Artificial antigen, antibody of fenvalerate and uses thereof
A technology of fenvalerate and artificial antigen, which is applied in the direction of animal/human protein, anti-animal/human immunoglobulin, serum albumin, etc., and can solve problems affecting food safety and environmental threats
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
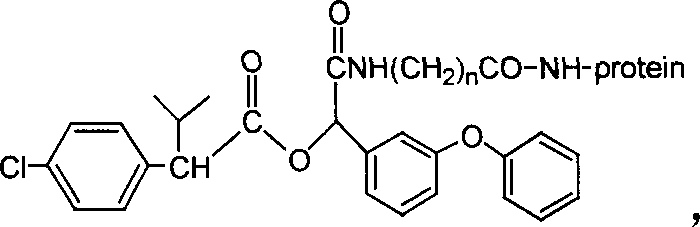
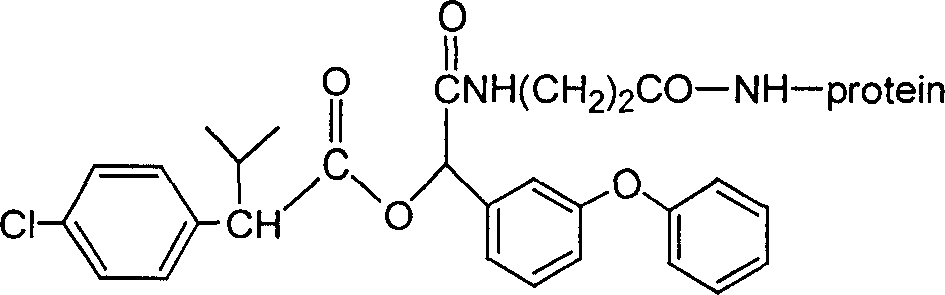
[0025] A kind of fenvalerate artificial antigen, the molecular structural formula is (in this case, n=2):
[0026]
[0027] This fenvalerate artificial antigen is prepared by coupling the fenvalerate hapten (QW-PS) and protein. The molecular structure of the hapten is: The binding ratio of hapten compound to protein is 5:1-100:1.
[0028] The above-mentioned fenvalerate hapten (QW-PS) was obtained according to the invention patent "Fenvalerate hapten compound, synthesis method and its use" that I applied at the same time. The specific synthesis method is as follows:
[0029] Synthesis of 2-cyano-3-phenoxybenzyl alcohol: Add 7.20g (0.14mol) sodium cyanide and 20ml water into a 250ml two-necked flask. After stirring to dissolve, add 40ml toluene and 20.60g (0.1mol) m-benzene Oxybenzaldehyde, 0.65g tetrabutylammonium bromide, 15ml of 36~38% hydrochloric acid was added dropwise at room temperature, after the addition, the reaction was continued for 1.5hr, and then 12.5ml of water was...
Embodiment 2
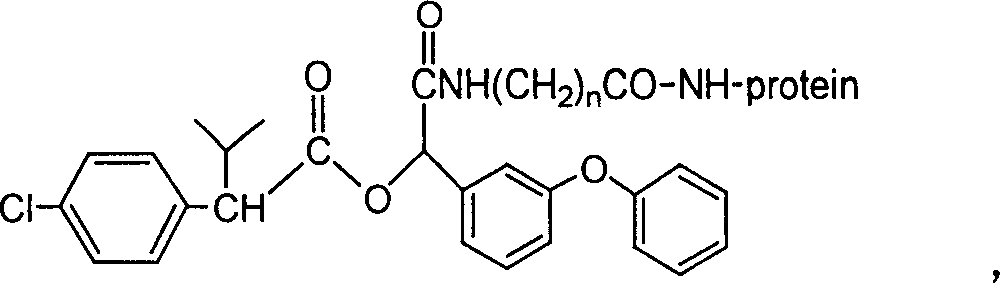
[0063] A kind of fenvalerate artificial antigen, the molecular structural formula is (in this case, n=5):
[0064]
[0065] This fenvalerate artificial antigen is prepared by coupling the fenvalerate hapten (QW-He) and protein. The molecular structure of the hapten is: The binding ratio of hapten compound to protein is 5:1-100:1.
[0066] The above-mentioned fenvalerate hapten (QW-He) was obtained according to the invention patent "Fenvalerate hapten compound, synthesis method and its use" which I applied at the same time. The specific synthesis method is as follows:
[0067] Synthesis of 2-cyano-3-phenoxybenzyl alcohol: Add 7.20g (0.14mol) sodium cyanide and 20ml water into a 250ml two-necked flask. After stirring to dissolve, add 40ml toluene and 20.60g (0.1mol) m-benzene Oxybenzaldehyde, 0.65g tetrabutylammonium bromide, cooled to below 0 degrees with an ice water bath, dropwise add 15ml of 36~38% hydrochloric acid, control the speed, about 30min to drip, continue to react f...
Embodiment 3
[0086] Example 3 Establishment and identification of fenvalerate enzyme-linked immunosorbent assay method
[0087] 1. Establishment of fenvalerate ELISA method and its working conditions and basic parameters
[0088] Using direct competition enzyme-linked immunosorbent assay method. The principle of the determination is that the compound prepared by coupling the pesticide molecule and the macromolecular carrier (such as protein) as the coated antigen is adsorbed on the solid-phase carrier (96-well microtiter plate) to prepare the solid-phase antigen, and then add it to the test Pesticides and corresponding enzyme-labeled antibodies. The solid-phase antigen, the pesticide to be tested and the enzyme-labeled antibody undergo a competitive binding reaction. If the content of the pesticide to be tested is high, the enzyme-labeled antibody bound to the solid-phase antigen will be less. On the contrary, there are more enzyme-labeled antibodies bound to the solid-phase antigen. The subst...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com