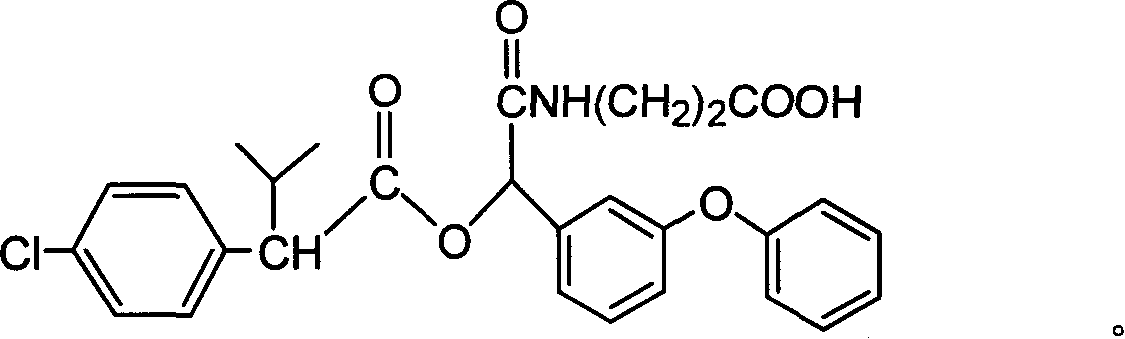
Cyfluthrin hapten compound, its synthesis method and use
A technology of fenvalerate and its synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems affecting food safety, environmental threats, etc., and achieves a simple synthesis method, good affinity, and cross-reaction low rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
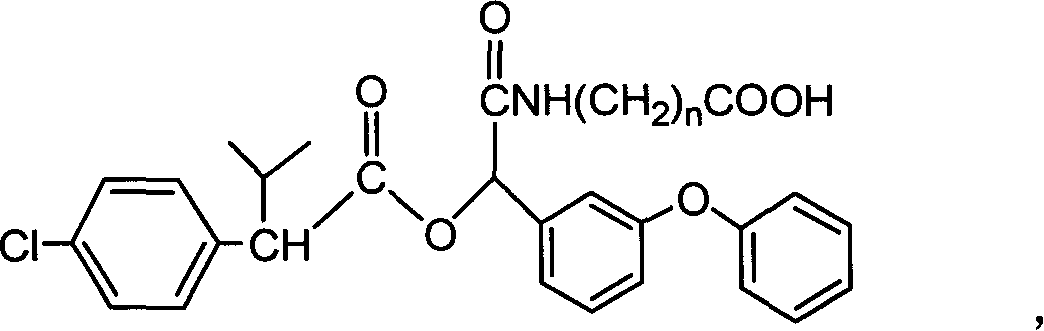
[0035] A kind of fenvalerate artificial hapten, molecular structural formula is (n=2 at this moment):
[0036]
[0037] It is used as the raw material of the antigen system for animal immunization.
[0038] The synthetic method of above-mentioned fenvalerate artificial hapten is as follows:
[0039] 1) Synthesis of 2-cyano-3-phenoxybenzyl alcohol: Add 7.20g (0.14mol) sodium cyanide and 20ml water into a 250ml two-necked flask, stir and dissolve, then add 40ml toluene, 20.60g (0.1mol) m-phenoxybenzaldehyde (2), 0.65g tetrabutylammonium bromide, add dropwise 15ml36~38% hydrochloric acid at room temperature, continue to react for 1.5hr after adding, then add 12.5ml water, dissolve the solid wherein, use The aqueous layer was separated by a separatory funnel to obtain a pale yellow organic phase (mainly containing compound (3)).
[0040] Put the light yellow organic phase into a 250ml three-neck flask, add 23.0ml (0.18mol) of 36-38% hydrochloric acid, stir overnight at room t...
Embodiment 2
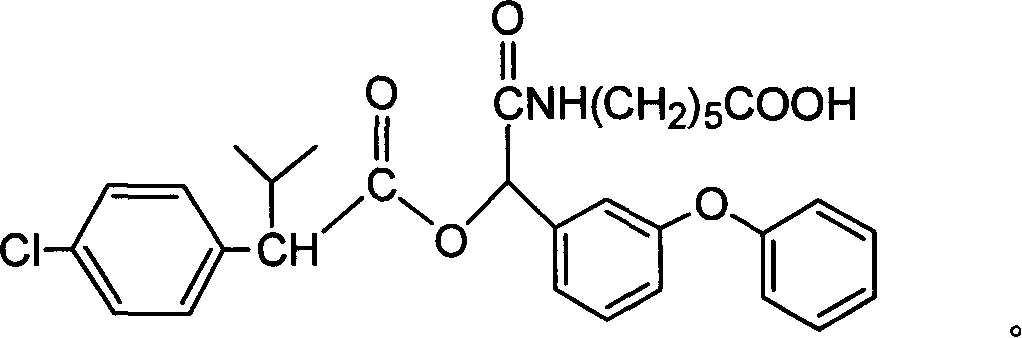
[0051] A kind of fenvalerate artificial hapten, molecular structural formula is (n=5 at this moment):
[0052]
[0053] It is used as the raw material of the antigen system for animal immunization.
[0054] The synthetic method of above-mentioned fenvalerate artificial hapten is as follows:
[0055] 1) Synthesis of 2-cyano-3-phenoxybenzyl alcohol Add 7.20g (0.14mol) sodium cyanide and 20ml water into a 250ml two-necked flask, stir to dissolve, add 40ml toluene, 20.60g (0.1mol) methane Phenoxybenzaldehyde (2), 0.65g of tetrabutylammonium bromide, cooled to below 0 degrees with an ice-water bath, dropwise added 15ml of 36-38% hydrochloric acid, controlled the speed, finished dropping in about 30 minutes, and continued to react for 1.5 minutes after adding hr, and then add 12.5ml of water to dissolve the solid therein, and separate the water layer with a separatory funnel to obtain a light yellow organic phase (mainly containing compound (3)).
[0056] Put the light yellow o...
Embodiment 3
[0067] Preparation of the immunogen:
[0068] The immunogen was synthesized using the carbodiimide method. Dissolve compound (1) (QW-PS) or (QW-He) (50-80 micromoles) in 1-2 mL of N,N-dimethylformamide, and then add an equivalent amount of Dicyclohexylcarbodiimide and N-hydroxysuccinimide, let it react overnight at room temperature, centrifuge, take 500-800 μL of the supernatant and add it to 4-8 mL of 15-20 mg / mL bovine serum albumin carbonate In the buffer solution, it should be added slowly, and then react with magnetic stirring for 4 to 6 hours. After the reaction is completed, put it into a dialysis bag, first dialyze with distilled water for 2 to 4 times, and then use 0.8 to 0.9% normal saline After dialysis, aliquots were stored in a refrigerator at -20°C.
[0069] Identification of artificial antigens:
[0070] According to the proportion of hapten, carrier protein and coupling product used in the immune antigen reaction of synthetic fenvalerate, carry out ultraviol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com