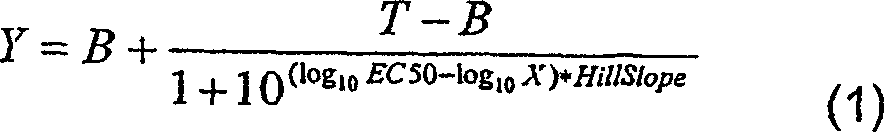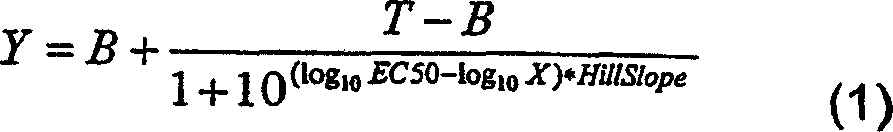Evaluation of adjuvanted vaccines
A vaccine and immune activity technology, applied in the field of adjuvant vaccine evaluation, can solve the problems that are not yet fully understood and the impact of the reaction is complex
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0145] Preparation of Allergen Vaccine with Aluminum Gel Adjuvant
[0146] Lyophilized allergens are dissolved in aqueous buffer and diluted to the desired concentration. Under stirring, "aluminum glue" (1, 3%) was added to the resulting allergen solution, followed by sterile water. The resulting solution was allowed to stand until the next day, and then the buffer was slowly added with stirring to produce the final allergen aluminum hydroxide gel.
Embodiment 1
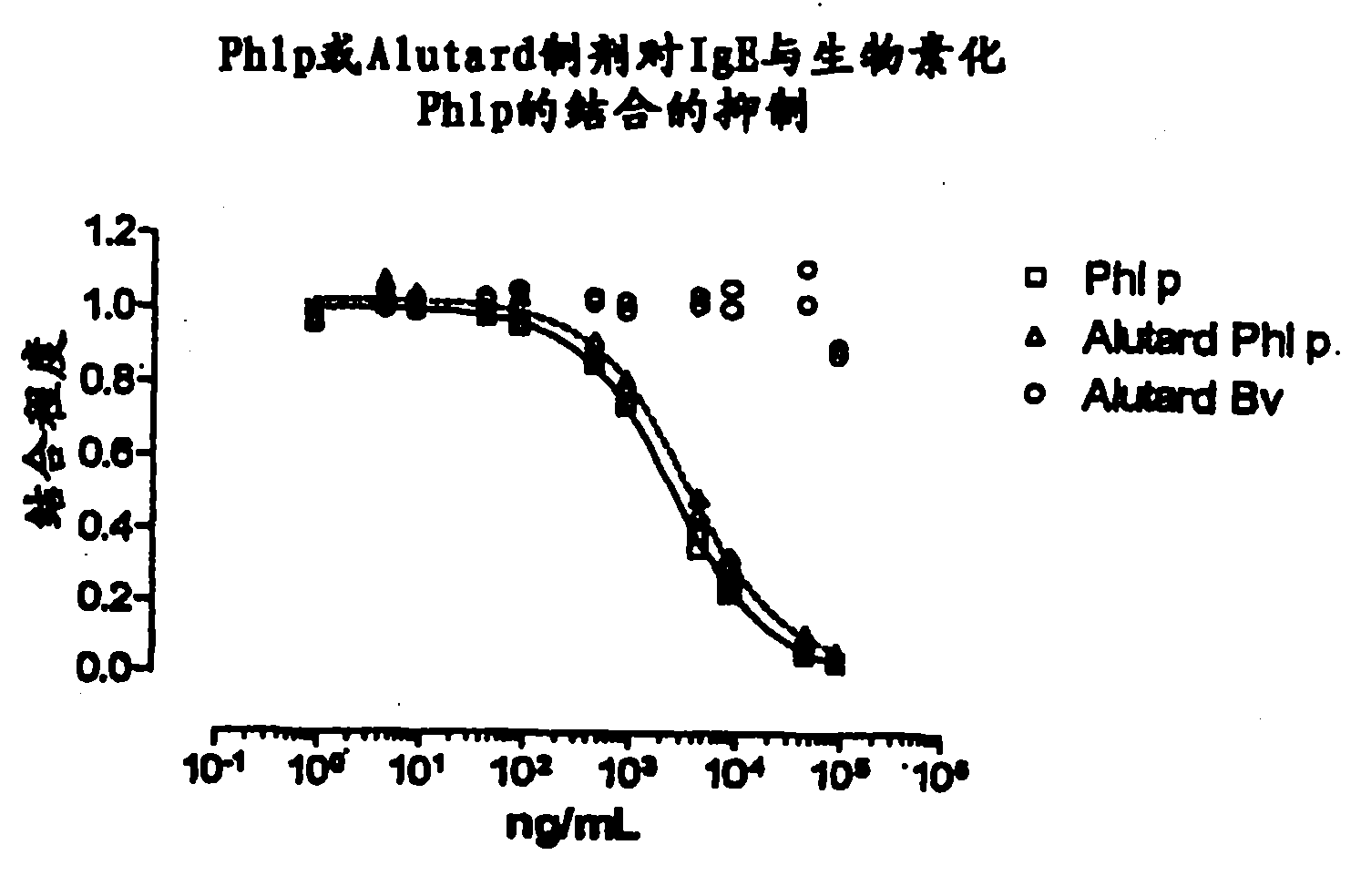
[0149] IgE Inhibition Test on Allergens in Solution and Adsorbed on Aluminum Hydroxide Gel Adjuvant
[0150] method
[0151] On the ADVIA centaur device, IgE inhibition tests were performed. Serial dilutions (performed with TECAN (P-05-07F294)) of the inhibitor (antigen in solution or antigen-gel adjuvant vaccine) were mixed with a fixed amount of biotinylated antigen and then mixed with a solid-phase adsorbed IgE was incubated together. The amount of biotinylated allergen bound to the solid phase was estimated from the light emitted after incubation with acridinium ester-labeled streptavidin. Raw data were processed in Excel and transferred to GraphPad Prism v.4.0 for final analysis (curve fitting, graphing and statistical comparison). Fit the data to a 4-parameter logarithmic function (Equation 1):
[0152] Y = B + T - B 1 ...
Embodiment 2
[0161] Histamine Release Test of Allergen Adsorbed on Aluminum Hydroxide Gel Adjuvant
[0162] method
[0163] Histamine is measured in an ELISA-based method on the basis of competition between the histamine to be tested and its enzyme conjugate, which is used as a substrate for coating Antibody-bound tracers on microwells. The monoamine histamine is too small to completely occupy the binding site on the antibody. Accordingly, high affinity monoclonal antibodies against modified histamines have been obtained. The histamine in the sample must be derivatized in the same manner as the histamine of the conjugate. This can be easily and reproducibly achieved using acylating agents at slightly basic pH. Acylated histamine and the histamine-alkaline phosphatase conjugate in the sample compete for binding to a limited number of antibody sites when added to the microtiter wells. After incubation, the wells are washed to remove unbound components. Bound enzyme activity was then me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com