Recombined attenuated live vaccine for preventing and treating I-type infection of herpes simplex virus and preparation method thereof
A herpes simplex virus and attenuated live vaccine technology, applied in the field of biomedicine, can solve the problems of difficult to obtain pathogenicity, time-consuming and laborious, etc., and achieve the effects of difficult virulence recovery, reduction of morbidity, and control of recurrence and transmission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
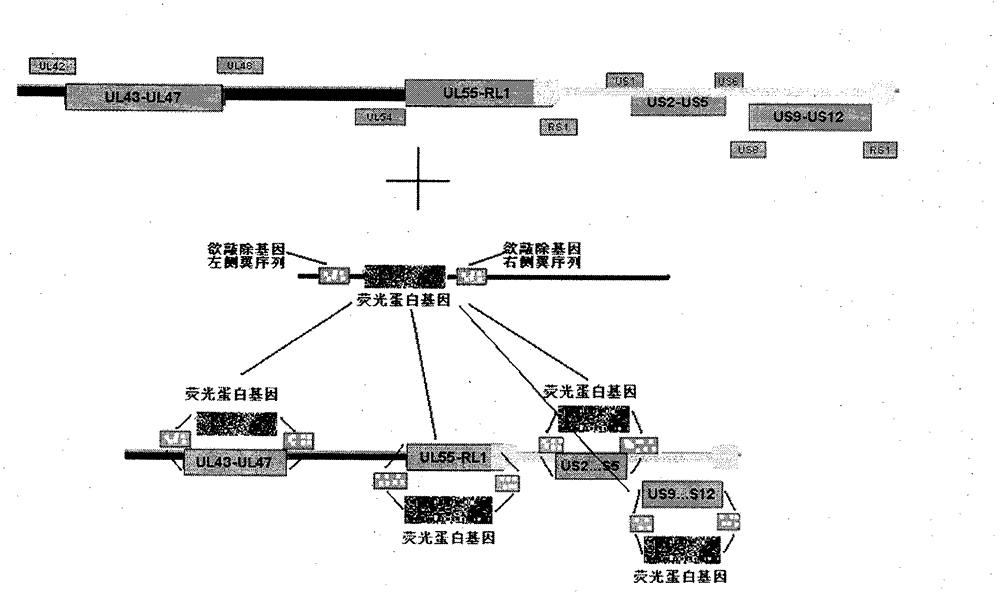
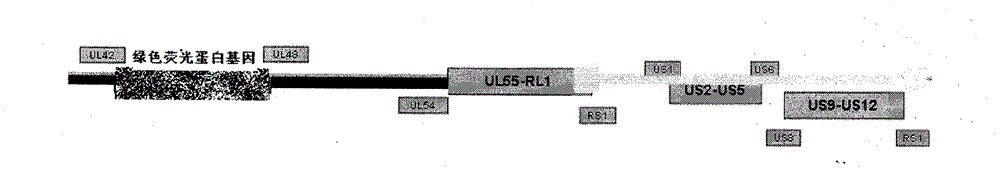
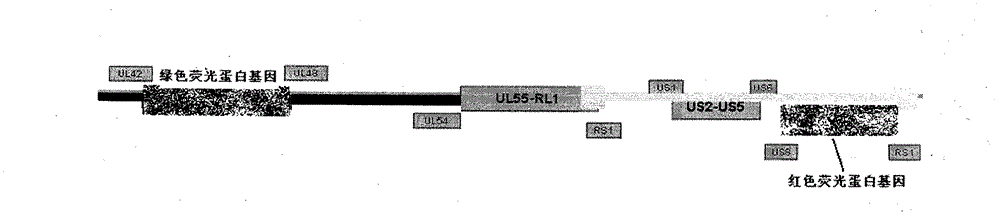
[0023] Example 1 Construction of recombinant herpes simplex virus attenuated live vaccine JSH01L
[0024] 1. Isolation of wild-type HSV-1
[0025] (1) Take the herpes fluid and secretions of patients with clinical HSV infection, add 1ml of PBS buffer solution and filter with a 0.45 micron filter.
[0026] (2) Take a six-hole plate, pass 2×10 per hole 5 For each Vero cell, 200 microliters of virus filtrate was added to each well of 5 wells, and one well was reserved as a control.
[0027] (3) Put it into a 37°C carbon dioxide incubator and culture it for 48-72 hours, then observe the cytopathic effect (CPE) in the well.
[0028] (4) After the cells in the wells of the six-well plate have complete CPE, put the medium and the cells into the centrifuge tube together.
[0029] (5) Freeze and thaw the centrifuge tube three times at 37°C-minus 80°C, centrifuge at 5000 rpm for 10 minutes, transfer the supernatant into a 2ml EP tube, and put it in a minus 80°C refrigerator for later...
Embodiment 2
[0076] The pathogenicity of embodiment 2 recombinant herpes simplex virus attenuated live vaccine JSH01L reduces
[0077] 1. Experimental materials:
[0078] Wild-type herpes simplex virus type I: the titer is measured after being amplified with Vero cells, and the titer is about 1×10 9 IU / ml;
[0079] Recombinant live attenuated herpes simplex virus vaccine JSH01L: the titer after amplification with Vero cells is about 1×10 9 IU / ml;
[0080] Ordinary level experimental mice: 30, female, weighing 20 ± 2g, each of which is marked on different parts of its body, and the corresponding marks are converted into a total of 30 Arabic numerals from 1 to 30, and divided according to the principle of randomization 10 rats / group were divided into three groups: control group, normal dose group and high dose group.
[0081] 2. Experimental operation:
[0082] The control group was given 20 microliters of wild-type herpes simplex virus type I via nasal infusion, the normal dose group w...
Embodiment 3
[0084] Embodiment 3 Live vaccine JSH01L is to the immune protection experiment of mouse
[0085] 1. Experimental materials:
[0086] Wild-type herpes simplex virus type I: the titer is measured after amplifying HSV-1 with Vero cells, and the titer is about 1×10 9 IU / ml;
[0087] Recombinant live attenuated herpes simplex virus vaccine JSH01L: the titer after amplification with Vero cells is about 1×10 9 IU / ml;
[0088] Normal cell lysate: normal cultured Vero cells in the petri dish were scraped off and then filtered after repeated freezing and thawing at -80°C and 37°C.
[0089] Ordinary level experimental mice: 30, female, weighing 20 ± 2g, each of which is marked on different parts of its body, and the corresponding marks are converted into a total of 30 Arabic numerals from 1 to 30, and divided according to the principle of randomization 15 rats / group were divided into negative group and positive group.
[0090] 2. Immunization and attack plan
[0091] Immunity dose:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com