A vaccine composition, a kit and applications of the composition and the kit
A technology of vaccine composition and antigen, which is applied in the direction of antiviral agents, medical preparations containing active ingredients, antibody medical ingredients, etc., can solve problems such as harm and loss, and achieve excellent immune effect, long immune period, good prevention and The effect of the control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0040] As an embodiment of the present invention, the canine parvovirus S0425 strain antigen in the vaccine composition is an inactivated whole virus antigen, and the content of the inactivated whole virus antigen is 10 5 -10 9 TCID 50 / ml.
[0041] As a preferred embodiment of the present invention, the canine parvovirus S0425 strain antigen is an inactivated whole virus antigen, and the content of the inactivated whole virus antigen is 10% before inactivation. 6 -10 8 TCID 50 / ml.
[0042] As a preferred embodiment of the present invention, the canine parvovirus S0425 strain antigen is an inactivated whole virus antigen, and the content of the inactivated whole virus antigen is 10% before inactivation. 7 TCID 50 / ml.
[0043] As an embodiment of the present invention, the other antigens include canine distemper virus antigen, canine adenovirus type I antigen, canine adenovirus type II antigen, canine leptospira antigen, canine coronavirus antigen, canine parainfluenza...
Embodiment 1
[0090] Example 1 Isolation, identification and assay of canine parvovirus
[0091] 1.1 Isolation and identification of canine parvovirus
[0092] The wild strain of CPV was isolated from clinically infected animals with canine parvovirus. The clinical symptoms of infected animals were: anorexia, depression, elevated body temperature, vomiting, loose stools, sticky stools and even tomato sauce-like bloody stools, and dehydration in the later stage of the disease Symptoms, etc., were used as the criteria for selecting animals.
[0093] Dilute the intestinal content of sick dogs to 10% V / V suspension with serum-free RPMI-1640 culture medium, filter and sterilize with a 0.22 μm filter membrane, and inoculate the filtrate with F81 monolayer cells (purchased The virus was isolated from Shanghai Enzyme Detection Technology Co., Ltd., and the cytopathy of the drawing appeared in 24 to 48 hours, and the cytopathy reached more than 80% in about 96 hours. The virus was collected by free...
Embodiment 2
[0099] The preparation of embodiment 2 vaccine composition
[0100] 2.1 Preparation of CPV inactivated whole virus antigen
[0101] The CPV S0425 strain of embodiment 1 is inactivated through the final concentration of 0.025% V / V BPL (beta-propiolactone), and according to "Chinese Veterinary Pharmacopoeia" (Chinese Veterinary Drug Committee, 2010 edition three, China Agricultural Press , 2010) method to carry out sterility test, mycoplasma test and exogenous virus test to CPV, the results show that: after the CPV S0425 strain was inactivated, it was not polluted by bacteria and mold, nor was it infected by mycoplasma and exogenous virus. good.
[0102] 2.2 Preparation of vaccine composition
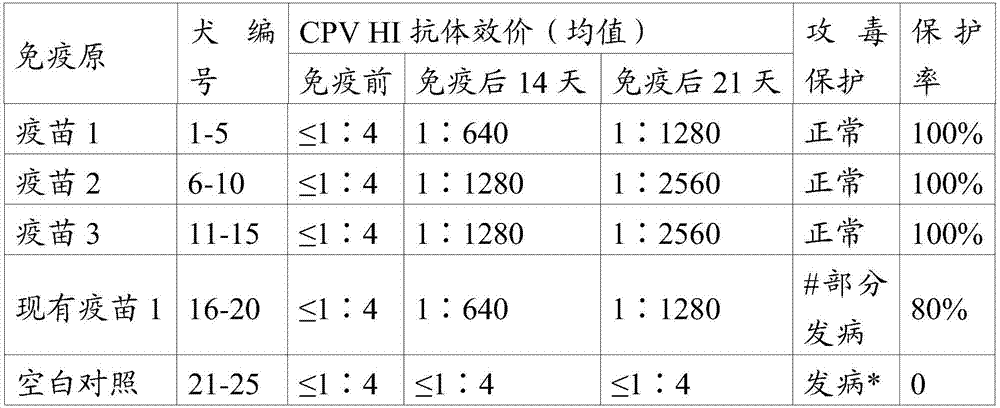
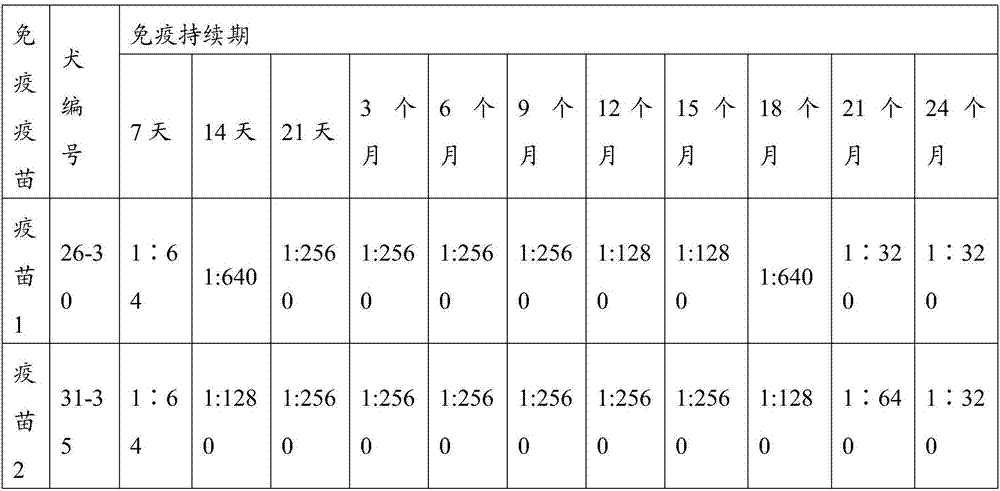
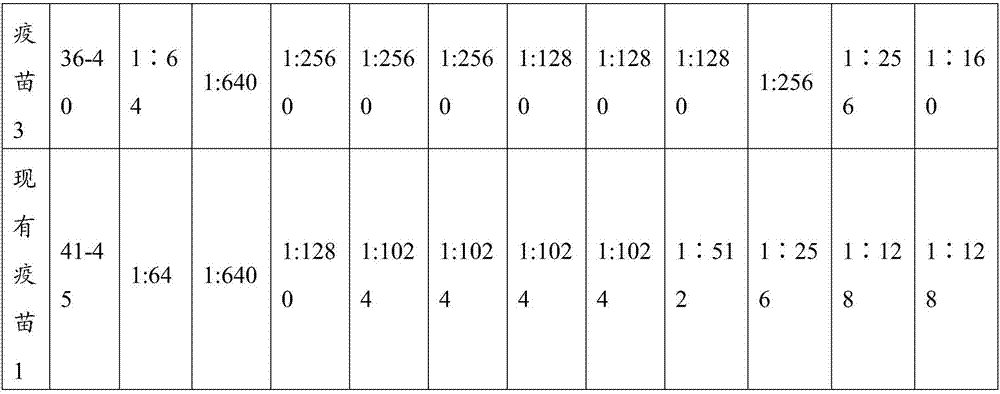
[0103] The CPV S0425 strain antigen prepared in Example 2.1, other antigens in the prior art, and PET GEL A adjuvant (purchased from SEPPIC, France, with a final concentration of 5%) were prepared according to the components and contents shown in Table 1 with physiological saline soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com