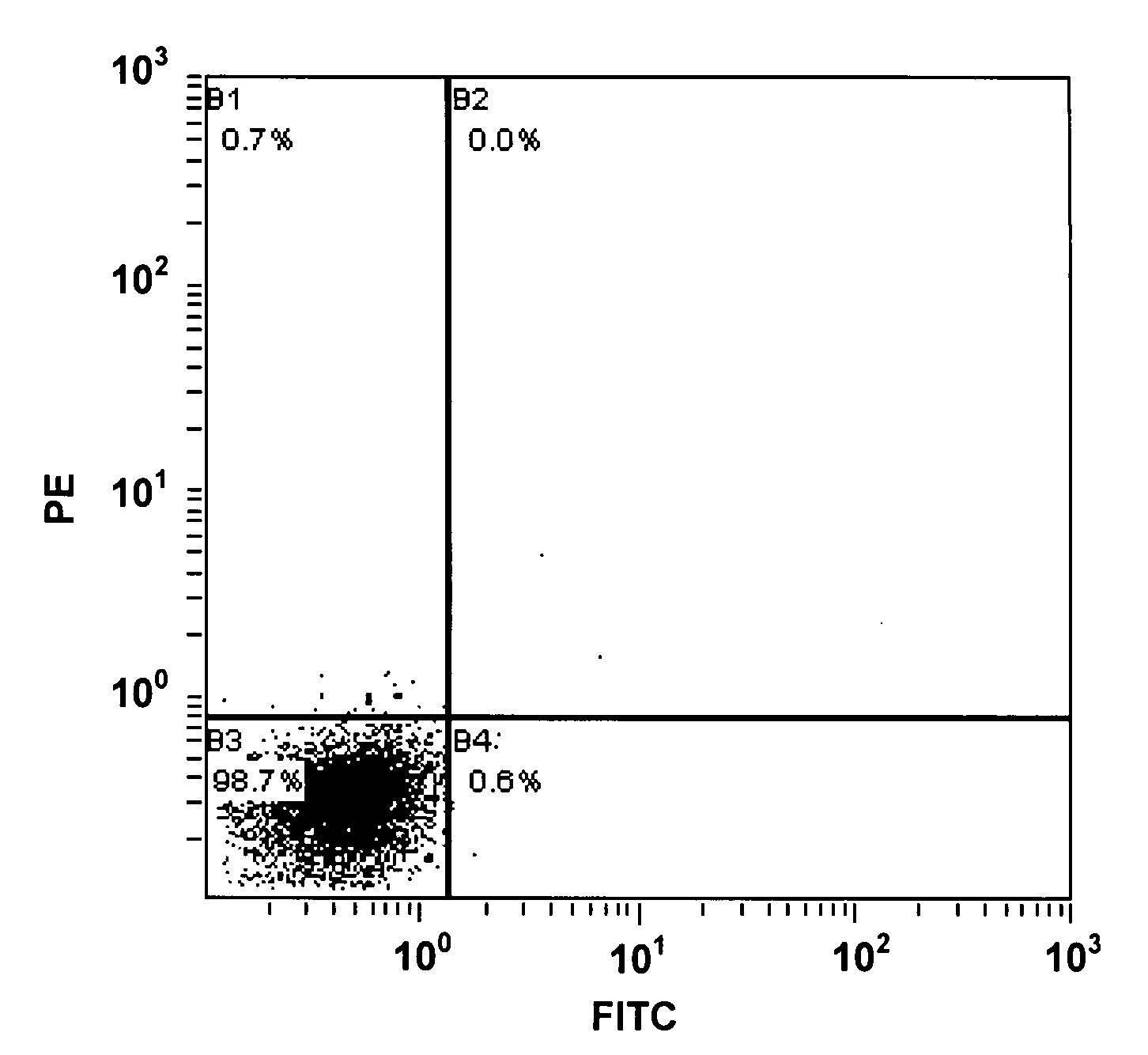
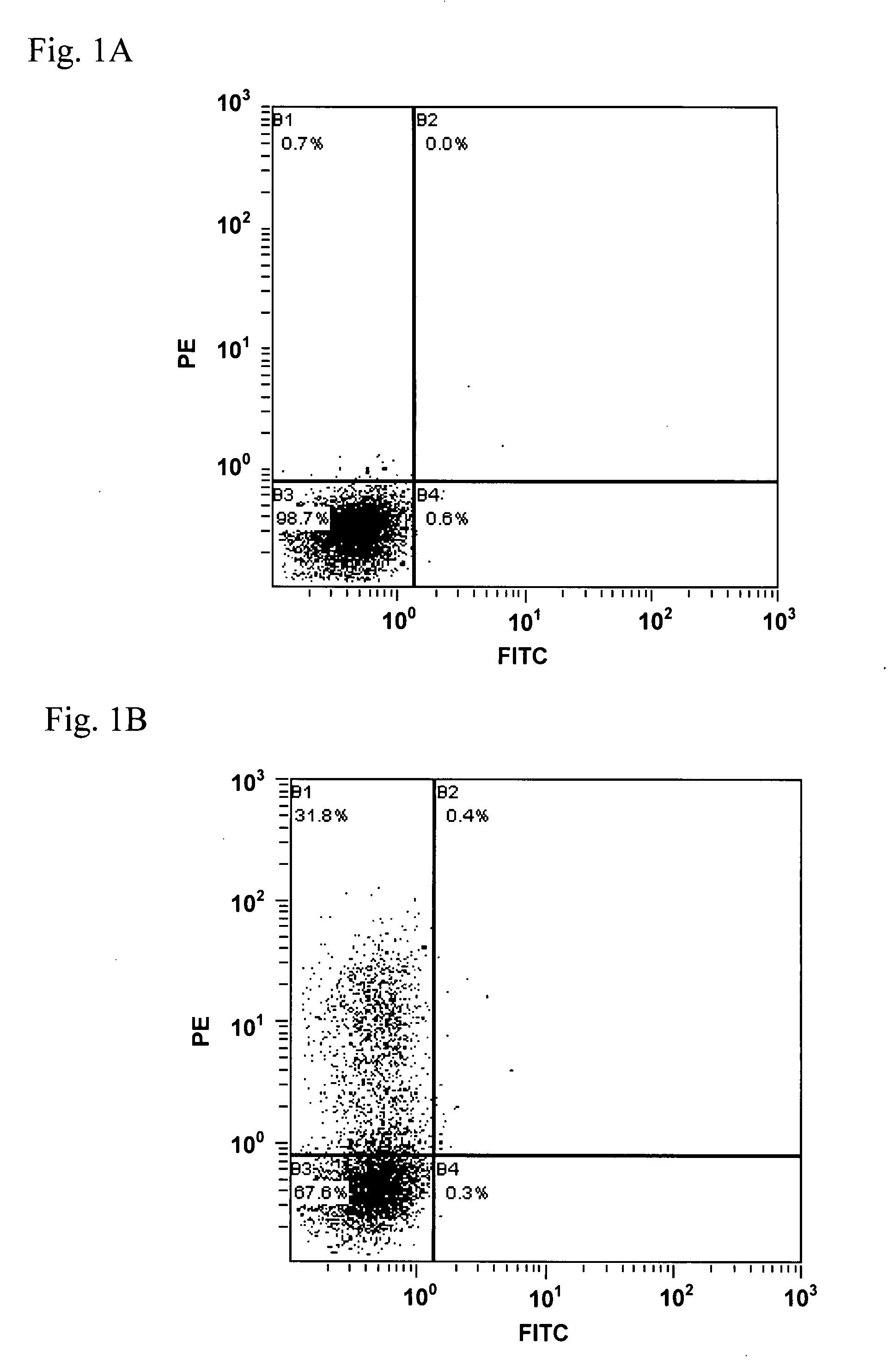
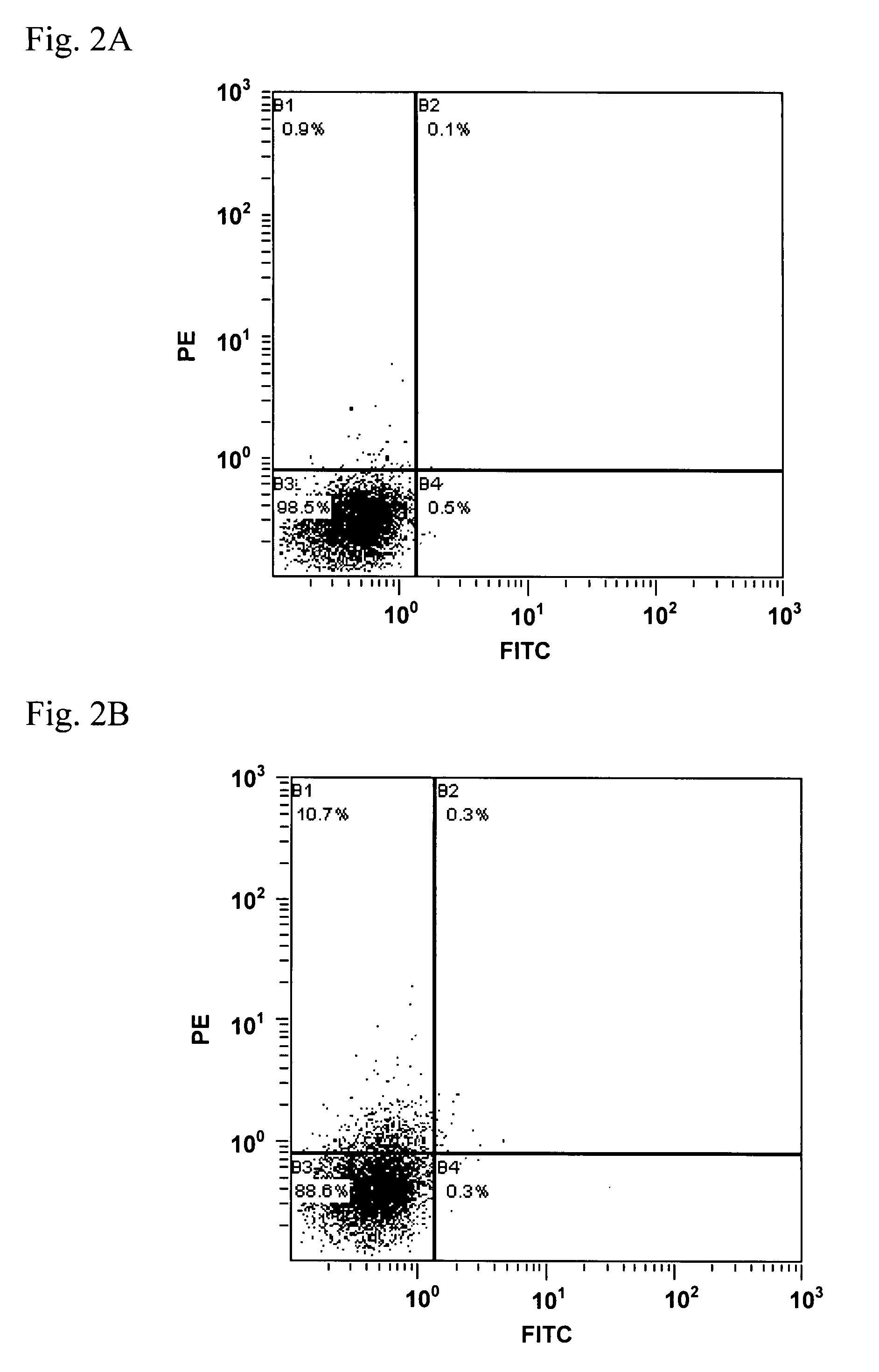
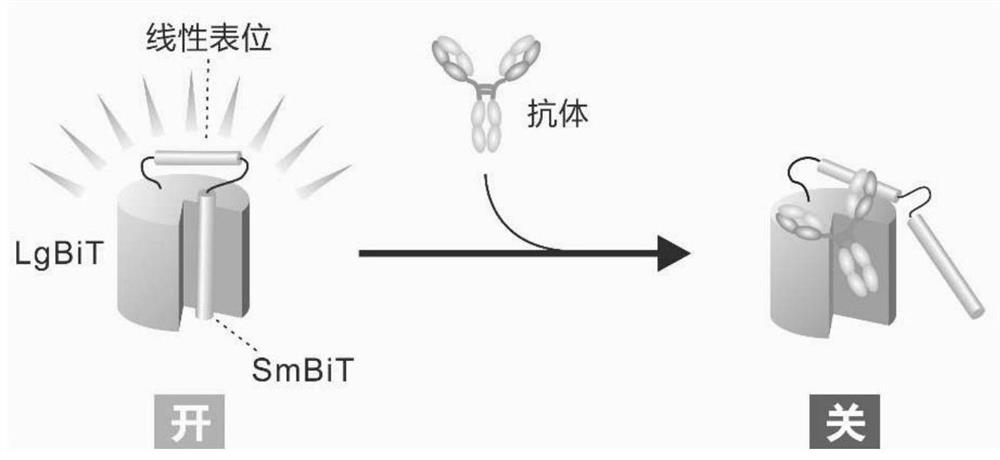
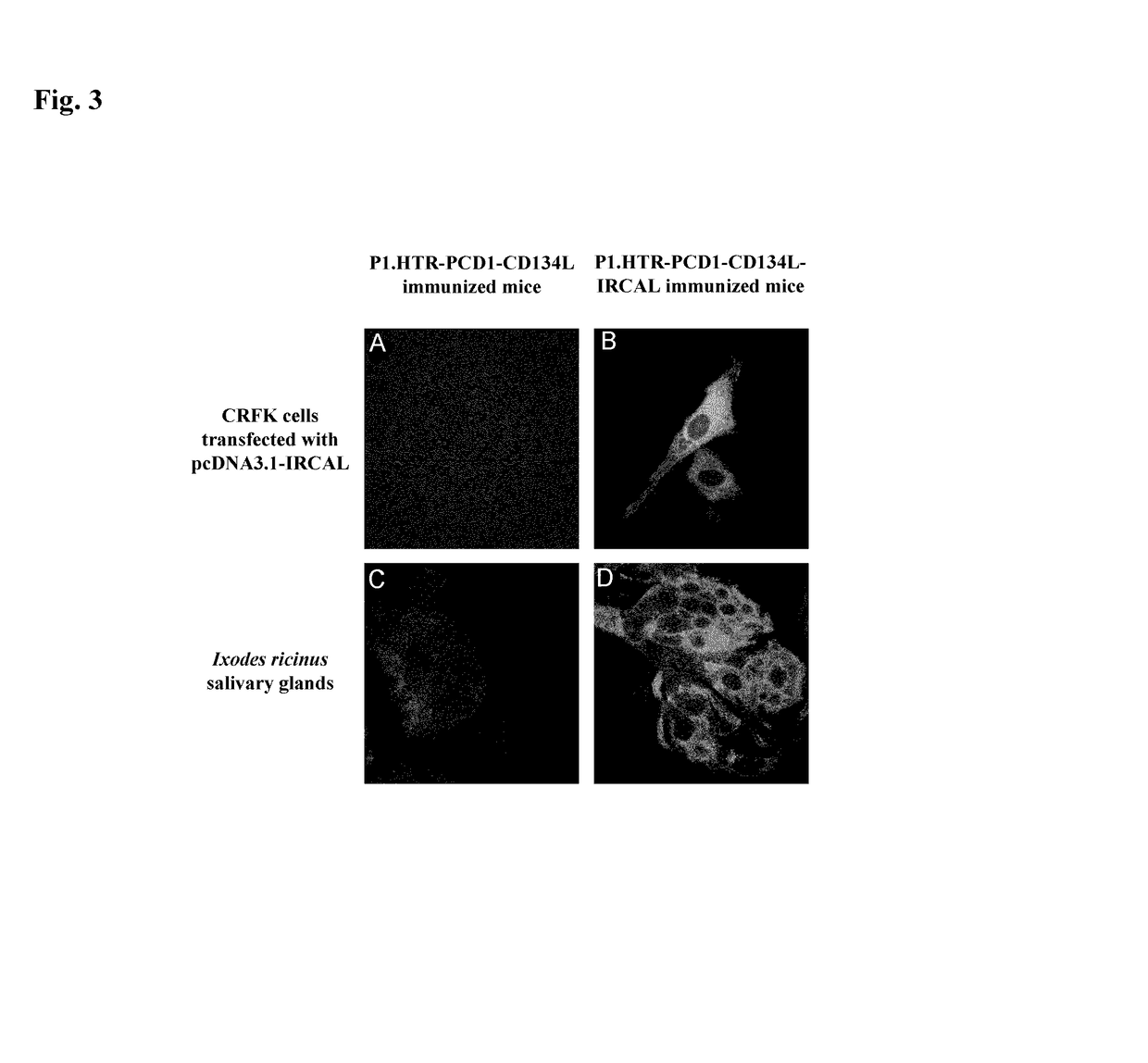
Patents
Literature
30 results about "Partial antigen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Partial antigen an antigen that does not produce antibody formation, but gives specific precipitation when mixed with the antibacterial immune serum. pollen antigen the essential polypeptides of the pollen of plants extracted with a suitable menstruum, used in diagnosis, prophylaxis, and desensitization in hay fever.
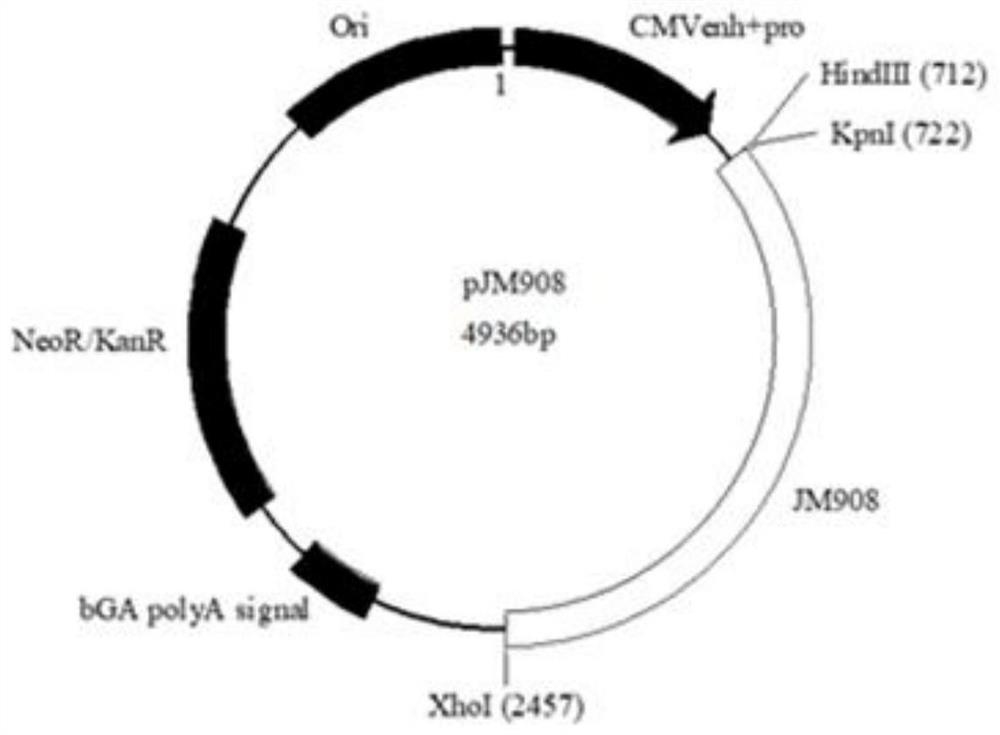
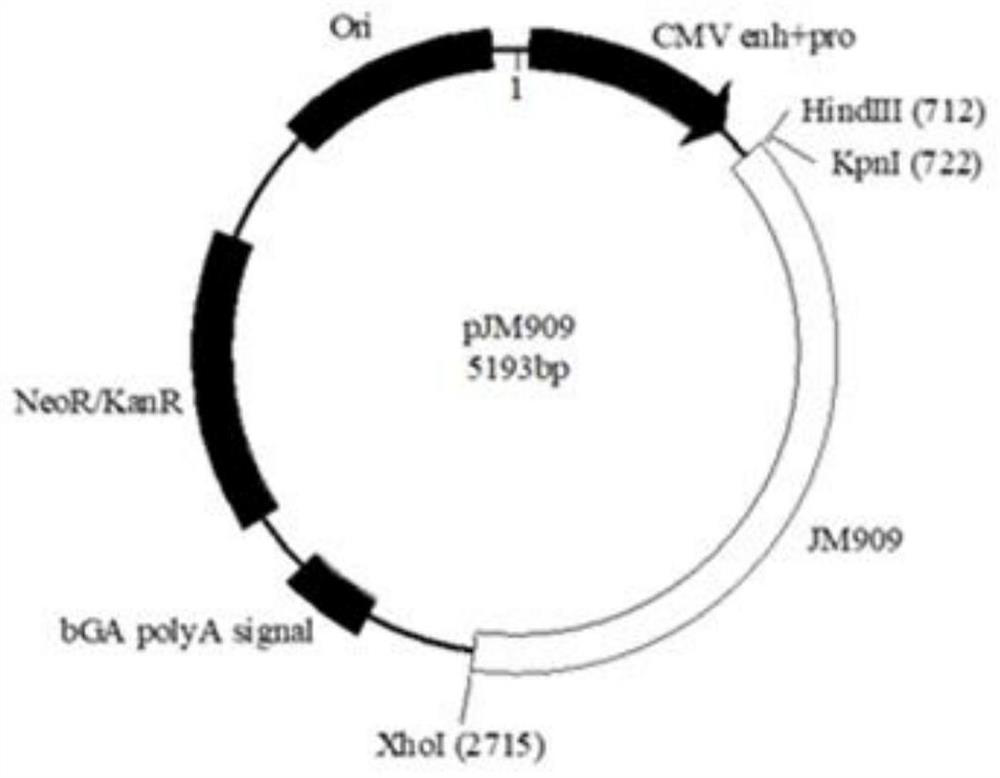
Microorganisms as carriers of nucleotide sequences coding for antigens and protein toxins, process of manufacturing and uses thereof
ActiveUS20110287037A1Patient compliance is goodSuitable for oralAntibacterial agentsBacteriaCell specificMutated protein
The invention relates to a microorganism as a carrier of nucleotide sequences coding for antigens and protein toxins comprising the following components: (I) at least one nucleotide sequence coding for at least one complete or partial antigen of at least one wild-type or mutated protein; and (II) at least one nucleotide sequence coding for at least one protein toxin and / or at least one protein toxin subunit; and (III) a) at least one nucleotide sequence coding for at least one transport system which enables the expression of the expression products of component (I) and component (II) on the outer surface of the microorganism and / or enables the secretion of the expression products of component (I) and component (II); and / or coding for at least one signal sequence which enables the secretion of the expression products of component (I) and component (II); and / or (III) b) optionally, at least one nucleotide sequence coding for at least one protein for lysing the microorganism in the cytosol of mammalian cells and for intracellularly releasing plasmids or expression vectors, which are contained in the lysed microorganism; and (IV) at least one nucleotide sequence for at least one activation sequence for the expression of one or more of components (I) to (III), wherein said activation sequence can be activated in the microorganism and / or is tissue cell-specific, tumor cell-specific, macrophage-specific, dendrite-specific, lymphocyte-specific, function-specific or non-cell-specific”; wherein any of components (I) to (IV) can be present either once or several times and either identical or different. Also disclosed are a process of manufacturing thereof, corresponding plasmids or expression vectors and uses of the microorganism as a medicament.
Owner:SOCIUM THERAPEUTICS INC
Synthesis of polypeptide antibody from anti-human myocardial troponin I, its production and use
InactiveCN1982337AIntuitive single assayImmunoglobulins against animals/humansBiological testingProtein iPartial antigen
CTnI synthetic polypeptide antibody, its production and use are disclosed. The process is carried out by synthesizing polypeptide by 98-110 amino-acid at N end of amino-acid sequence RQLHARVDKVDEE, taking it as partial antigen to connect with protein carrier, preparing artificial immunogen, immunizing for animal, inducing organism effectively, generating immune response to obtain antibody, specific combined reacting cTnI synthetic polypeptide antibody with natural myoepicardial protein I molecule and coating cellulose nitrate film by the antibody, and reducing by tri-sodium citrate to obtain the final product. It's fast and has better sensitivity and specificity, and it can be used for acute myocardial infarction early diagnostic technology.
Owner:INST OF RADIATION MEDICINE CHINESE ACADEMY OF MEDICAL SCI
Hemolytic streptococcus diagnostic immunochromatography reagent, kit, and detection method
ActiveUS20160370368A1High-sensitivity and quick testingRequires preparationImmunoassaysOrganic acidNitrite
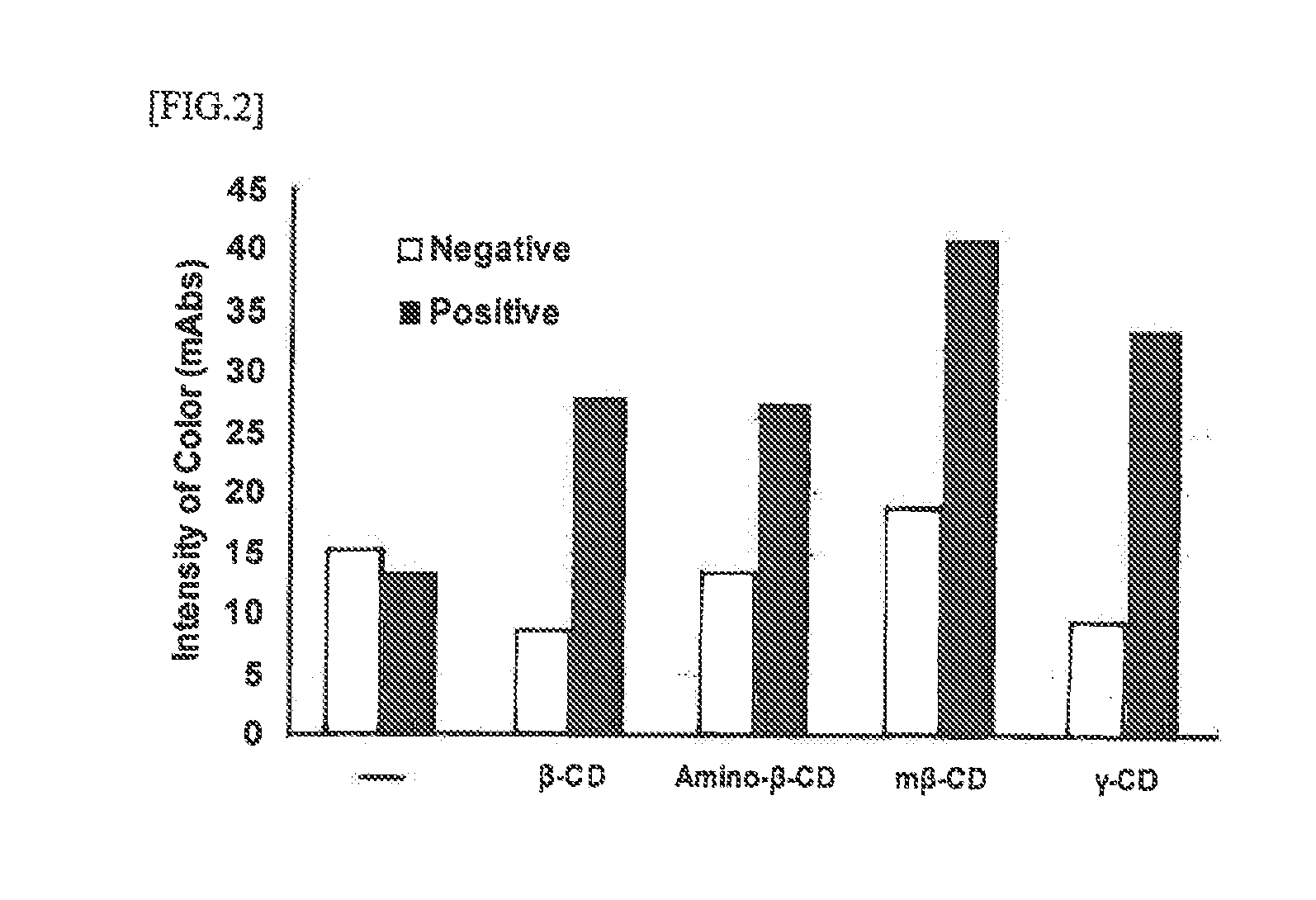
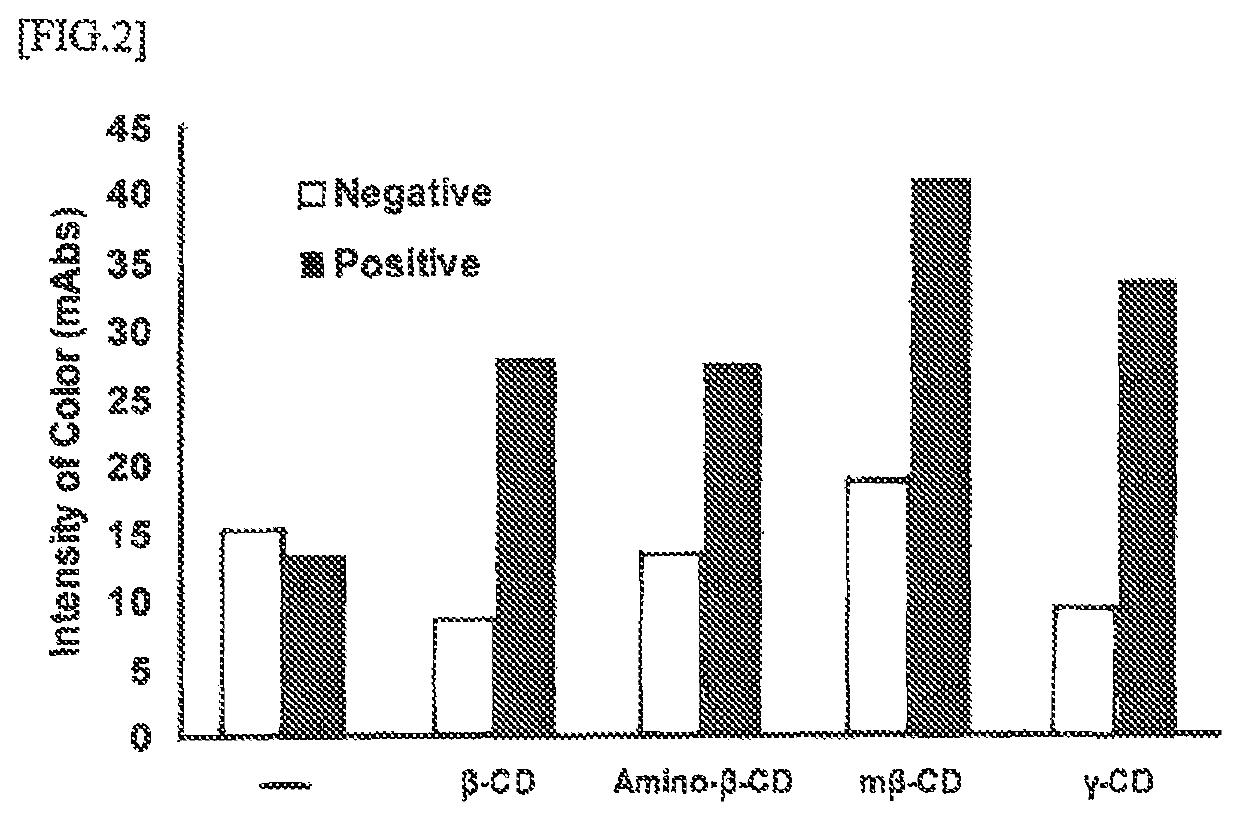
Disclosed herein is an immunoassay method for detecting the detection target antigen in an analyte through extraction with an extraction agent. The method is characterized in that the detection is performed in the presence of a cyclic oligosaccharide. The method is also characterized in the use of an immunochromatography kit for detecting gram-positive bacteria in an analyte, and that is configured from an analyte dilution solution, a sample dropping part, an antigen extracting part, a labeling substance retaining part, an immunochromatography medium having a detection part, and an absorption part, and in which an organic acid is retained in the antigen extracting part. The immunochrornatography kit contains a cyclic oligosaccharide and a nitrite compound by containing at least one of a cyclic oligosaccharide, a nitrite compound, and a mixture thereof in the analyte dilution solution and / or the sample dropping part.
Owner:TANAKA PRECIOUS METAL IND
Virus-like particle containing RNA virus nucleic acid and its preparing method and use
InactiveCN1587420AImprove stabilityImprove the simulation effectMicrobiological testing/measurementVector-based foreign material introductionPartial antigenCoat protein
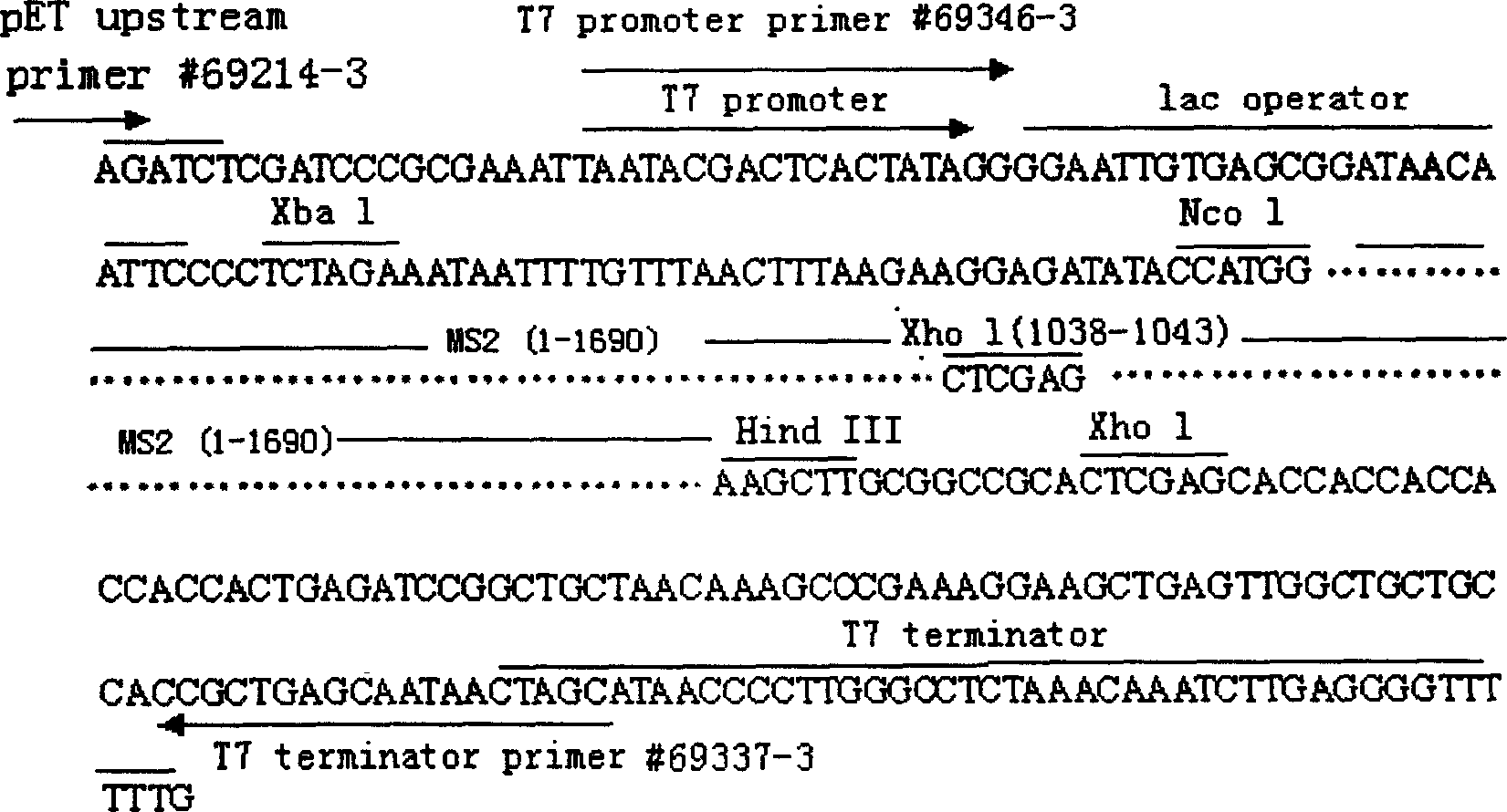
The present invention belongs to the field of virus gene engineering, and relates to virus-like particle containing RNA virus nucleic acid and its preparation process and application. The virus-like particle is RNase resistant, and contains coat protein and inside recombinant gene sequence including partial MS2 bactereophage sequence, RNA virus nucleic acid sequence and partial RNA virus antigen. The virus-like particle of the present invention may be used as stable and reliable RNA quality controlling and standard article without biological infectious danger. The present invention provides also RT-PCR reagent kit amplification segment suitable for detecting virus RNA in several methods. The virus-like particle containing partial RNA virus antigen may be used as the mold for research of the interaction between virus and host cell, as antisense RNA tool for directional transportation of small molecule medicine, and in vaccine research.
Owner:BEIJING HOSPITAL
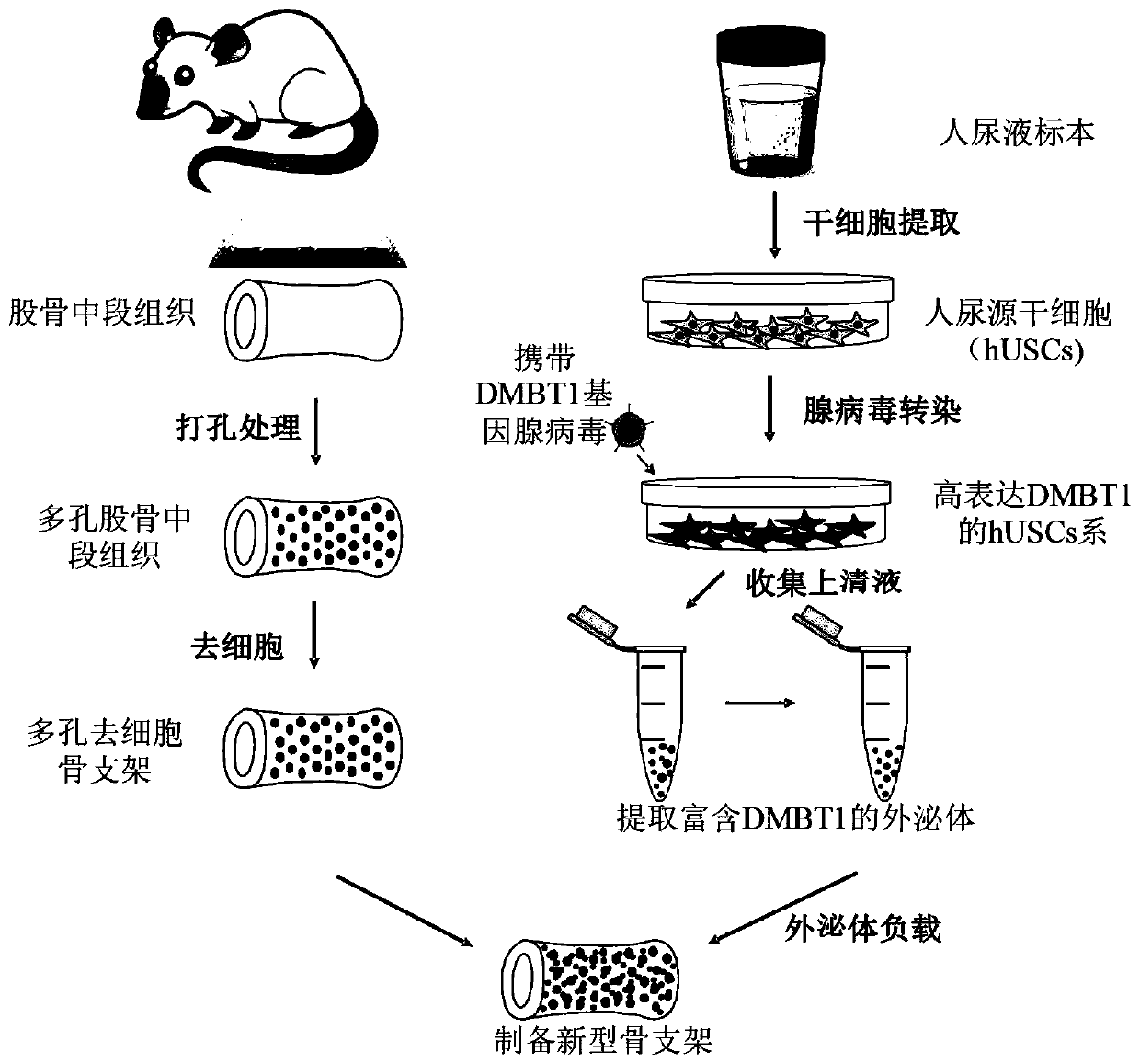
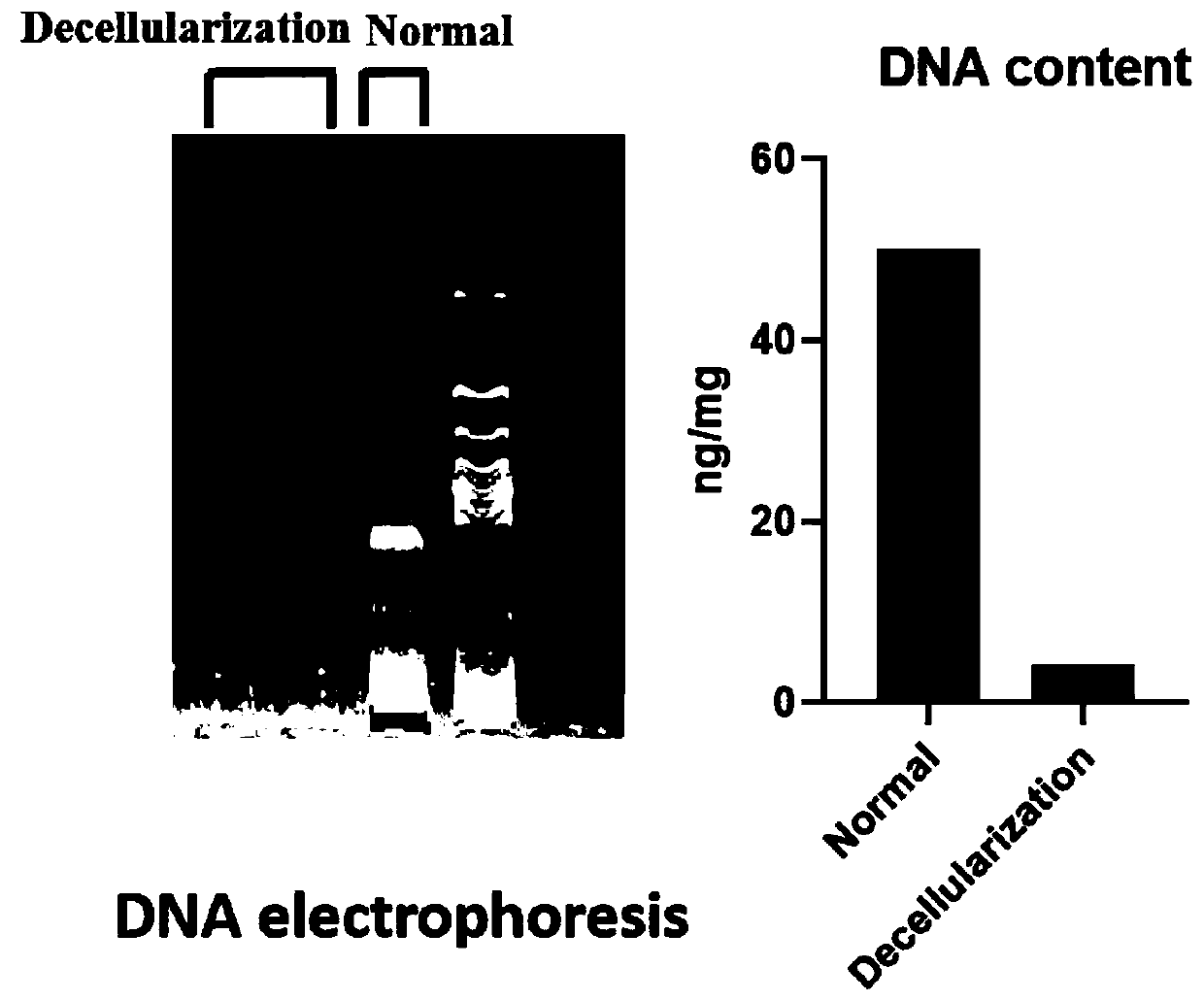
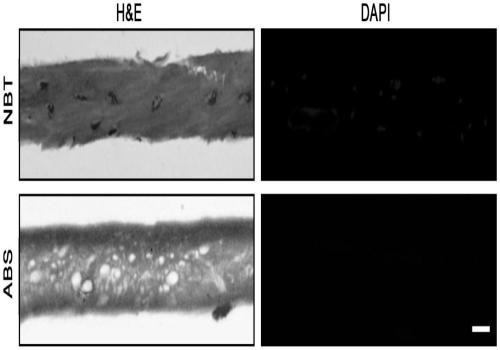
Novel bone tissue engineering scaffold and preparation method thereof
The invention relates to the technical field of biomedical tissue engineering, in particular to a novel bone tissue engineering scaffold and a preparation method thereof. The bone scaffold comprises abone material and an exosome-loaded fibrin gel compound, the bone material is provided with holes, and the gel compound is distributed in the holes. The invention researches a bone material which ishighly similar to a natural bone matrix and has osteogenesis and vascularization activities. The porous decellularized tissue engineering scaffold is closer to a normal bone, has good biomechanical properties, is suitable for bone defect repair of a load bearing area, and maximally retains inherent components of the scaffold. According to the method, cell components with most antigens in tissues can be effectively removed, the immunological rejection reaction of grafts is reduced, the approximate morphological structure of the tissues can be maintained, and most tissue matrix components and bioactive factors are retained.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
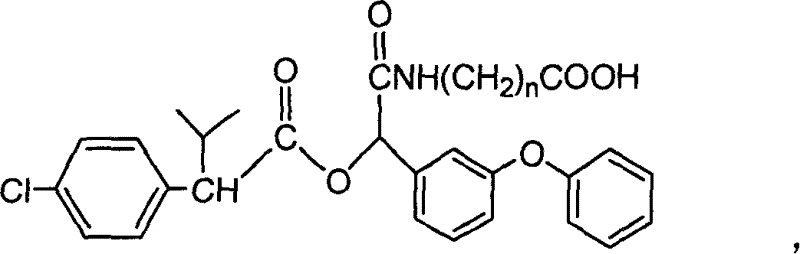
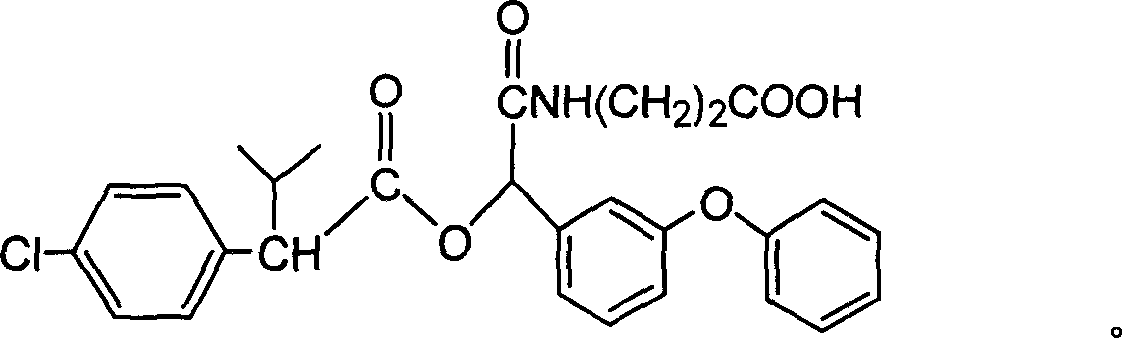
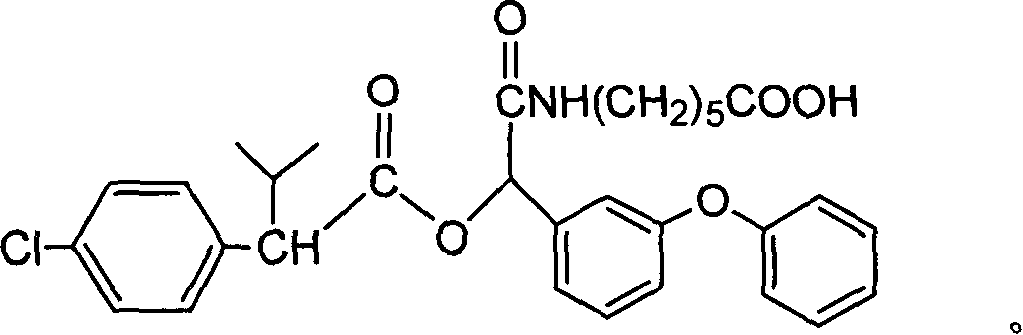
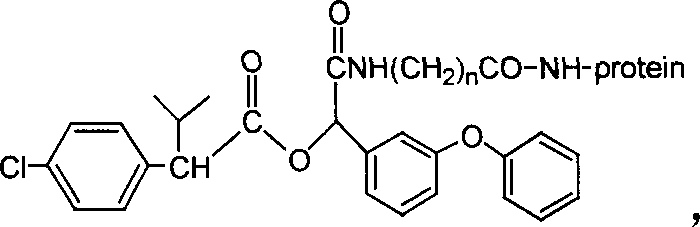
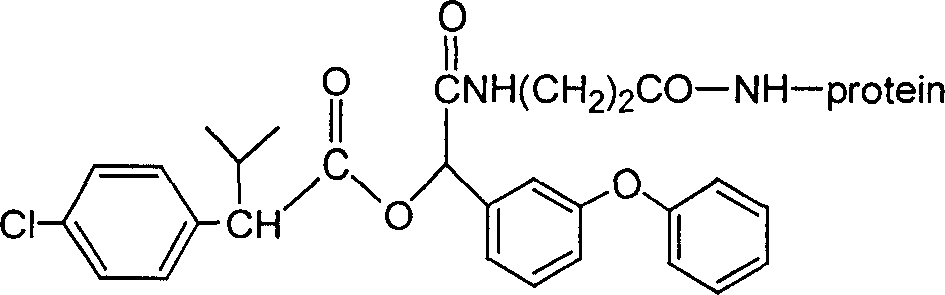
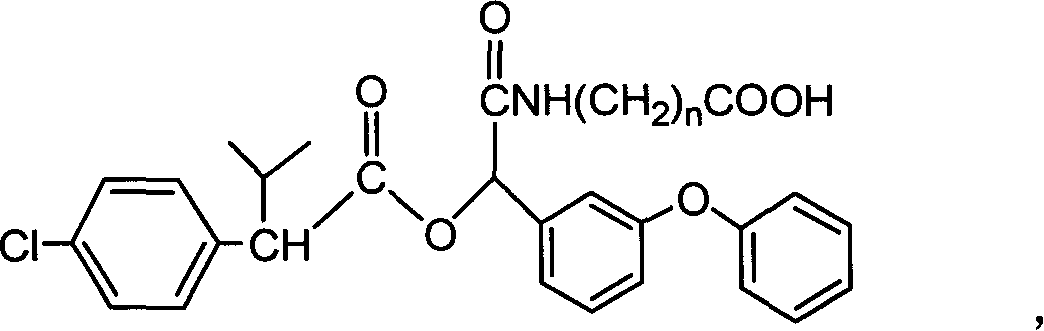
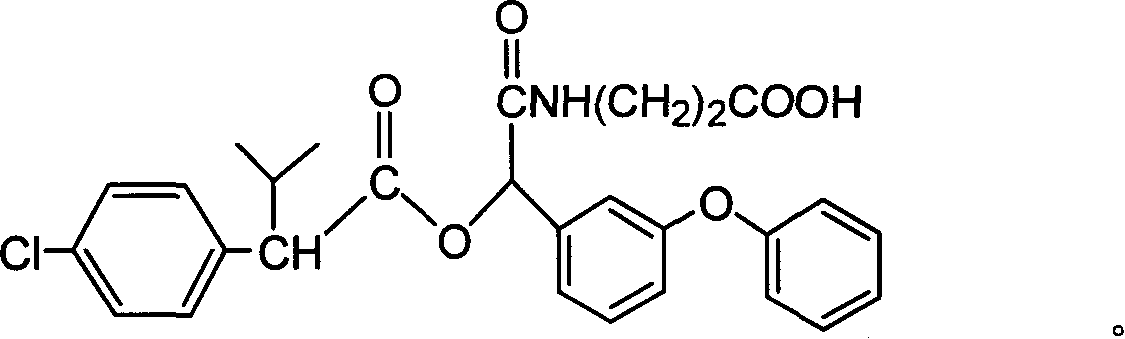
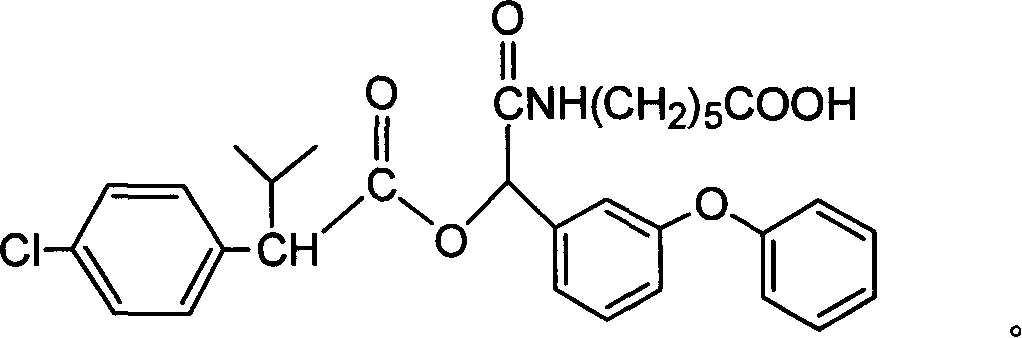
Cyfluthrin hapten compound, its synthesis method and use
InactiveCN1789238ARetain chemical structureGood potencyOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsPartial antigen
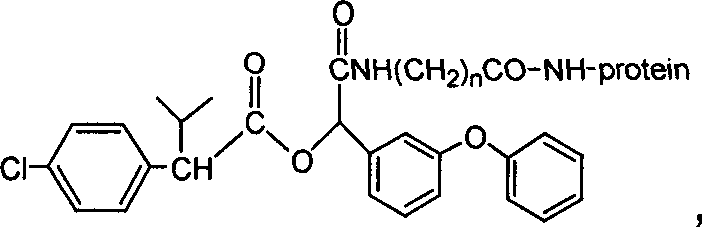
The invention discloses a fenvalerate artificial partial antigen, the molecular structure is in the right, and n=1-5. The invention also provides the method for synthesizing fenvalerate artificial partial antigen, and the application of the product as raw material of antigen system for animal immunization.
Owner:ZHEJIANG UNIV
Combined vaccine for anthrax and black death
InactiveCN1966074AImprove complianceReduce the number of immunizationsAntibacterial agentsFungiEpitopeMicrobiology
The invention relates to a coupled vaccine for protecting human or animal from bacillus anthracis and Yersinia, wherein it comprises bacillus anthracis PA antigen, Yersinia V antigen, and Yersinia F1, or their protective epitope. The antigen is recombined protein separated and / or purified. The DNA of integral or partial PA antigen, integral or partial F1 antigen, and integral or partial V antigen can be directly used as nucleic acid vaccine.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
A vaccine composition, a kit and applications of the composition and the kit
ActiveCN107537033AEasy to solveImprove control effectAntiviralsAntibody medical ingredientsDiseasePartial antigen
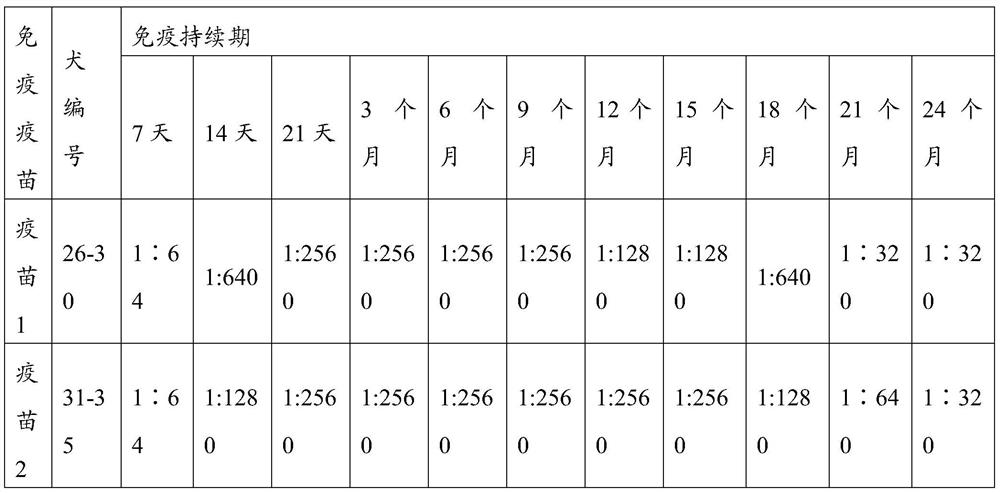
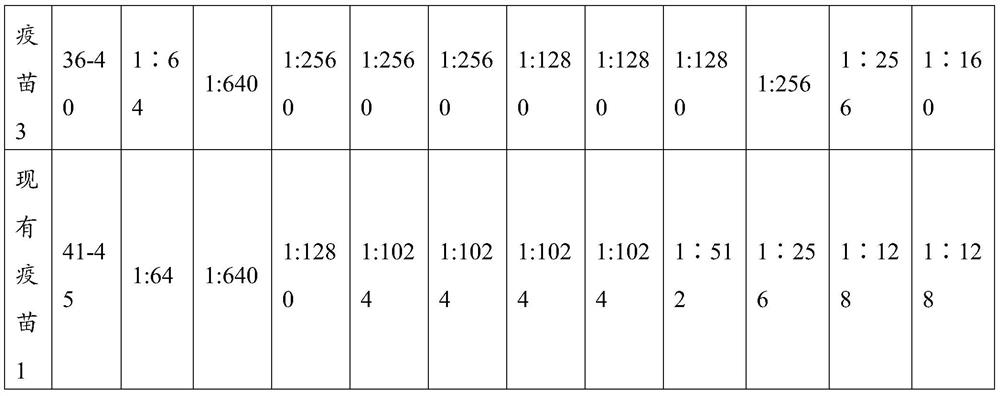
The invention relates to a vaccine composition comprising an immunizing dose of a canine parvovirus antigen and an immunizing dose of another antigen, and relates to a kit comprising an effective amount of the canine parvovirus antigen and an effective amount of another antigen. The composition and the kit can be effectively used for preparation of medicines preventing and / or treating canine parvovirus infection related diseases. The composition and the kit can effectively prevent present epidemic canine parvovirus wild strains, and synergistic effects with some antigens in other antigens aregenerated, thus further improving immunization effects of a combined vaccine.
Owner:PU LIKE BIO ENG
Moderately hydrolyzed formula powder for inducing oral immune tolerance and preparation method
InactiveCN108142562AOral immune tolerance hasBalanced Amino Acid ProfileMilk preparationVegetable oilWhey protein powder
The invention provides moderately hydrolyzed formula powder for inducing the oral immune tolerance. The powder is prepared from, by weight, 3-15% of moderately hydrolyzed whey protein powder, 2-10% ofmoderately hydrolyzed casein, 60-75% of glucose syrup, 10-25% of mixed vegetable oil, 0.1-0.5% of docosahexoenoic acid oil, 0.1-0.5% of arachidonic acid oil, 0.5-10% of caprylic / capric triglyceride,0.1-0.5% of anhydrous cream, 0.1-0.6% of multi-vitamin and 0.4-2.2% of composite minerals. The formula powder has the advantages that the aminogram is balanced, sensitization is reduced, partial antigens are retained, and the formula powder has oral immune tolerance; the fatty acid spectrum is balanced, and the palatability is enhanced by adding short-chain fat.
Owner:青岛德能食品有限公司
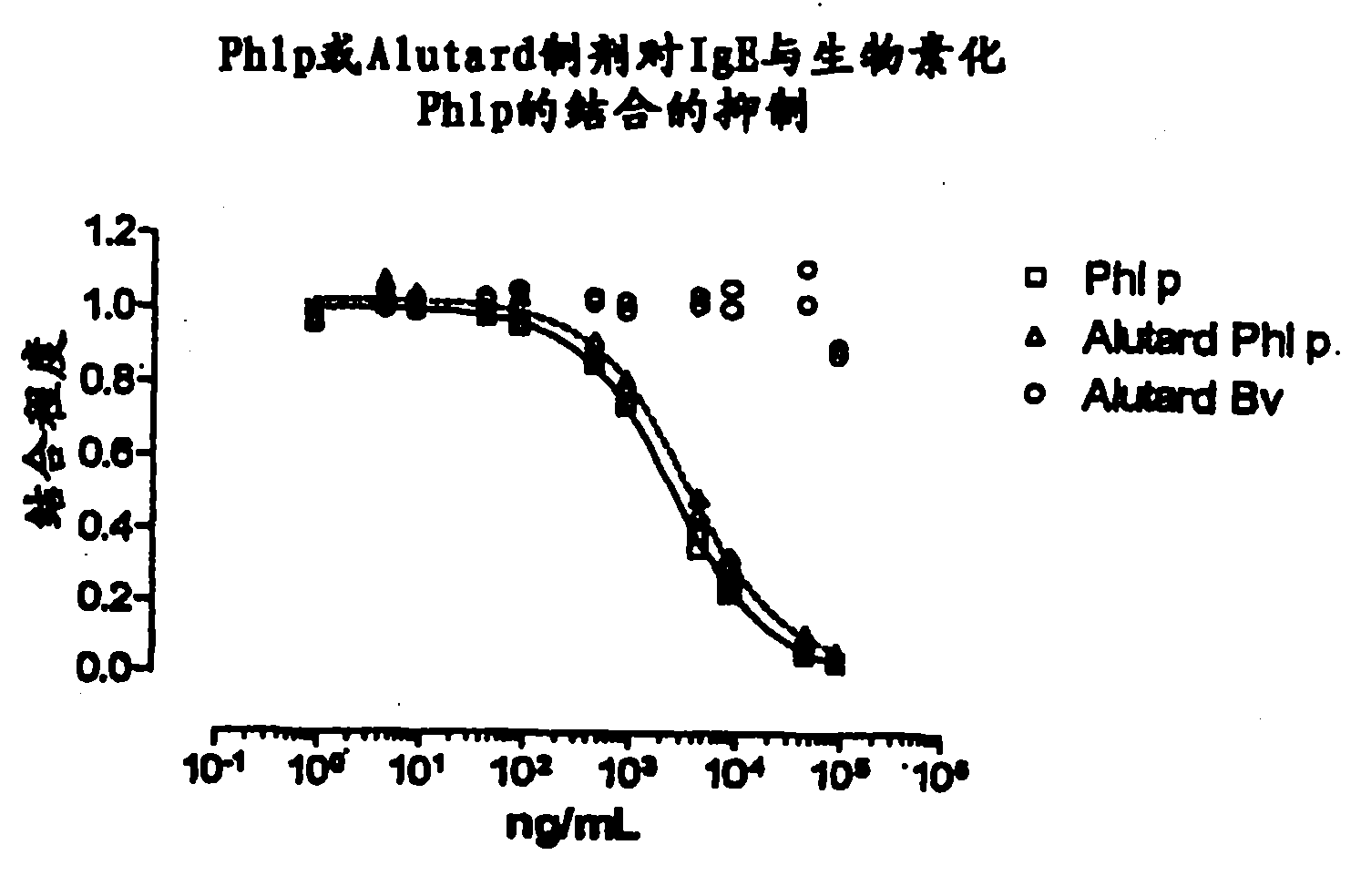
Evaluation of adjuvanted vaccines
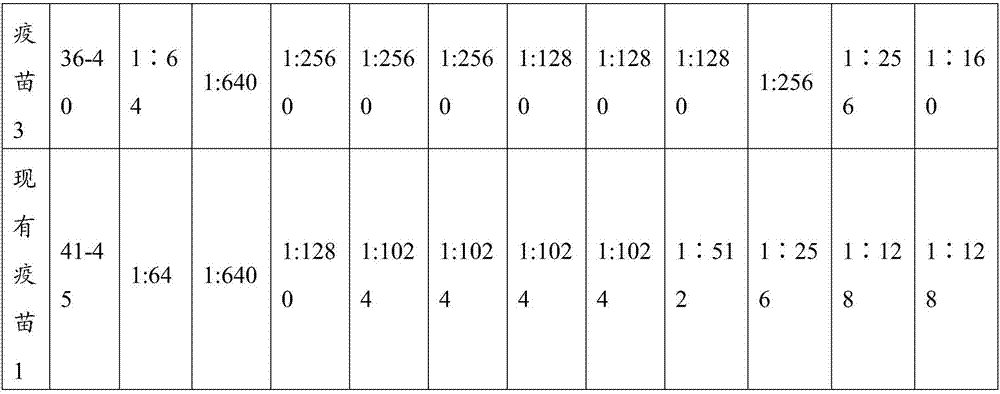
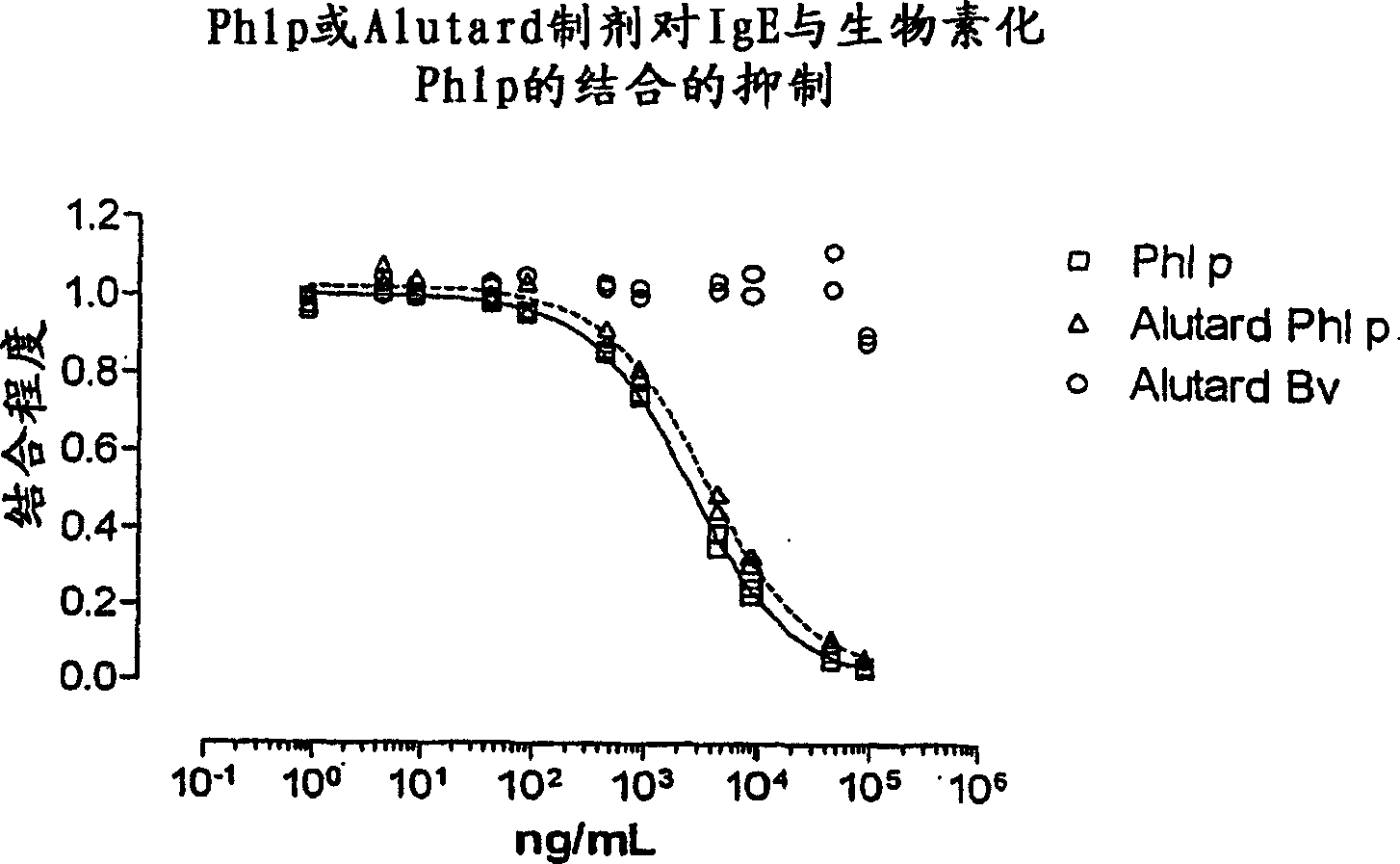
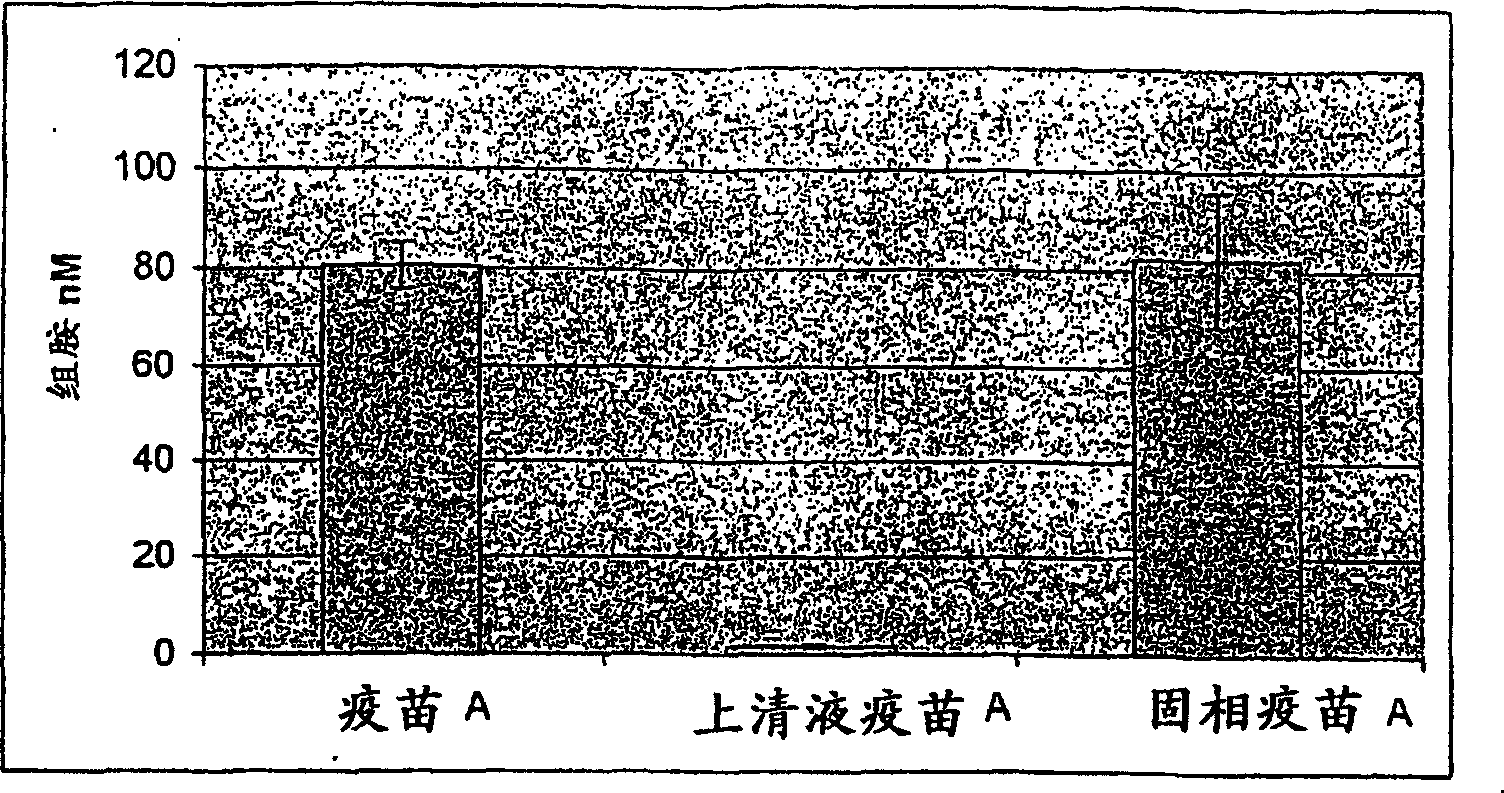
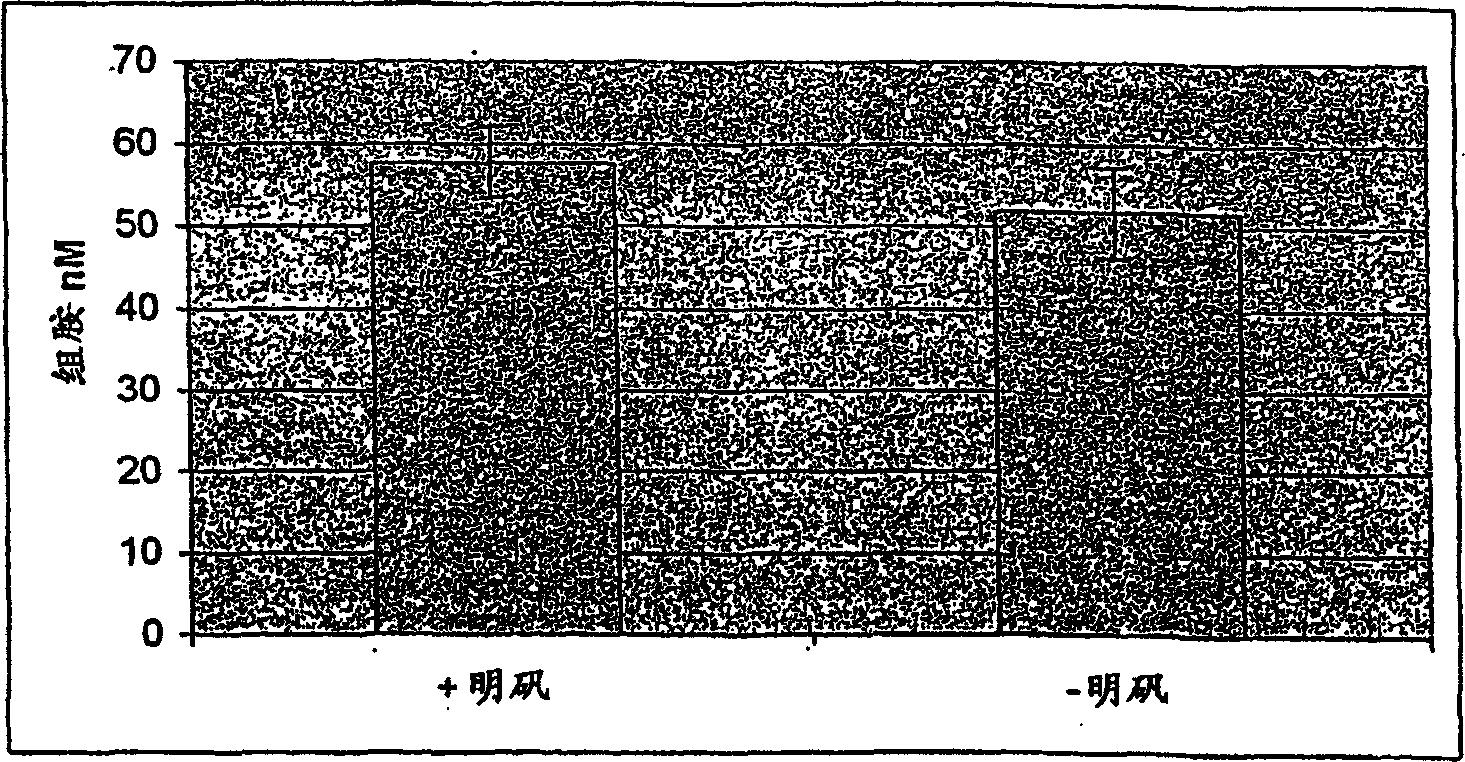
The invention relates to an in vitro method of evaluating the immunological activity of a vaccine preparation in the form of a mixture of a molecular antigen and a carrier, wherein the mixture comprises a liquid phase and a solid phase, to which at least a part of the antigen is attached, the method comprising the steps of i) subjecting the vaccine to one or more measurements selected from the group consisting of: 1) the immunological activity of the mixture, 2) the immunological activity of antigen in the liquid phase, 3) the immunological activity of antigen in the solid phase, 4) the immunological activity of antigen in the liquid phase upon a treatment of the mixture to displace the antigen from the solid phase, and 5) the immunoligical activity of antigen in the solid phase upon a treatment of the mixture to displace the antigen from the solid phase, wherein the immunological activity measurement is selected from the group consisting of antibody binding capacity using an immunoassay employing an antigen-specific antibody bound to an antibody solid phase, b) ability to activate effector cells and c) potential for inducing anaphylaxis; and ii) using the measurement results.
Owner:ALK ABELLO SA
NK cell engaging antibody fusion constructs
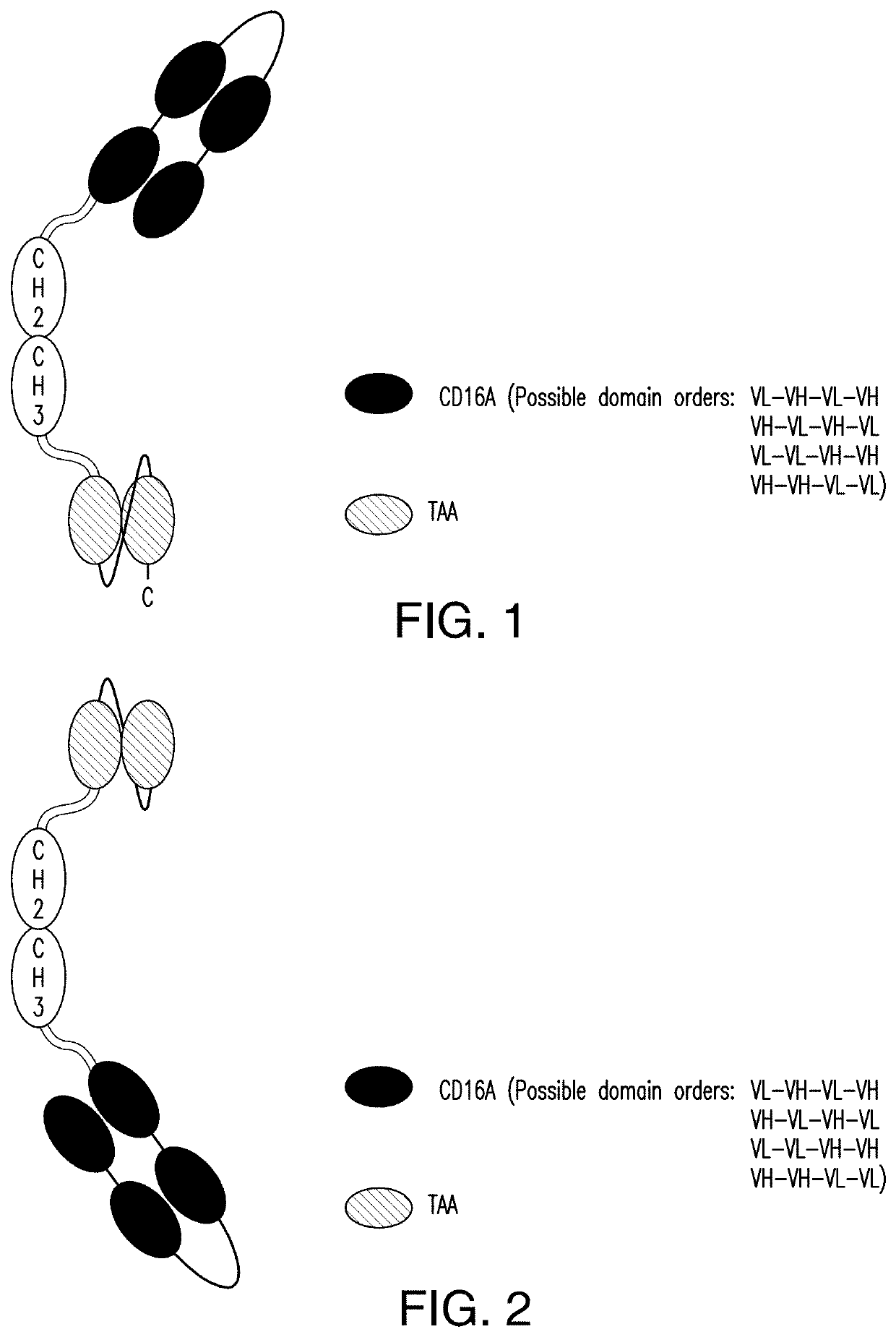
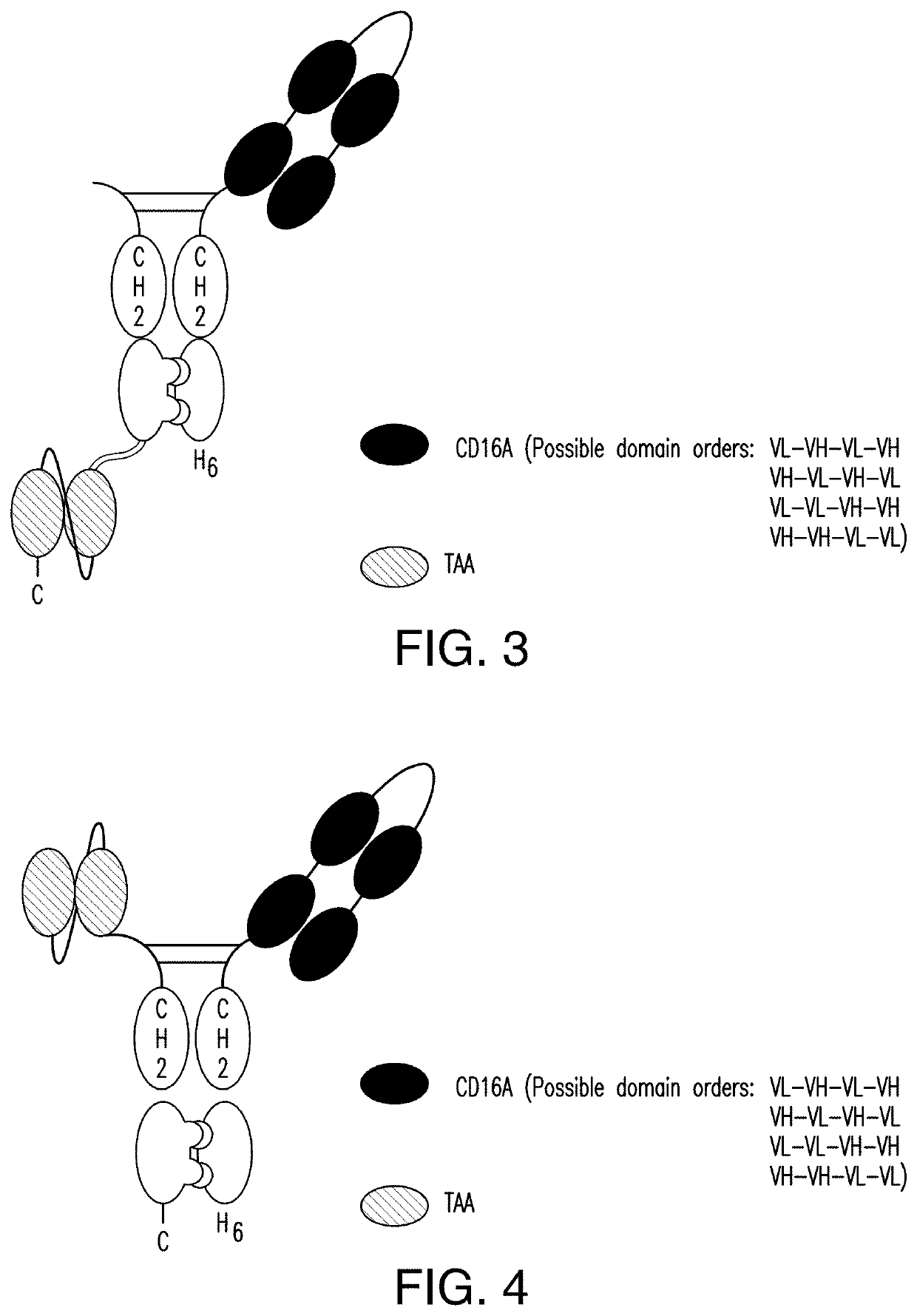
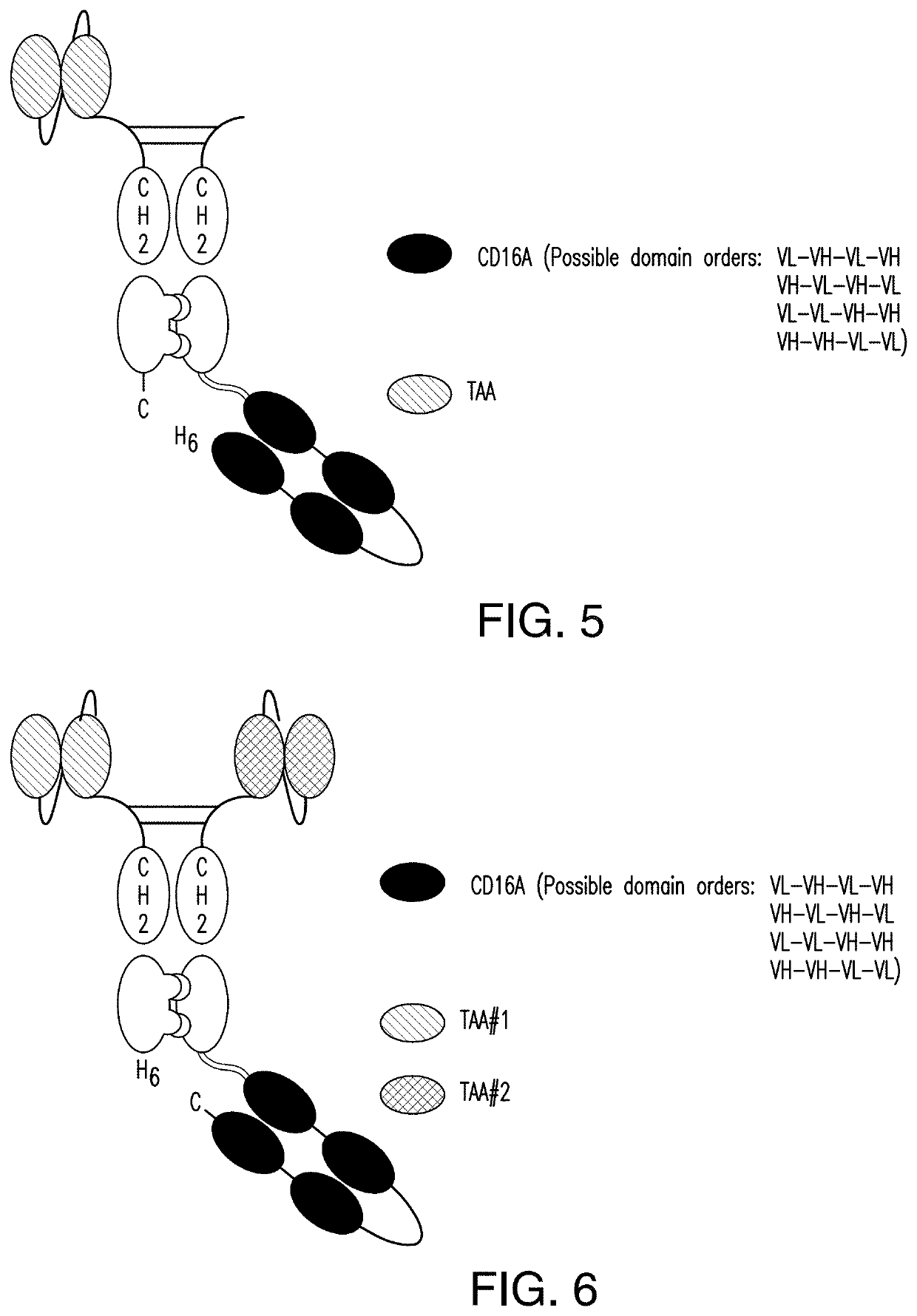
ActiveUS11001633B2Avoid inductionHigh affinityAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsHeavy chainNatural Killer Cell Inhibitory Receptors
Owner:AFFIMED GMBH
Hemolytic Streptococcus diagnostic immunochromatography reagent, kit, and detection method
Owner:TANAKA PRECIOUS METAL IND
Artificial antigen, antibody of fenvalerate and uses thereof
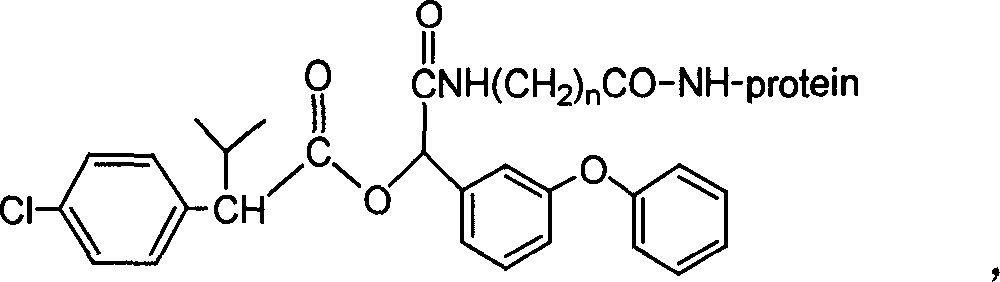
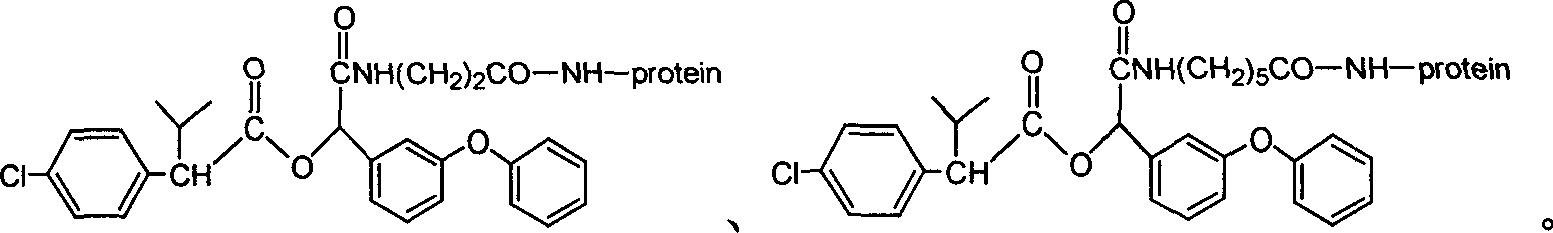
The invention discloses a fenvalerate artificial antigen, with the molecular structural formula is as formulaó±, n=1-5, employing formula ó� as partial antigen, covalent coupling with protein for synthesis; the combination ratio between the partial antige and protein is 5:1-100:1. The invention also discloses the monoclonal or polyclonal immune globulin G which is got from immunizating mouse or rabbit by the above said fenvalerate artificial antigen and can occur specific immunological reaction with fenvalerate, the said fenvalerate specific antibody can be used to check the residual quantity of fenvalerate in sample. The invention also discloses a detecting agent box of direct and indirect competeing enzyme and immunoadsorption for fenvalerate residual analysis. The got antibody is used to detect fenvalerate by ELISA method, with the lowest detecting limit being 4.0í‚1.5 ug / l (0.004ppm), and the detecting sensitivity is high.
Owner:ZHEJIANG UNIV
Method of immunizing animal, composition for immunization, method for producing antibody, method for producing hybridoma and method for producing monoclonal antibody
ActiveUS20090041761A1Easy to produceProduce efficientlyAntibacterial agentsGenetic material ingredientsAntigenMonoclonal antibody
It is an object of the present invention to a method whereby a humoral immune response is induced more efficiently in producing an antibody against an antigen protein by gene immunization. A fusion gene composed of a gene encoding the full-length of a part of the antigen protein or a gene encoding a chaperonin subunit or a chaperonin subunit linkage linked thereto is administered to express the fusion gene in the animal, thereby inducing a humoral immune response to an antigen protein by administering. An example of the chaperonin includes Escherichia coli GroEL. There is also provided with a composition for immunization, a method for producing an antibody, a method for producing a hybridoma, and a method for producing a monoclonal antibody.
Owner:SEKISUI CHEM CO LTD
Multifunctional protein molecule switch used for antibody detection
ActiveCN112694535AImprove signal-to-noise ratioStrong specificityAntibody mimetics/scaffoldsOxidoreductasesAntigen epitopeProtein detection
Owner:CHONGQING MEDICAL UNIVERSITY
Nk cell engaging antibody fusion constructs
ActiveUS20200109202A1Increased serum half-lifeEnhanced NK cell engaging functionalityAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsHeavy chainNatural Killer Cell Inhibitory Receptors
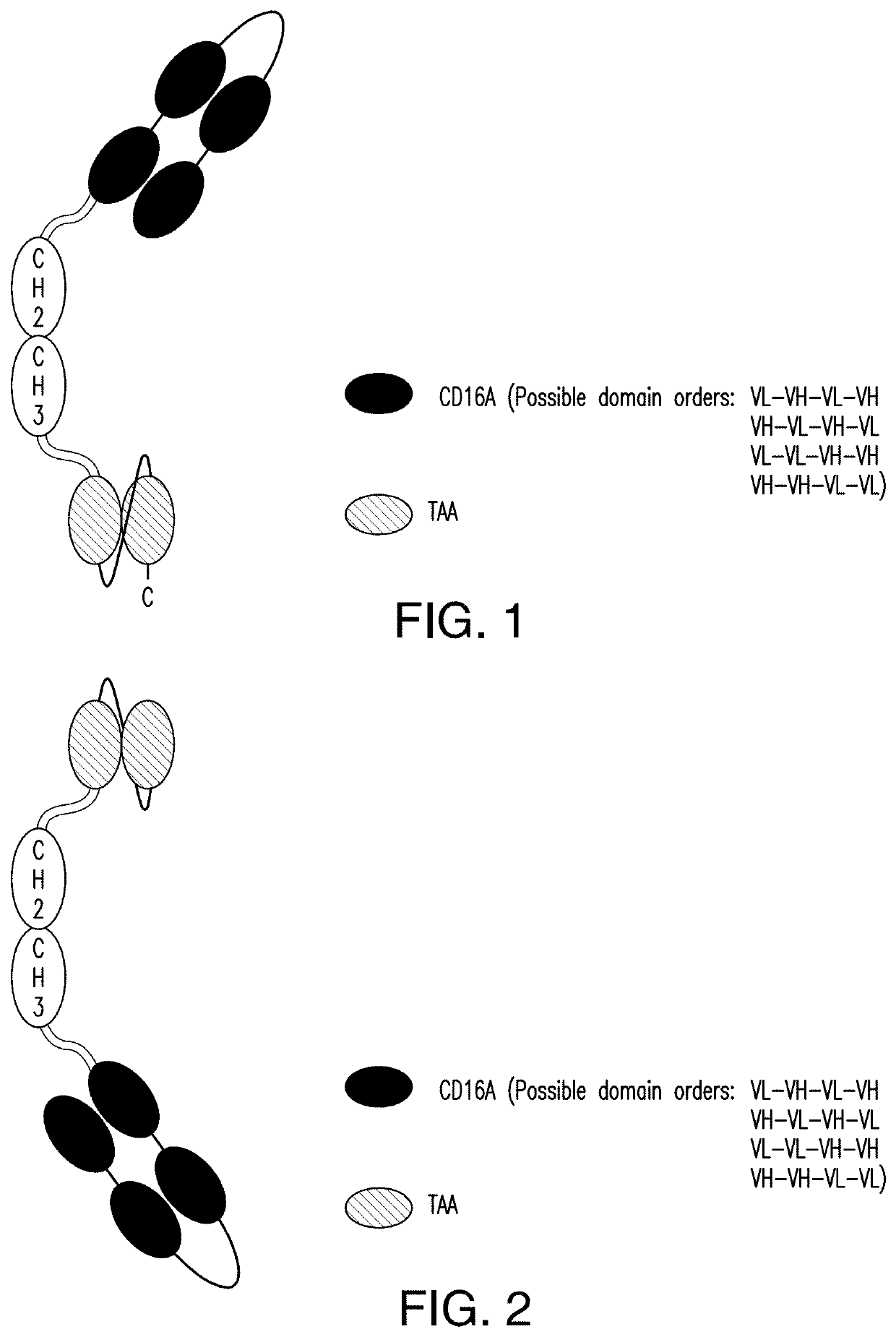
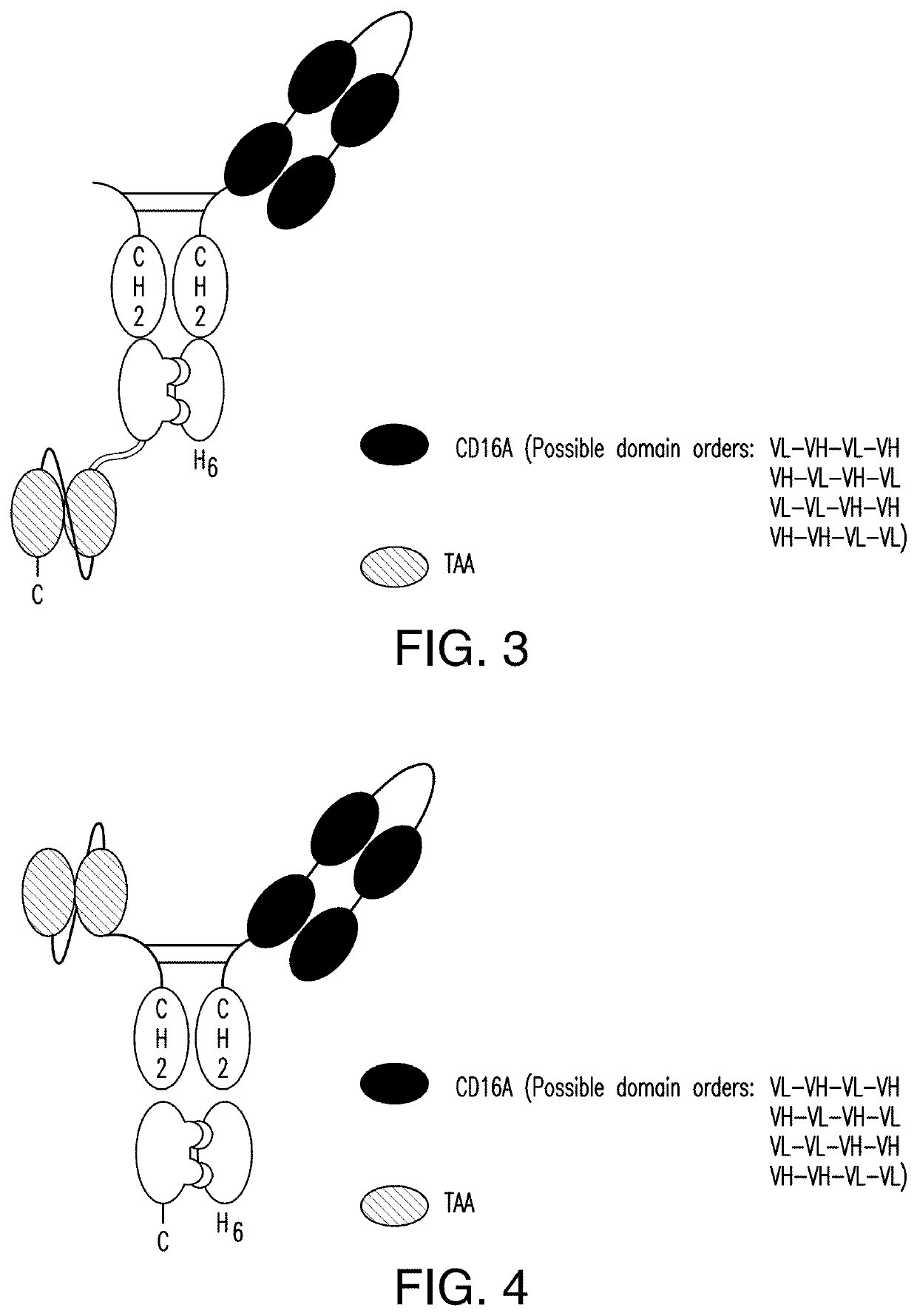
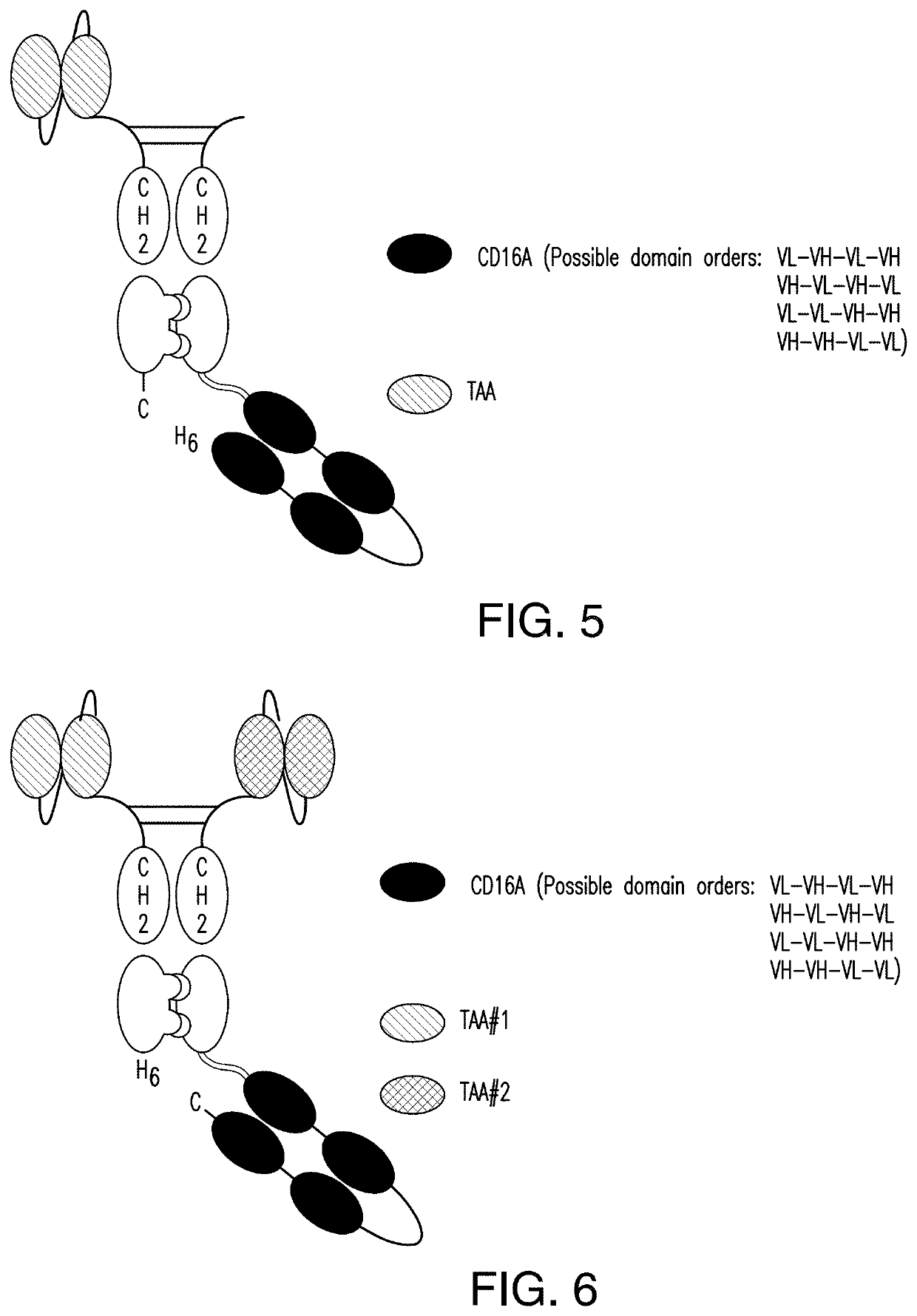
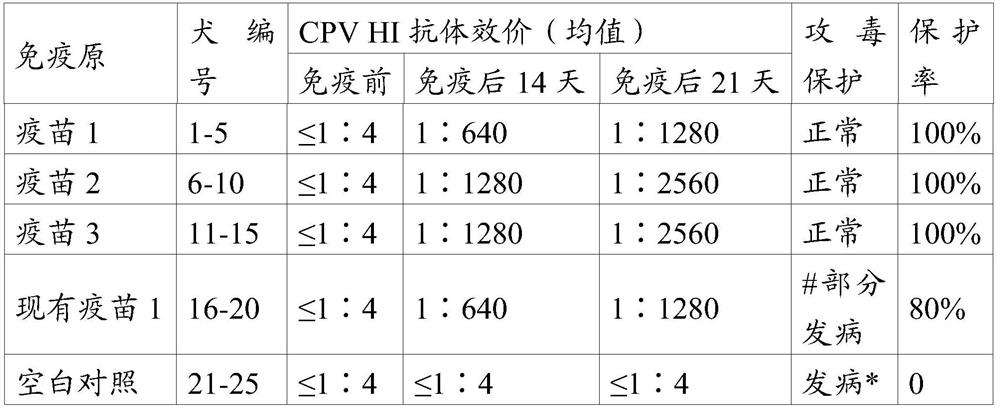
The invention relates to multispecific antigen-binding proteins for engaging natural killer (NK) cells for triggering NK cell cytotoxicity by engaging the CD16A (FcγRIIIA)expressed on NK cells, wherein the antigen-binding protein comprises at least two CD16A antigen-binding moieties and at least a further target antigen-binding moiety. The CD16A antigen-binding moiety comprises light chain and heavy chain variable regions linked one after another in a polypeptide chain and the variable region at the N-terminus of the polypeptide chain comprising the CD16A antigen-binding moiety is a light chain variable region.
Owner:AFFIMED GMBH
Vaccine composition, kit and application thereof
ActiveCN107537033BEasy to solveImprove control effectAntiviralsAntibody medical ingredientsDiseaseCanine parvovirus infection
The invention relates to a vaccine composition containing an immune amount of canine parvovirus antigen and an immune amount of other antigens, and also relates to a kit containing an effective amount of canine parvovirus antigen and an effective amount of other antigens, and the prepared vaccine composition Both the method and the kit can be effectively used for preparing medicines for preventing and / or treating diseases related to canine parvovirus infection. The vaccine composition and kit of the invention can effectively prevent the existing wild strains of the circulating canine parvovirus, and have a synergistic effect with some antigens in other antigens to further improve the immune effect of the combined vaccine.
Owner:PU LIKE BIO ENG
Immune memory enhanced preparations and uses thereof
PendingUS20220062406A1Enhance immune responseEliminate and reduce failureSsRNA viruses negative-senseSsRNA viruses positive-sensePartial antigenHistiocyte
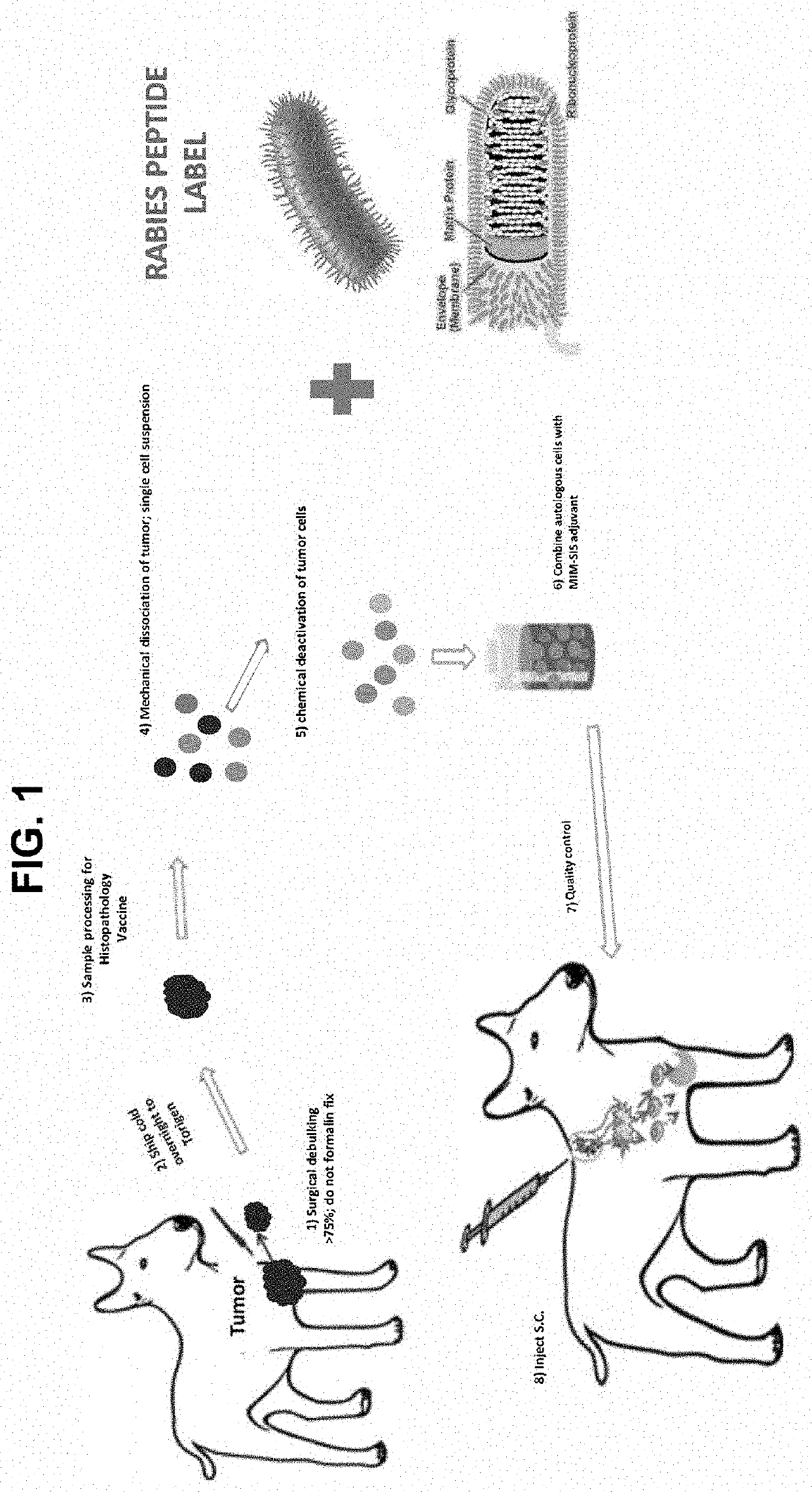
Formulations and preparations having immune memory enhanced properties are disclosed that provide for enhancing immune response against a tumor growth, cancer, infectious agent, bacteria, virus or other infectious or non-infectious agent. The vaccine formulation includes an immune memory invoking component, such as an antigen of an infectious agent, virus (e.g., Rabies), bacteria, prion, neo-antigen or other moiety antigen, and a targeted antigen (e.g., a harvested tumor tissue (B-cell, T-cell, epitopes)). The vaccine formulation / preparations may comprise a target infectious agent protein / peptide component (such as a SARS-Cov-2 spike protein epitope) mixed with, or fused to (or otherwise conjugated) an immune-memory associated viral antigen (such as Rabies, polio, or other peptide / protein antigen or peptide or fragment thereof).
Owner:TORIGEN PHARM INC
Method for producing antibody using “naked” expression vector expressing type II transmembrane fusion protein
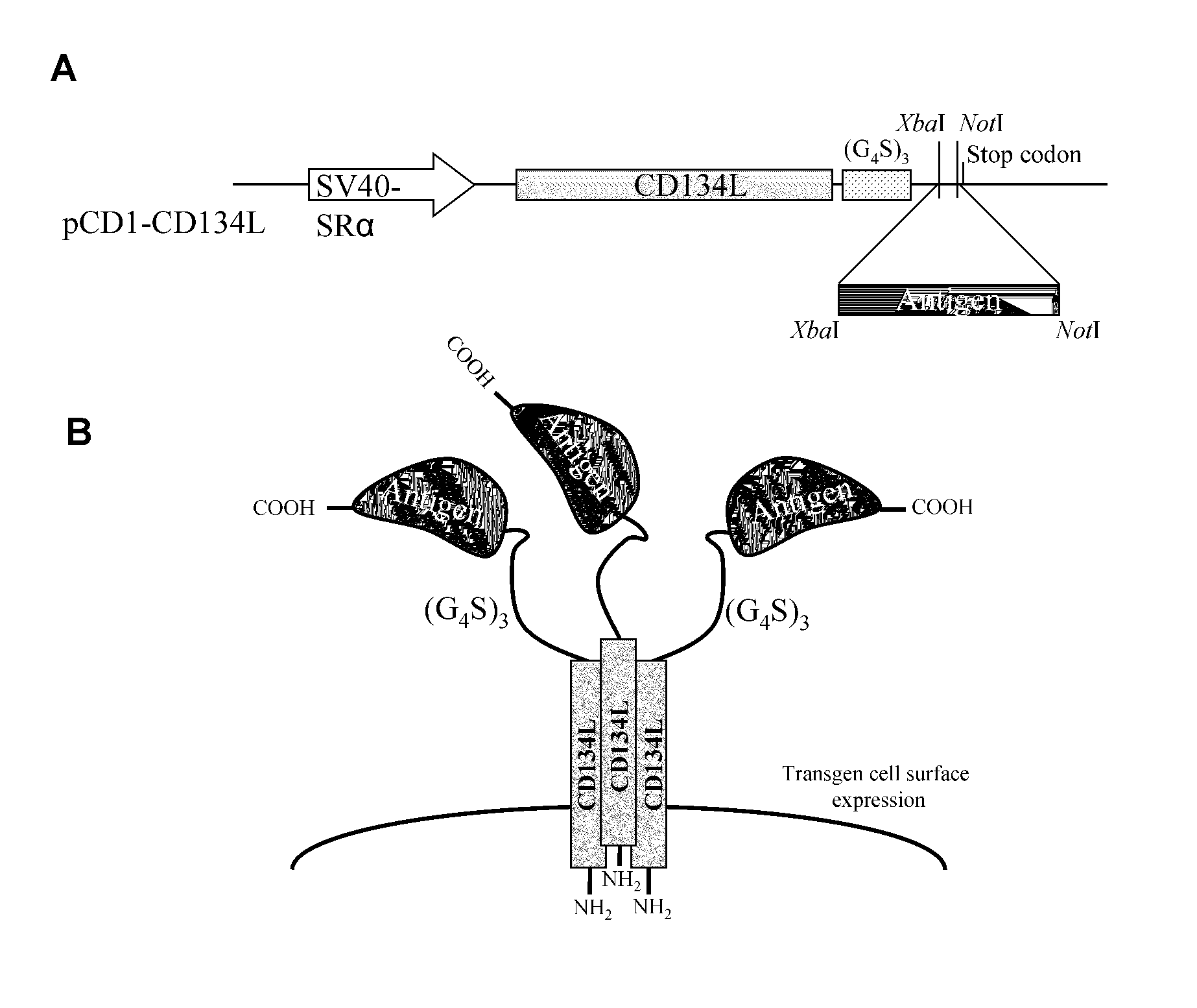
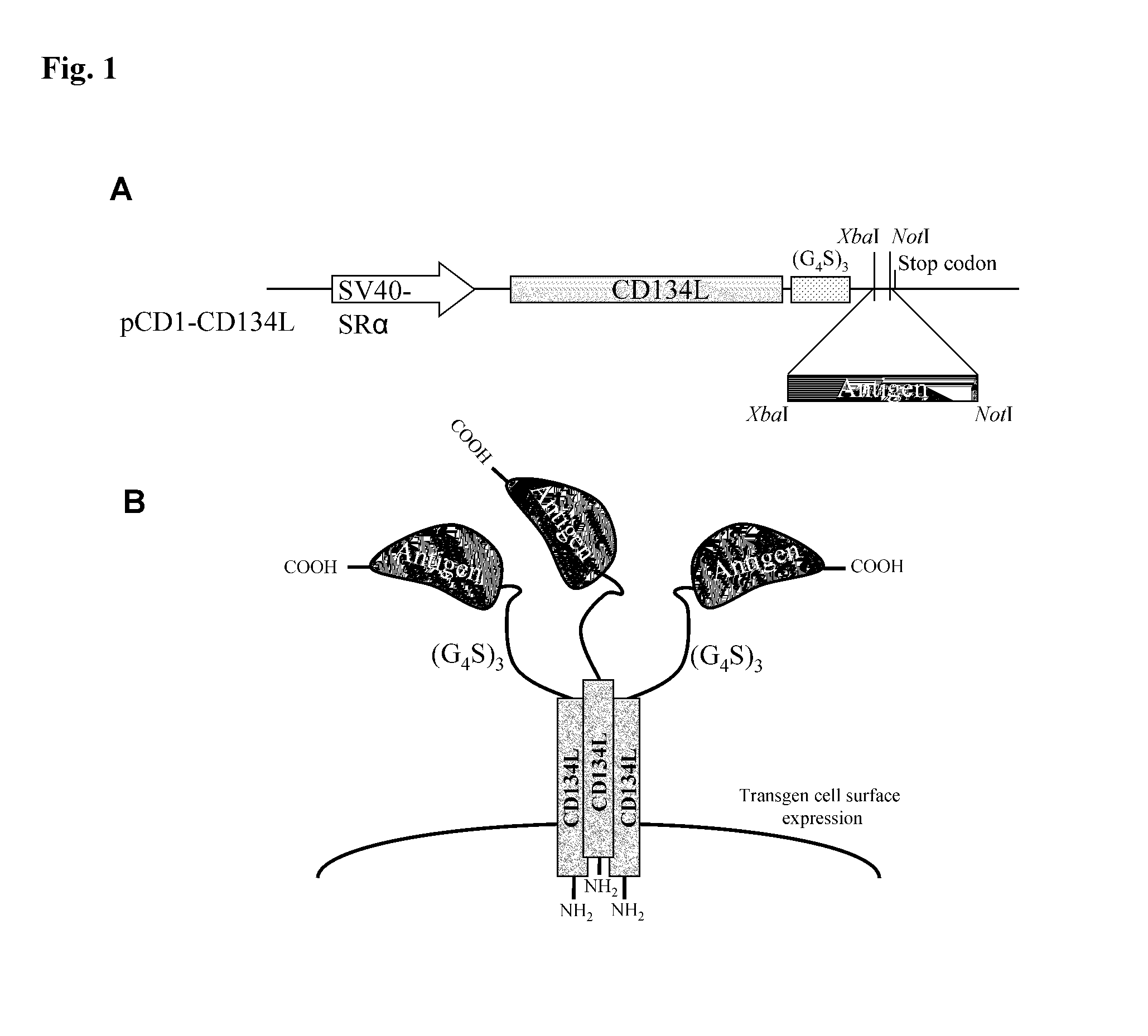
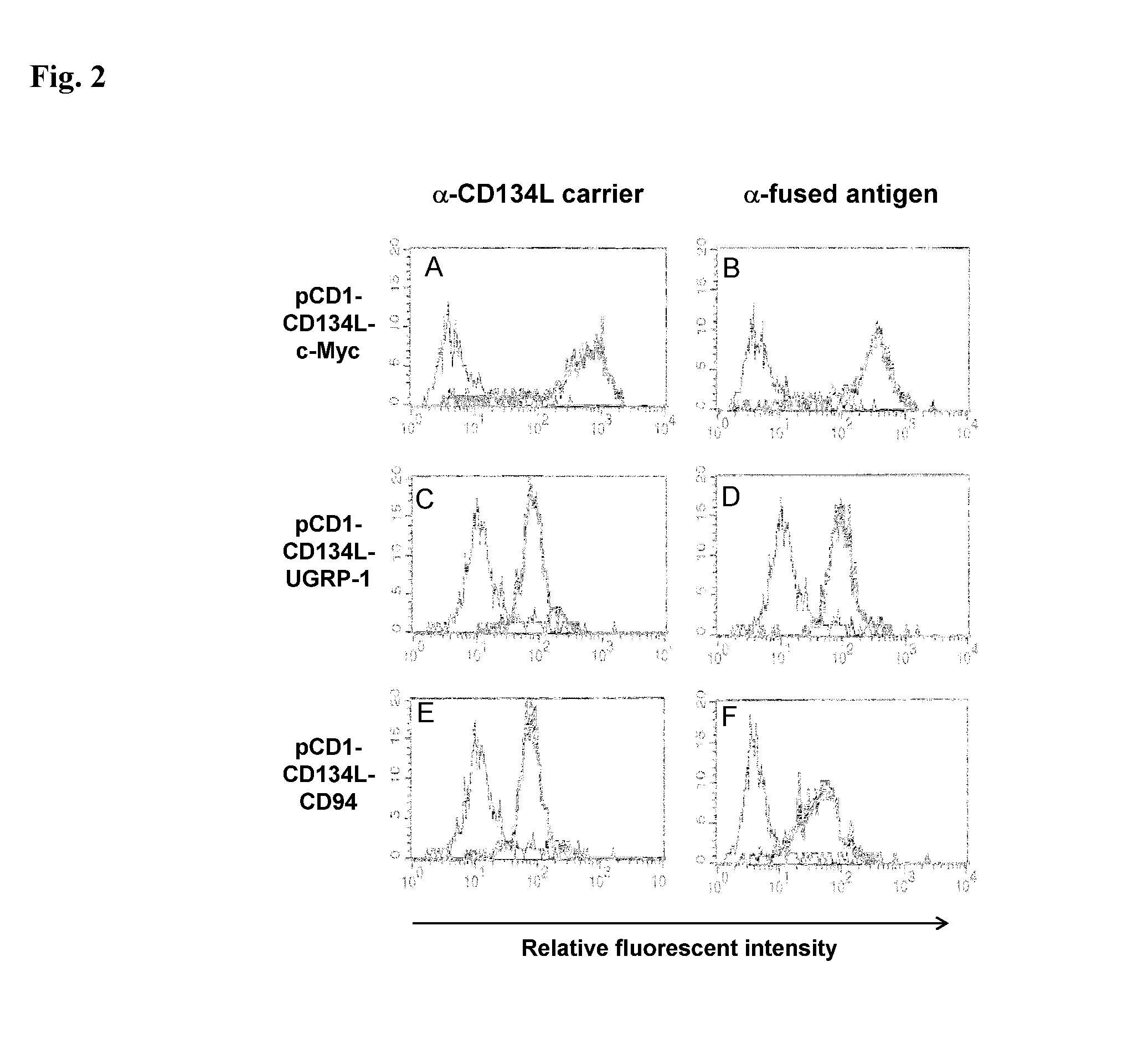
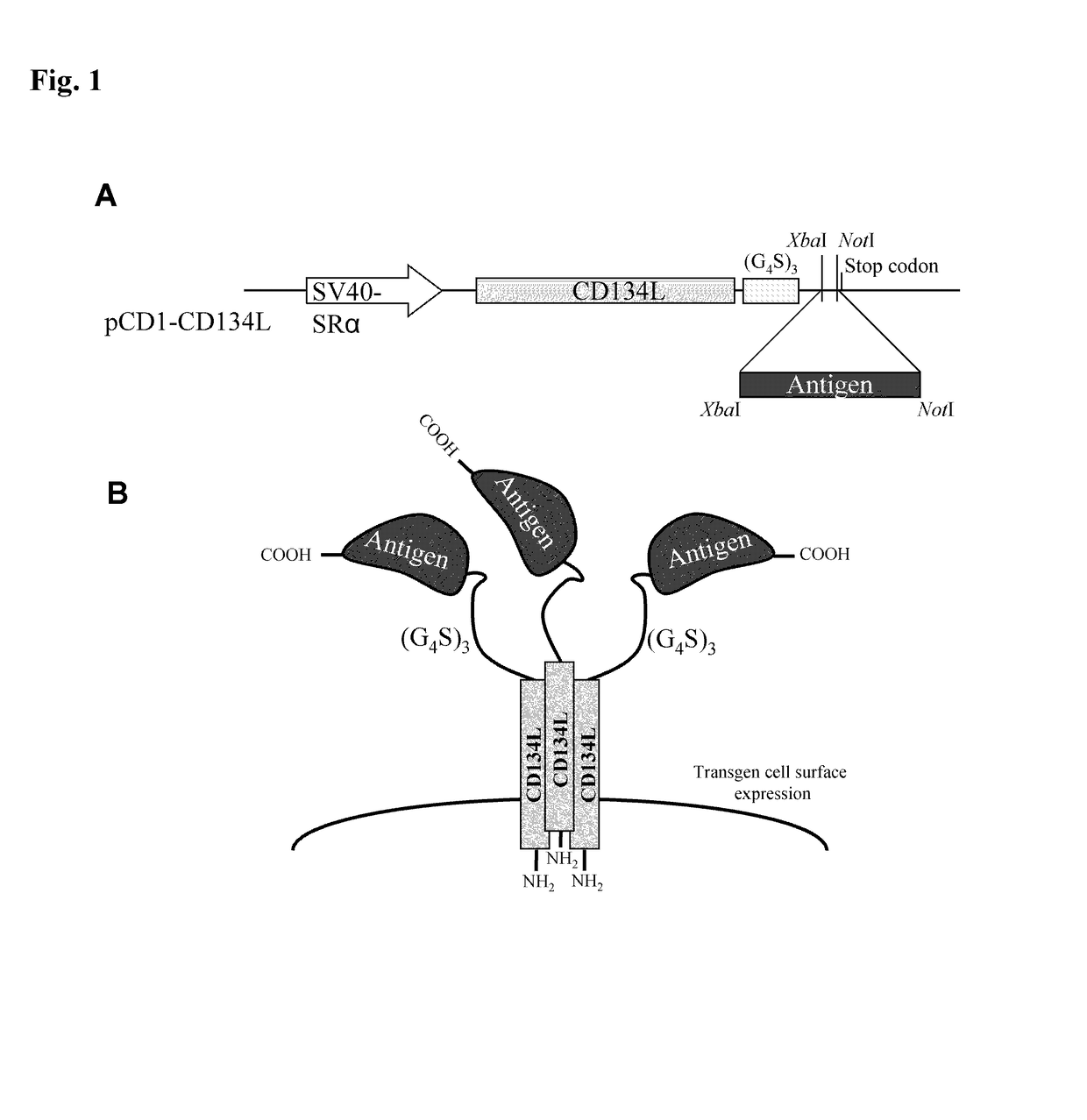
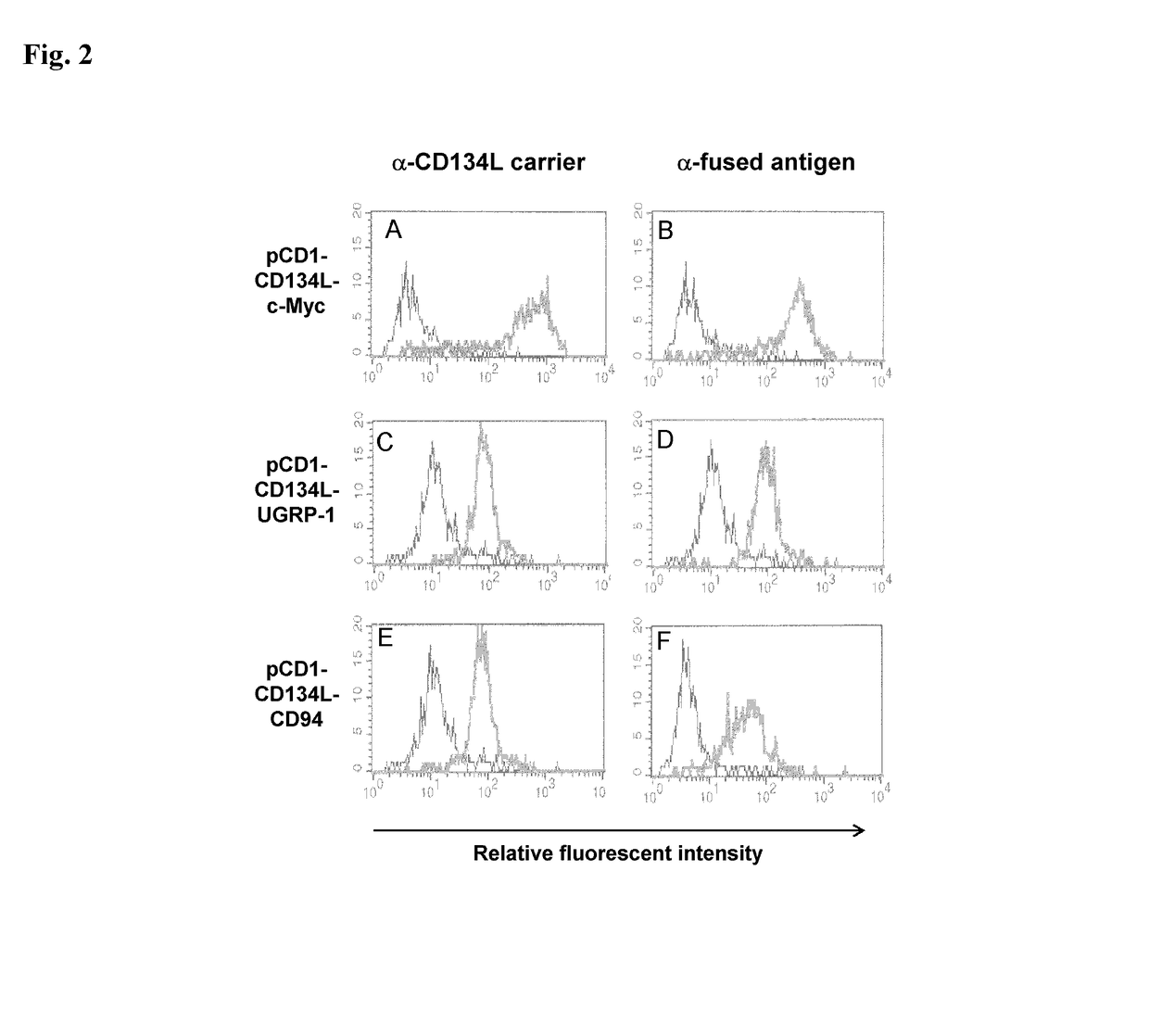
Methods are disclosed for generating antibodies and an expression vector used to express protein(s) which provoke the antibody response. The expression vector may be useful in generating an antibody directed to an antigen, comprising a gene in operable linkage with a promoter, which gene encodes upon expressing a fusion protein comprising (i) CD134L, a fragment or homologous protein thereof as N-terminal moiety of the fusion protein; and (ii) all or part of an antigenic protein as C-terminal moiety of the fusion protein. To generate the antibodies, the vector is injected into a subject animal, which produces a fusion protein, against which antibodies are generated.
Owner:R P SCHERER TECH INC
Method for producing antibody using “naked” expression vector expressing type II transmembrane fusion protein
Methods are disclosed for generating antibodies and an expression vector used to express protein(s) which provoke the antibody response. The expression vector may be useful in generating an antibody directed to an antigen, comprising a gene in operable linkage with a promoter, which gene encodes upon expressing a fusion protein comprising (i) CD134L, a fragment, homologous or orthologues protein thereof as N-terminal moiety of the fusion protein; and (ii) all or part of an antigenic protein as C-terminal moiety of the fusion protein. To generate the antibodies, the vector is injected into a subject animal, which produces a fusion protein, against which antibodies are generated.
Owner:R P SCHERER TECH INC
Method of immunizing animal, composition for immunization, method for producing antibody, method for producing hybridoma and method for producing monoclonal antibody
It is an object of the present invention to a method whereby a humoral immune response is induced more efficiently in producing an antibody against an antigen protein by gene immunization. A fusion gene composed of a gene encoding the full-length of a part of the antigen protein or a gene encoding a chaperonin subunit or a chaperonin subunit linkage linked thereto is administered to express the fusion gene in the animal, thereby inducing a humoral immune response to an antigen protein by administering. An example of the chaperonin includes Escherichia coli GroEL. There is also provided with a composition for immunization, a method for producing an antibody, a method for producing a hybridoma, and a method for producing a monoclonal antibody.
Owner:SEKISUI CHEM CO LTD
Methods of identifying and validating affinity reagents
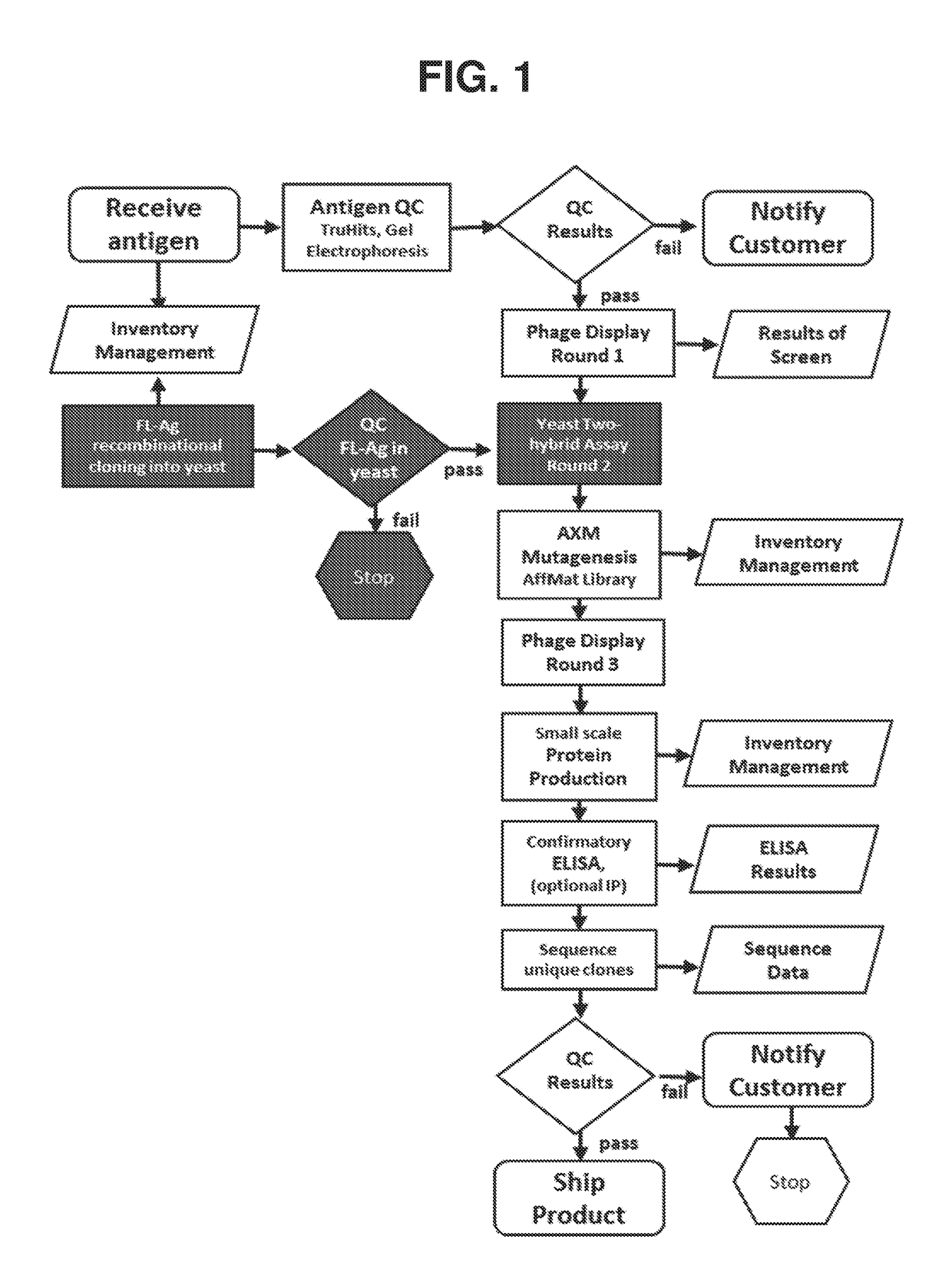
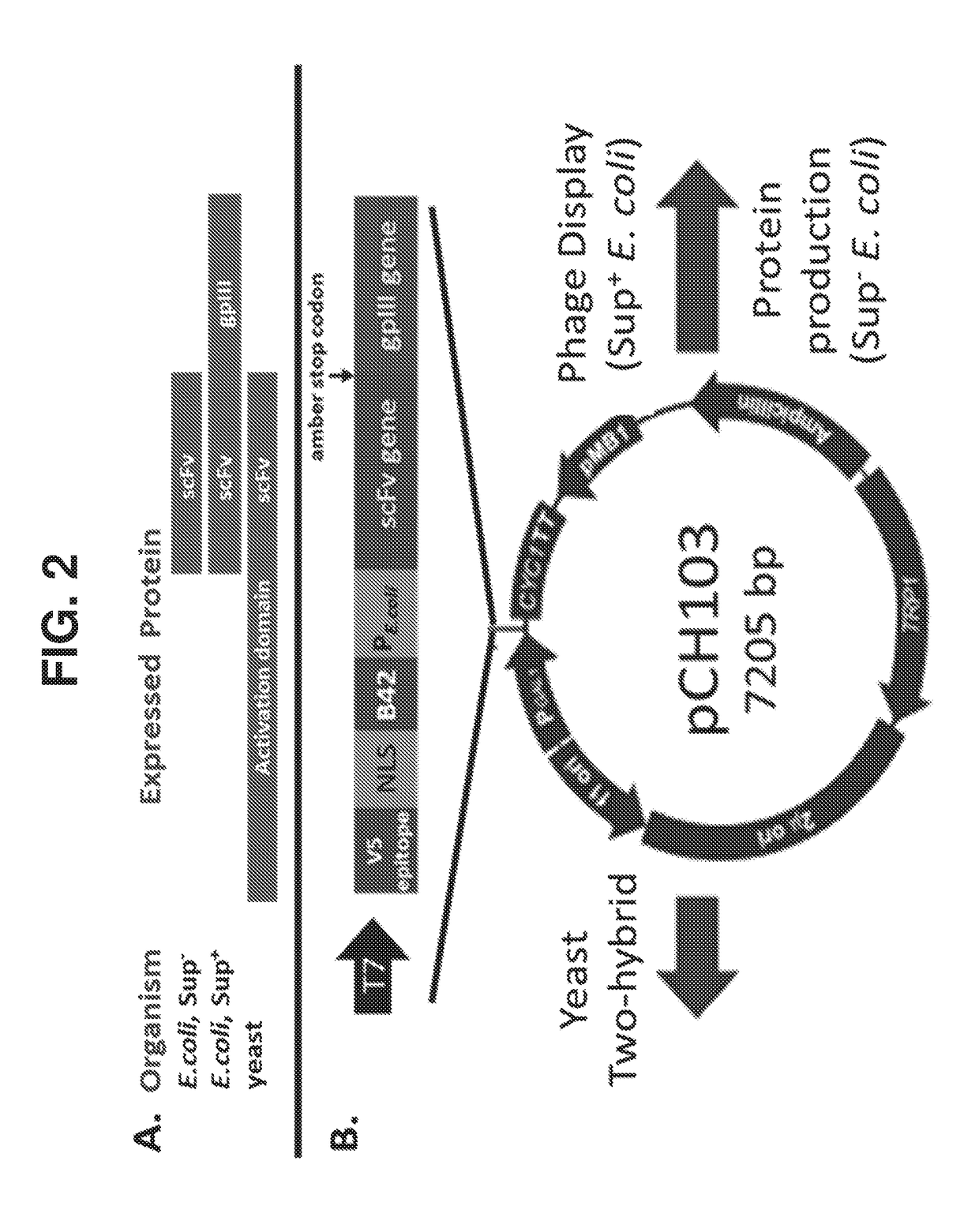
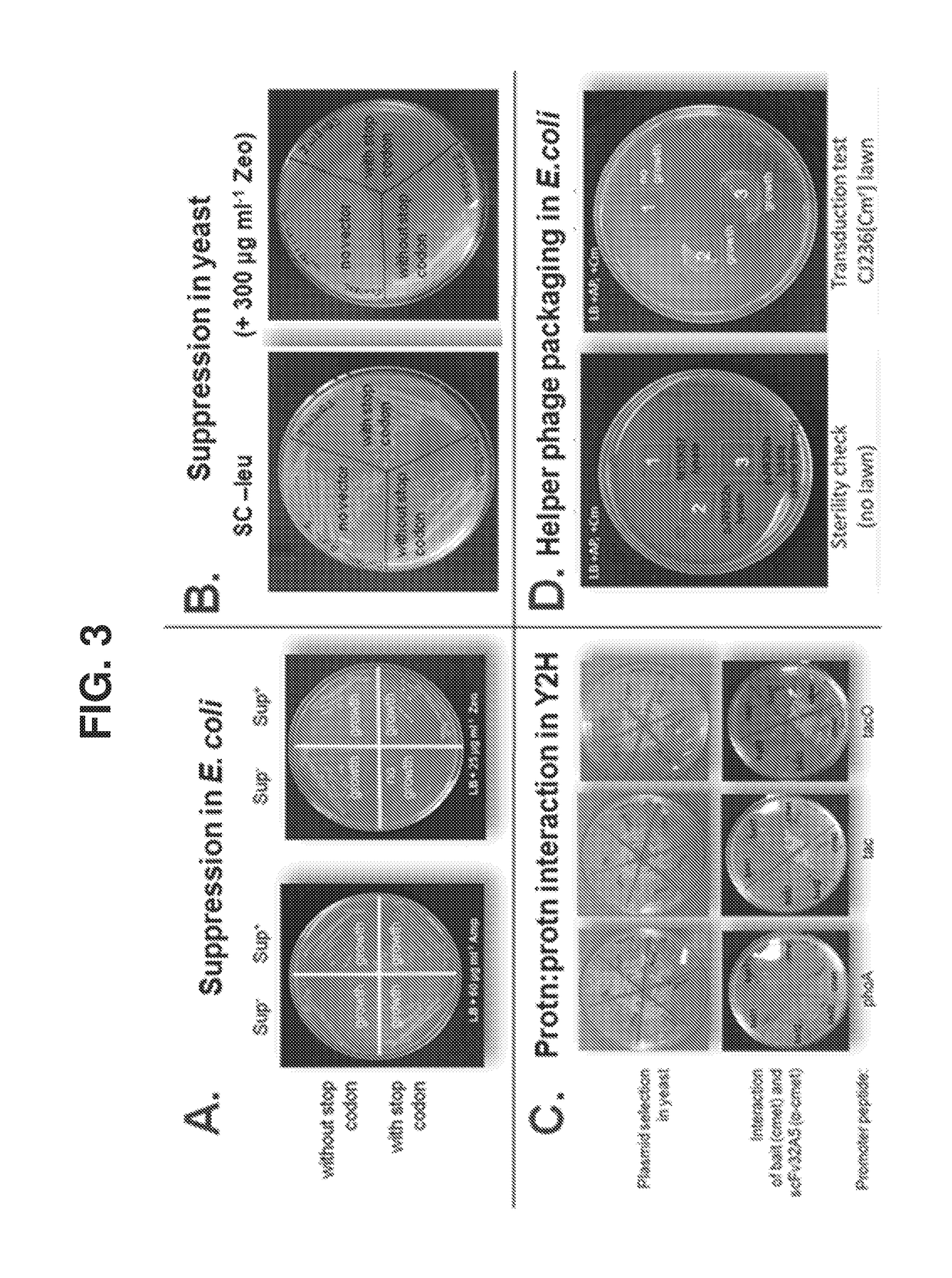
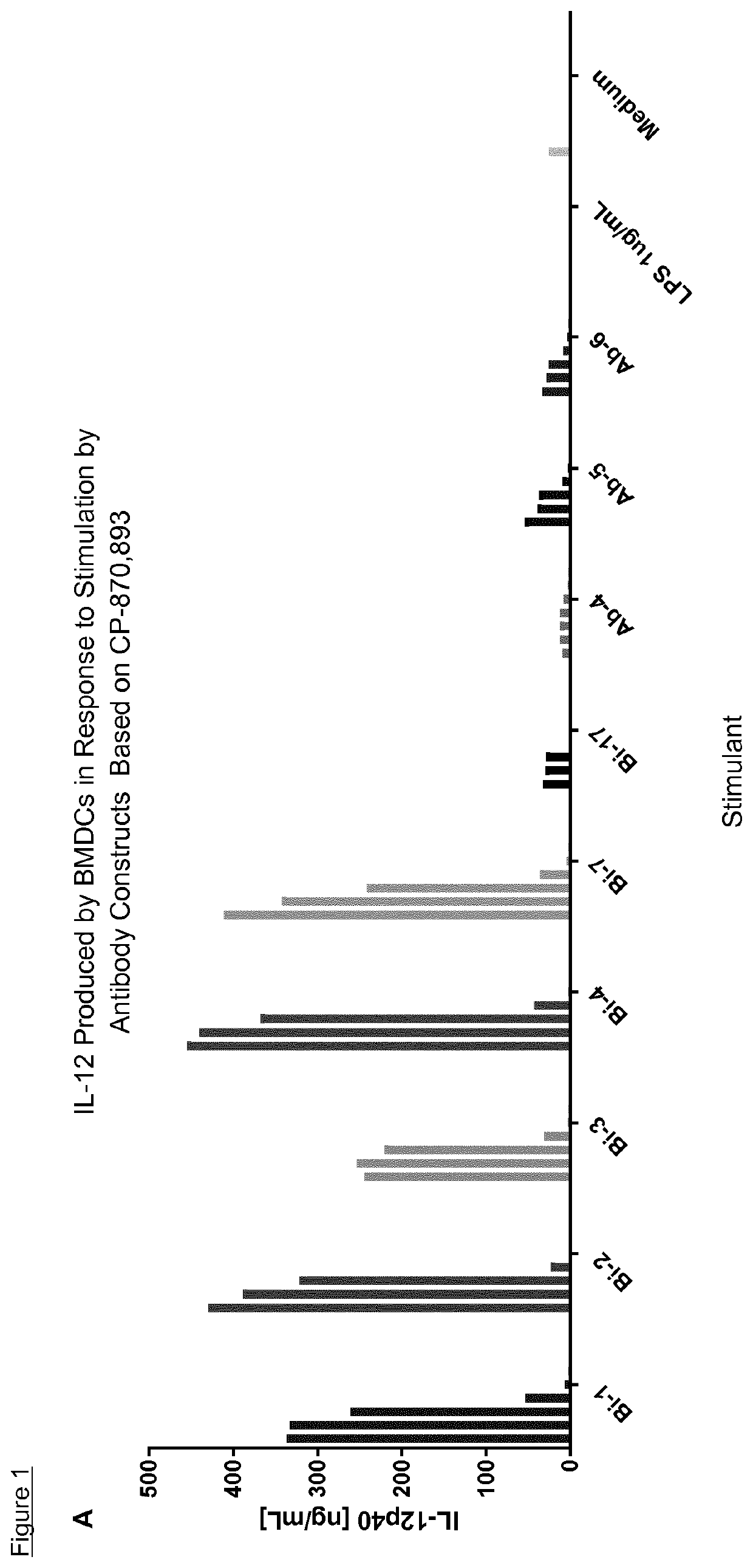
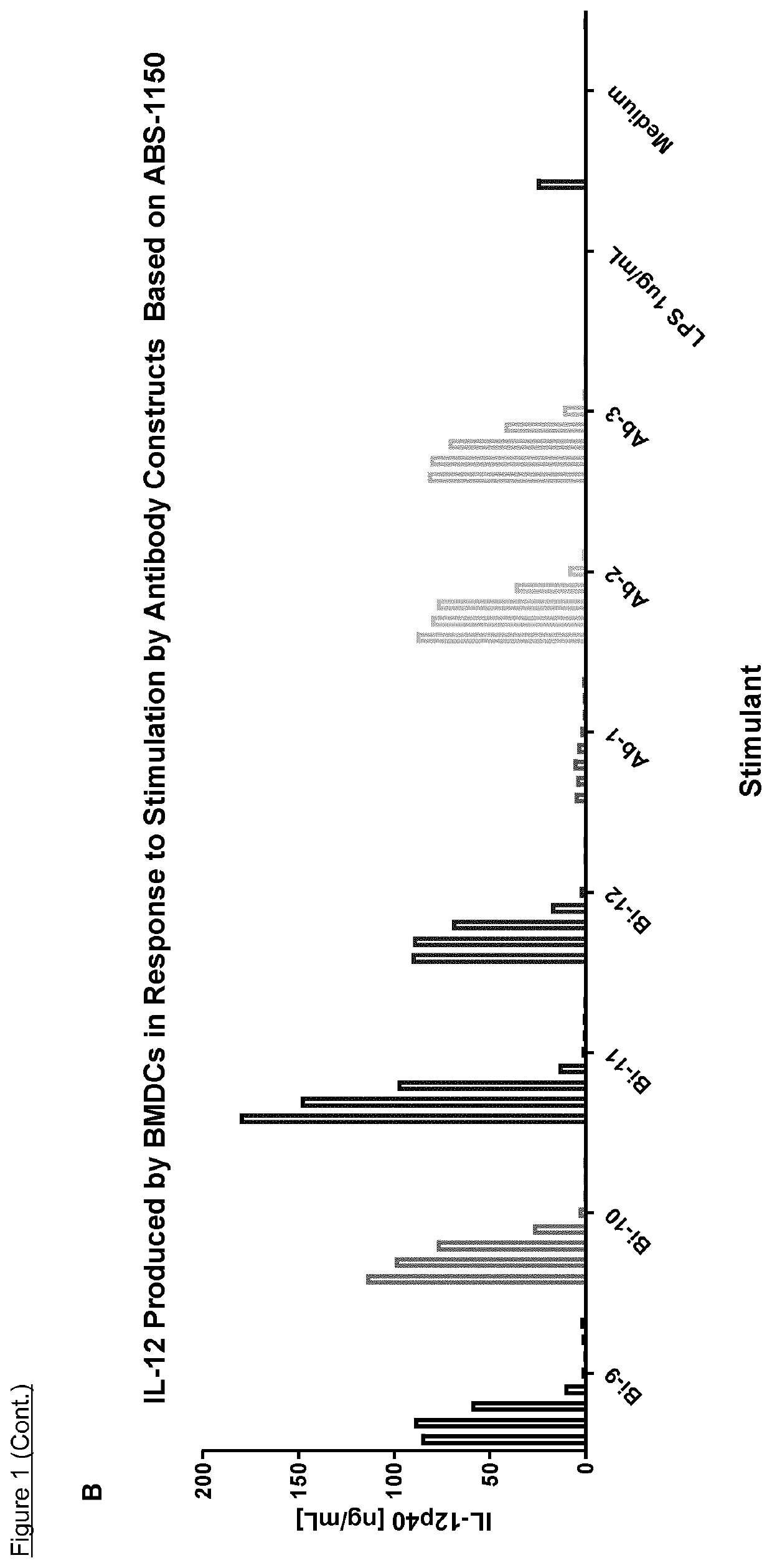
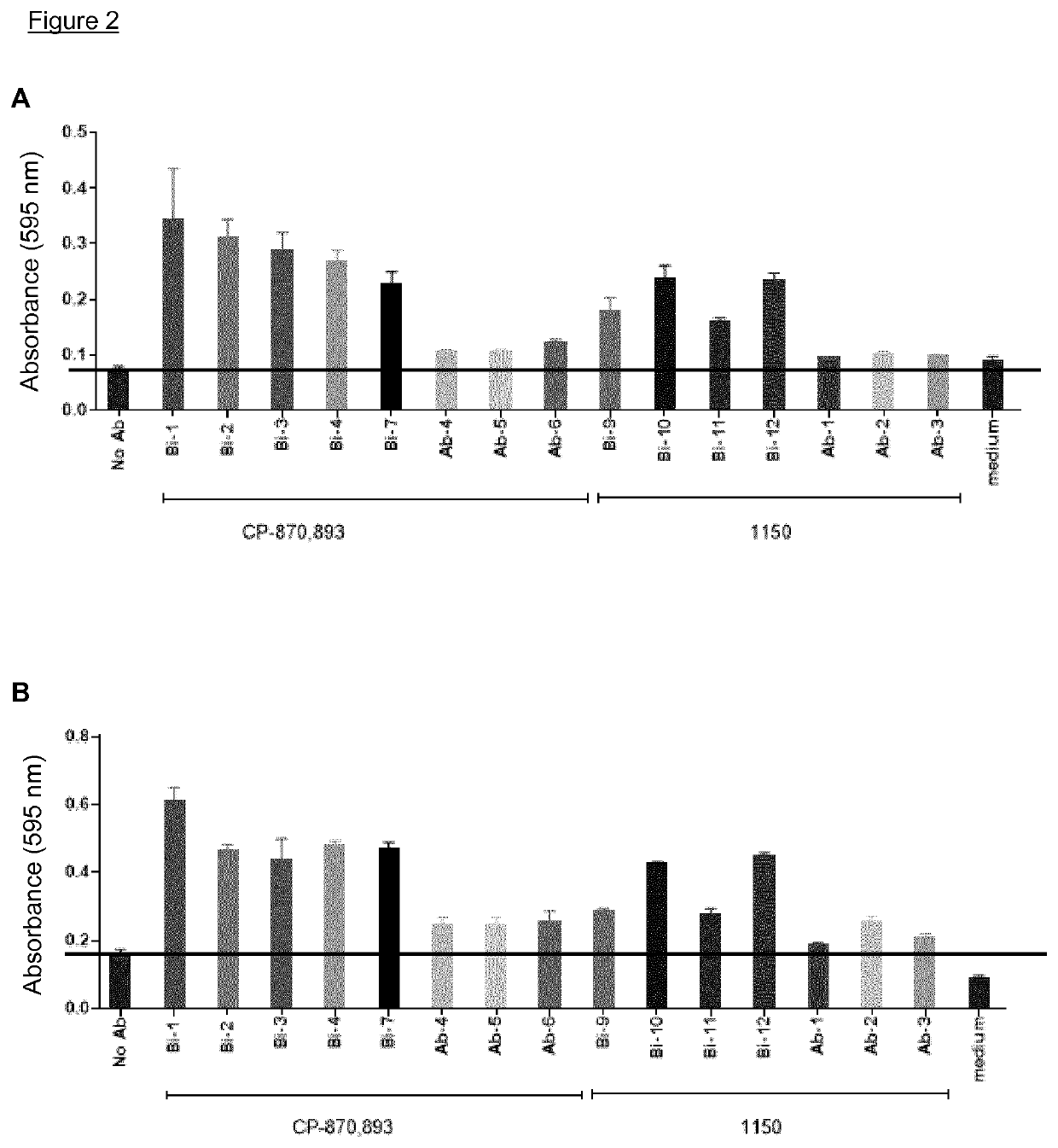
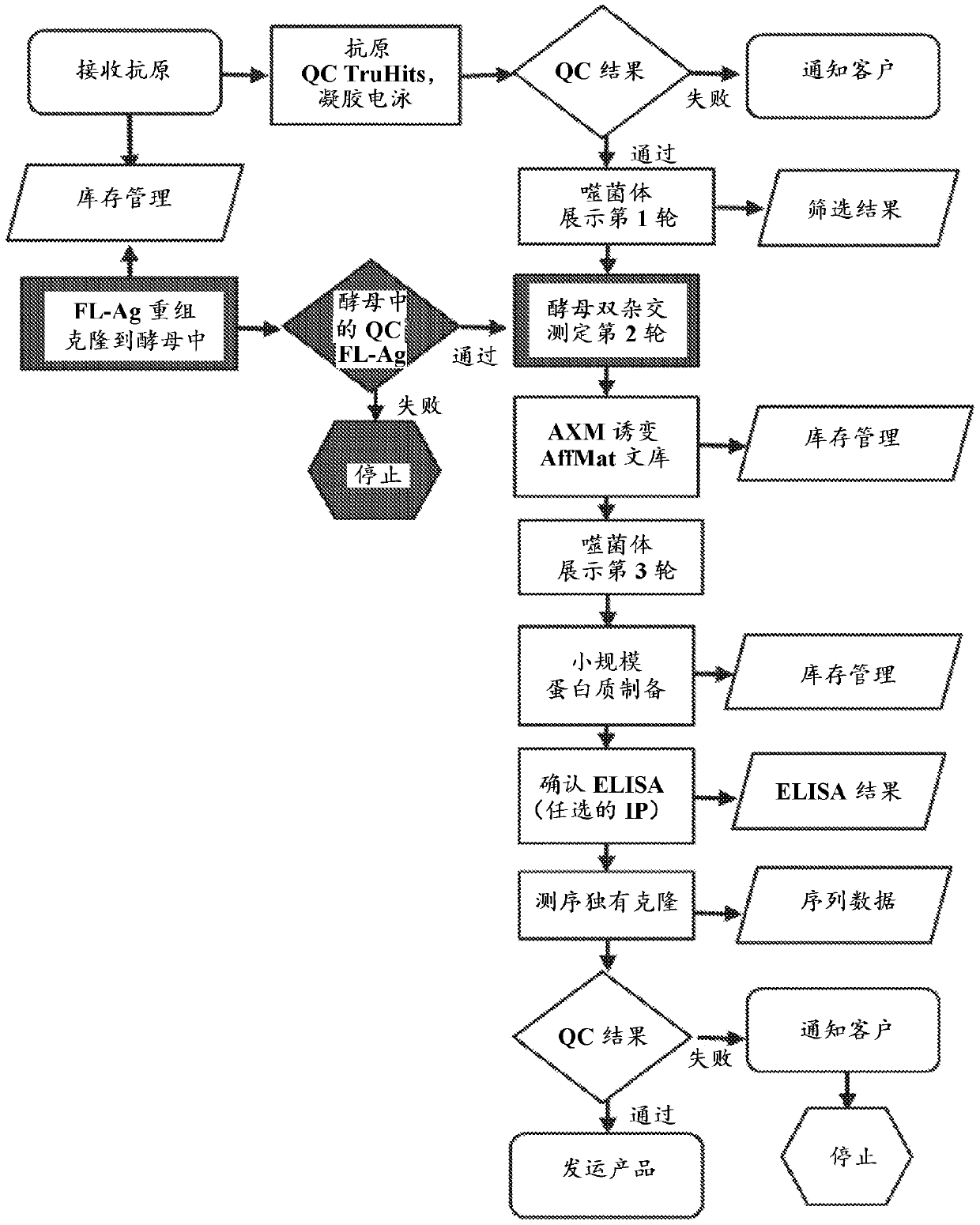
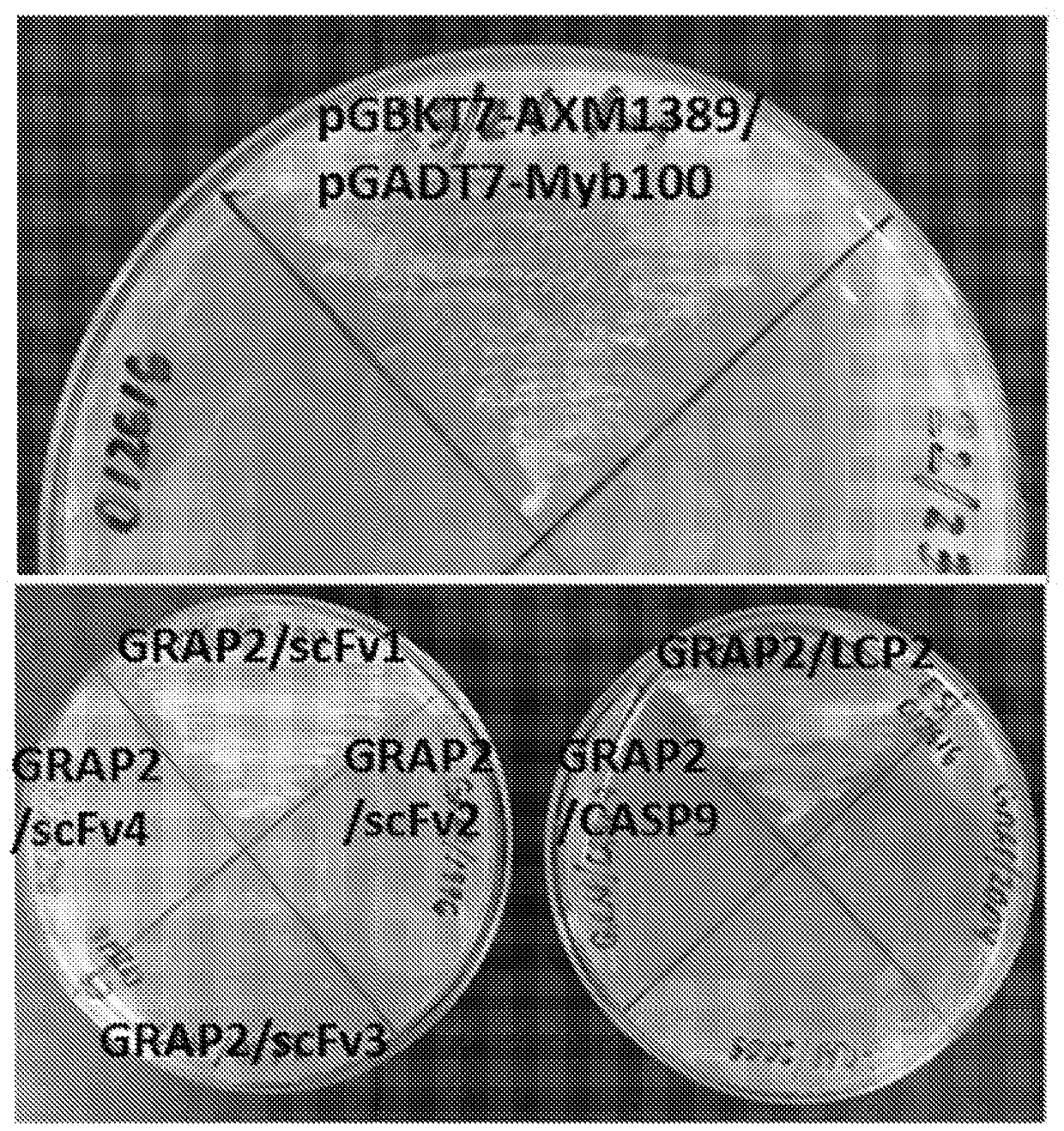
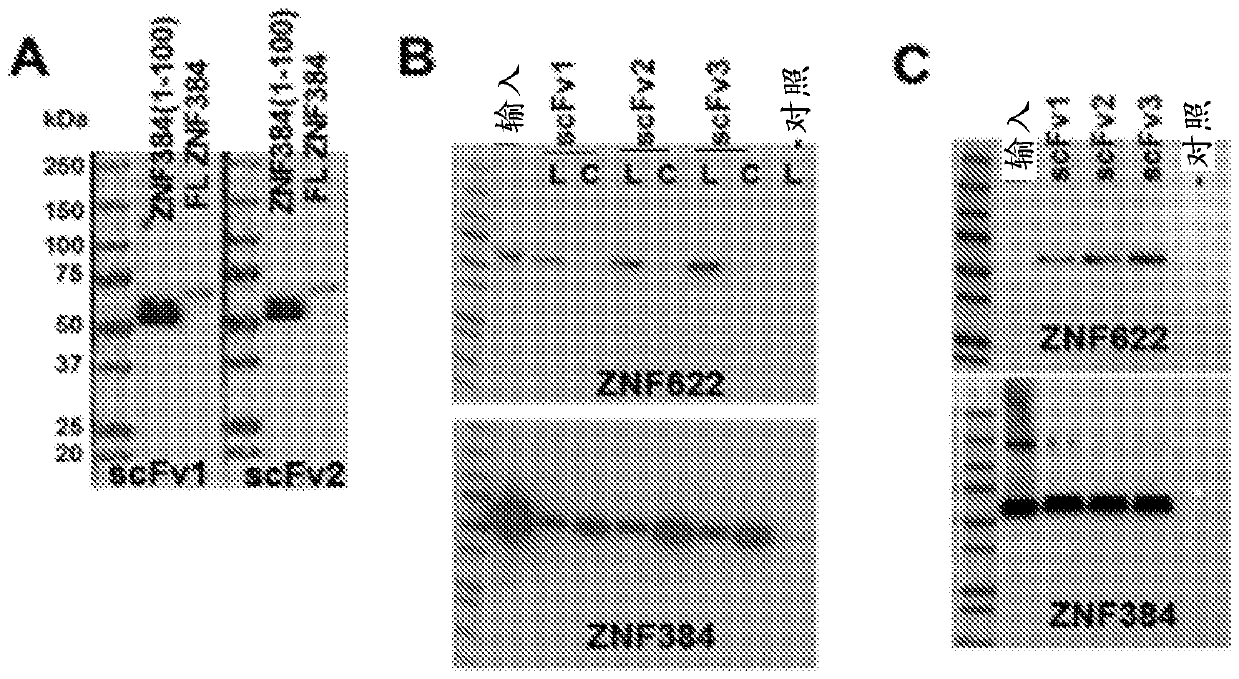
The invention features methods of identifying and validating affinity reagents, such as antibodies. The methods of the invention generally involve screening an antibody library by, for example, phage display on bacteria (e.g., E. coli) to identify particular antibody clones capable of binding a desired target polypeptide. Clones identified in this way can then be validated using yeast 2-hybrid. In some instances, antibodies identified by their capacity to binding a partial antigen can be validated by their capacity to bind to the full-length antigen. Validated clones can be further screened by additional rounds of phage display and / or yeast 2-hybrid. Between each round, additional variants of particular antibody clones can be generated and screened to identify variants that demonstrate higher binding affinity to the target of interest.
Owner:AXIOMX
Preparation method of viral vaccine, and pharmaceutical composition
ActiveCN113264991AAvoid serious side effectsAntagonize infectionSsRNA viruses positive-senseViral antigen ingredientsPartial antigenViral Vaccine
The invention provides a preparation method of a viral vaccine. The preparation method comprises the following steps: providing a coding sequence of a full-length or partial antigen peptide or protein of at least one virus, dividing the coding region of the full-length or partial antigen peptide or protein into one or more partially overlapped nucleic acid sequences, and connecting the one or more partially overlapped nucleic acid sequences in series to form a nucleic acid vaccine fragment. After the nucleic acid vaccine prepared by using the preparation method of the viral vaccine is injected into animals and human bodies, small-fragment antigen peptide vaccines are formed through in-vivo cell treatment, so serious side effects possibly caused by full-length protein vaccines can be effectively avoided, but the immunogenicity of antigen peptides or proteins can be maintained, and therefore, virus infection can be effectively antagonized. Meanwhile, a small-fragment antigen peptide component can be rapidly and correspondingly adjusted according to the mutation of the S protein of the new coronavirus, so the nucleic acid vaccine can also be effective to new coronavirus mutant strains. The invention also provides a pharmaceutical composition obtained through the preparation method of the viral vaccine.
Owner:SHANGHAI JENOMED BIOTECH CO LTD
Artificial antigen, antibody of fenvalerate and uses thereof
The invention discloses a fenvalerate artificial antigen, with the molecular structural formula is as formula I, n=1-5, employing formula II as partial antigen, covalent coupling with protein for synthesis; the combination ratio between the partial antige and protein is 5:1-100:1. The invention also discloses the monoclonal or polyclonal immune globulin G which is got from immunizating mouse or rabbit by the above said fenvalerate artificial antigen and can occur specific immunological reaction with fenvalerate, the said fenvalerate specific antibody can be used to check the residual quantity of fenvalerate in sample. The invention also discloses a detecting agent box of direct and indirect competeing enzyme and immunoadsorption for fenvalerate residual analysis. The got antibody is used to detect fenvalerate by ELISA method, with the lowest detecting limit being 4.0+ / -1.5 ug / l (0.004ppm), and the detecting sensitivity is high.
Owner:ZHEJIANG UNIV
Recombined attenuated live vaccine for preventing and treating I-type infection of herpes simplex virus and preparation method thereof
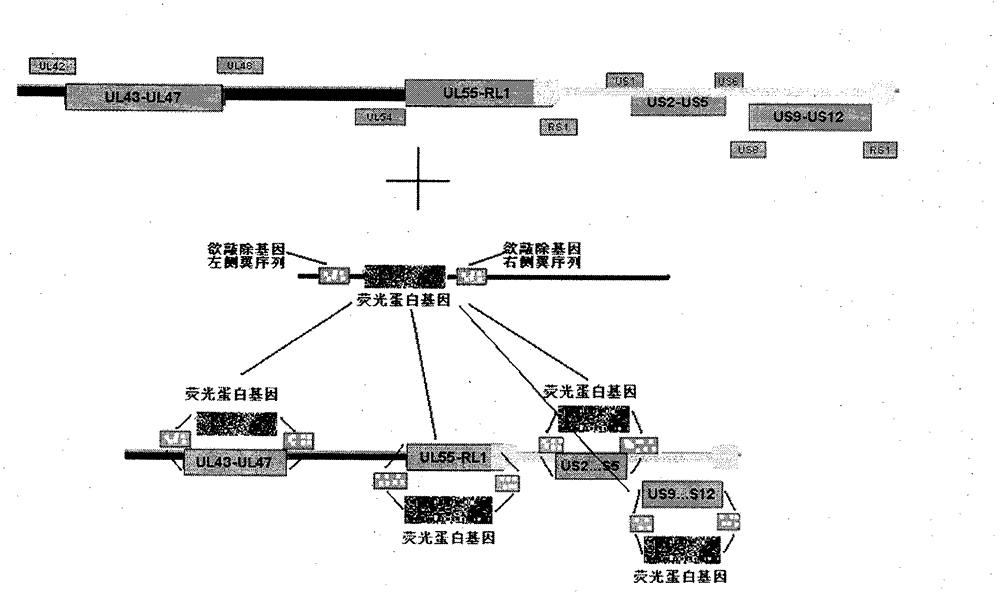
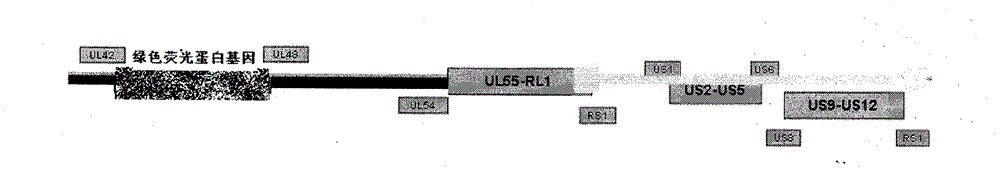
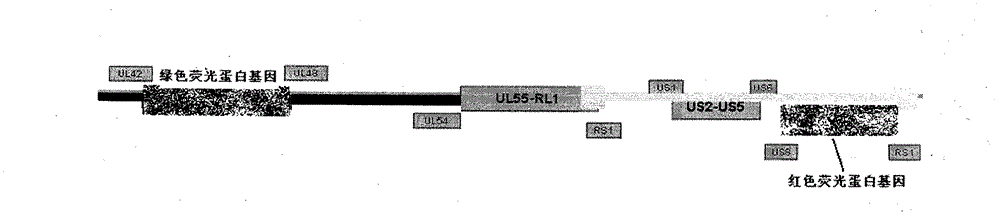
InactiveCN101926992BRelapse controlControl spreadInactivation/attenuationMicroorganism based processesPartial antigenWild type
The invention provides a recombined attenuated live vaccine for preventing and treating I-type or II-type infection of herpes simplex virus and preparation method thereof. The recombined attenuated live vaccine is characterized by omitting the UL43, UL44, UL45, UL46 and UL47 genes in the genome thereof; the method comprises the steps of: separating and identifying a wild-type herpes simplex virus; carrying out enlarged cultivation, extracting the complete genome of the virus, and designing a primer to amplify a homologous flanking sequence formulating the omitted gene segment; cloning the homologous flanking sequence and a fluorescent protein gene to a pShuttle-CMV carrier to carry out homologous recombination; selecting the recombined virus by taking the fluorescent protein gene as a mark; and carrying out plaque purification to obtain the gene-recombined attenuated live vaccine of the herpes simplex virus. The attenuated live vaccine prepared by the invention undergoes gene engineering recombination so that toxicity returning is not easy to happen, and the other biological characteristics of the attenuated live vaccine are similar to those of the wild-type herpes simplex virus.
Owner:樊科伟
Bi-specific conjugates
PendingUS20220000998A1Good effectLow costAntibody mimetics/scaffoldsImmunoglobulins against bacteriaCancer antigenPartial antigen
The present invention provides a conjugate comprising: i) at least one first specific binding molecule which binds CD40, wherein said first specific binding molecule is an agonist of CD40; and ii) at least one second specific binding molecule which binds a tag moiety, wherein said tag moiety is not a cancer antigen, wherein said first specific binding molecule and second specific binding molecule are antigen-binding proteins comprising an antigen-binding domain of an antibody and are covalently linked. The conjugate can be combined with a tag construct comprising: i) a tag moiety which is not a cancer antigen; and ii) an antigen, being a cancer antigen or an antigen derived from a pathogen; wherein said antigen is a polypeptide and said tag moiety is covalently linked to said antigen, for use in therapy, to stimulate an immune response by a subject against the antigen in question.
Owner:STRIKE PHARM AB
Methods of identifying and validating affinity reagents
The invention features methods of identifying and validating affinity reagents, such as antibodies. The methods of the invention generally involve screening an antibody library by, for example, phagedisplay on bacteria (e.g., E. coli) to identify particular antibody clones capable of binding a desired target polypeptide. Clones identified in this way can then be validated using yeast 2-hybrid. Insome instances, antibodies identified by their capacity to binding a partial antigen can be validated by their capacity to bind to the full-length antigen. Validated clones can be further screened byadditional rounds of phage display and / or yeast 2-hybrid. Between each round, additional variants of particular antibody clones can be generated and screened to identify variants that demonstrate higher binding affinity to the target of interest.
Owner:AXIOMX
Covalent heterobivalent antibody inhibitors and ligands
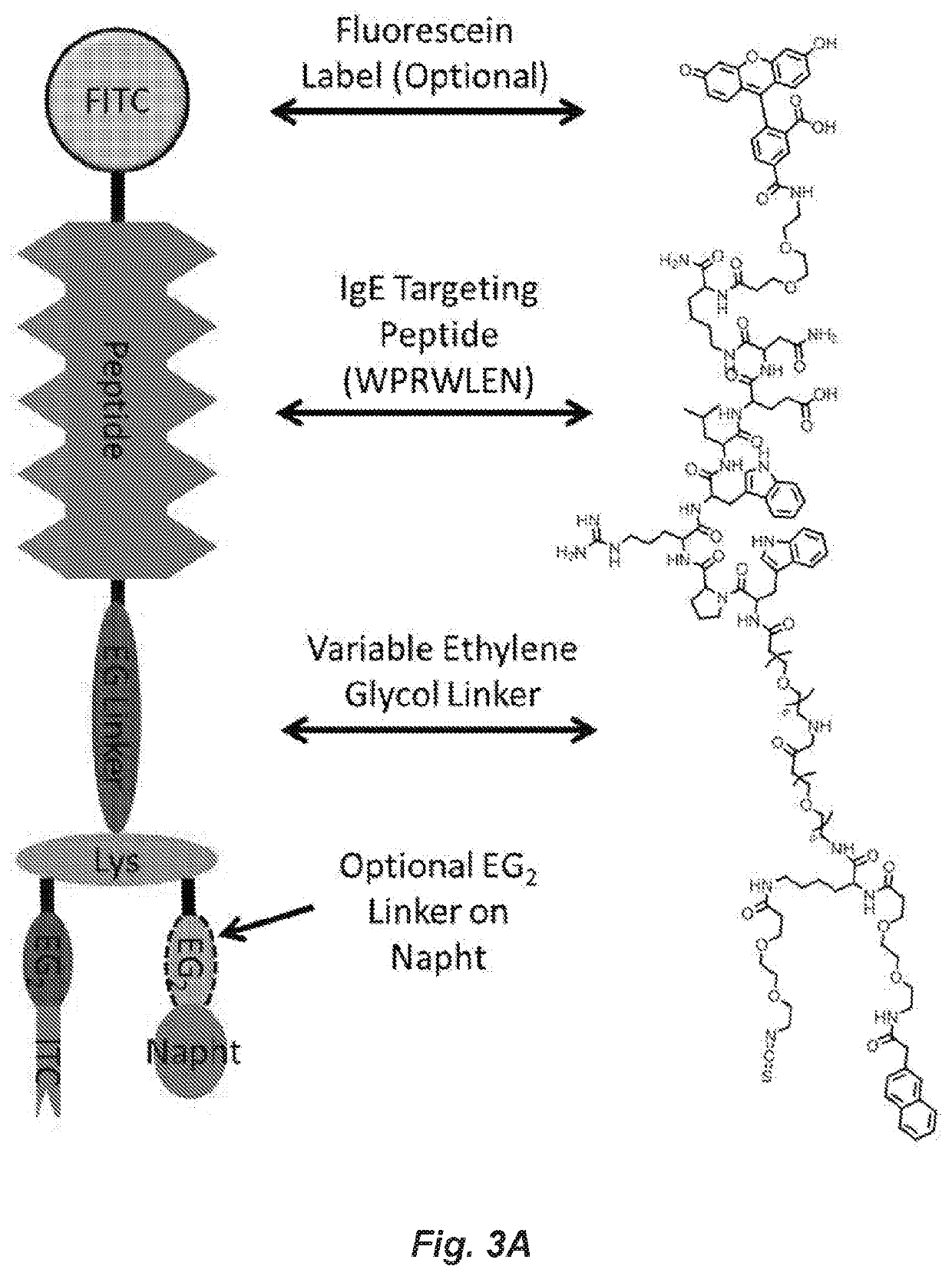
The invention provides a strategy for site specific covalent modification of antibodies using a specialized targeting covalent heterobivalent ligand (cHBL), and corresponding design for a covalent heterobivalent inhibitor (cHBI) that can be used to prevent Immunoglobulin E (IgE) mediated allergic reactions triggered by drug molecules, according to one embodiment. These molecules contain four important components: (1) an IgE antigen binding site (ABS) ligand that can be a mimotope for the allergen protein, a small molecule, or a peptidomimetic, (2) an appropriate linker, which can be any flexible or rigid chemical linker, providing spacing between the ABS binder and the other moieties, (3) a nucleotide binding site (NBS) ligand, and (4) a reactive moiety to form a covalent link with an amino acid side chain of target IgE antibodies.
Owner:UNIV OF NOTRE DAME DU LAC
Evaluation of adjuvanted vaccines
The invention relates to an in vitro method of evaluating the immunological activity of a vaccine preparation in the form of a mixture of a molecular antigen and a carrier, wherein the mixture comprises a liquid phase and a solid phase, to which at least a part of the antigen is attached, the method comprising the steps of i) subjecting the vaccine to one or more measurements selected from the group consisting of: 1) the immunological activity of the mixture, 2) the immunological activity of antigen in the liquid phase, 3) the immunological activity of antigen in the solid phase, 4) the immunological activity of antigen in the liquid phase upon a treatment of the mixture to displace the antigen from the solid phase, and 5) the immunoligical activity of antigen in the solid phase upon a treatment of the mixture to displace the antigen from the solid phase, wherein the immunological activity measurement is selected from the group consisting of antibody binding capacity using an immunoassay employing an antigen-specific antibody bound to an antibody solid phase, b) ability to activate effector cells and c) potential for inducing anaphylaxis; and ii) using the measurement results.
Owner:ALK ABELLO SA
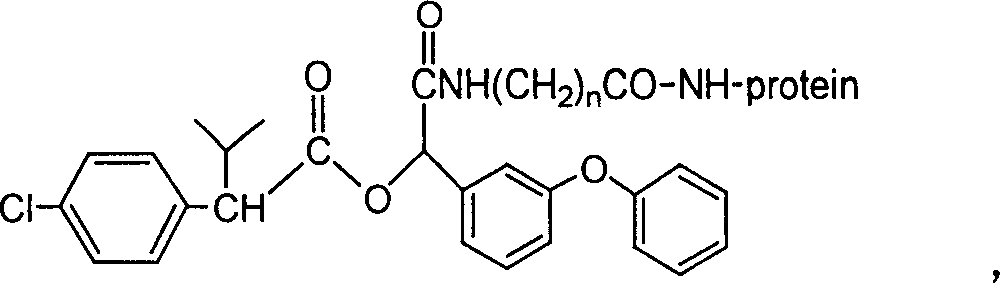
Cyfluthrin hapten compound, its synthesis method and use
InactiveCN100340543COrganic compound preparationCarboxylic acid amides preparationChemical compoundPartial antigen
The invention discloses a fenvalerate artificial partial antigen, the molecular structure is in the right, and n=1-5. The invention also provides the method for synthesizing fenvalerate artificial partial antigen, and the application of the product as raw material of antigen system for animal immunization.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com