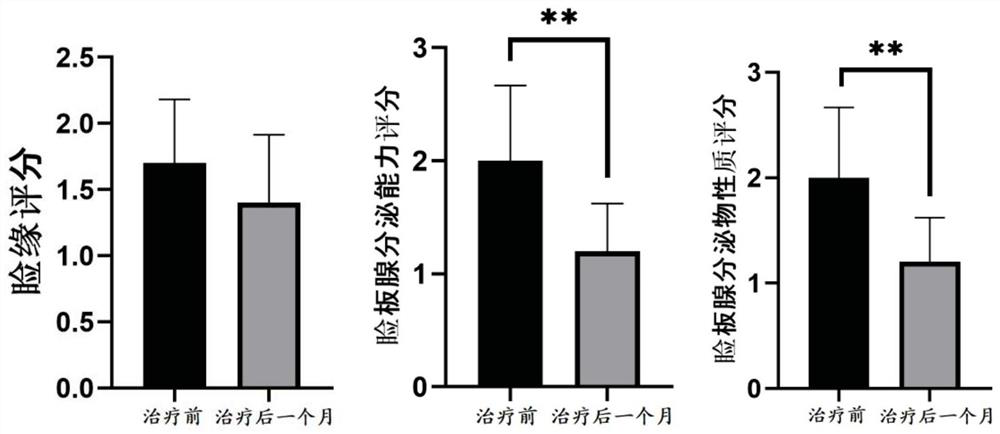
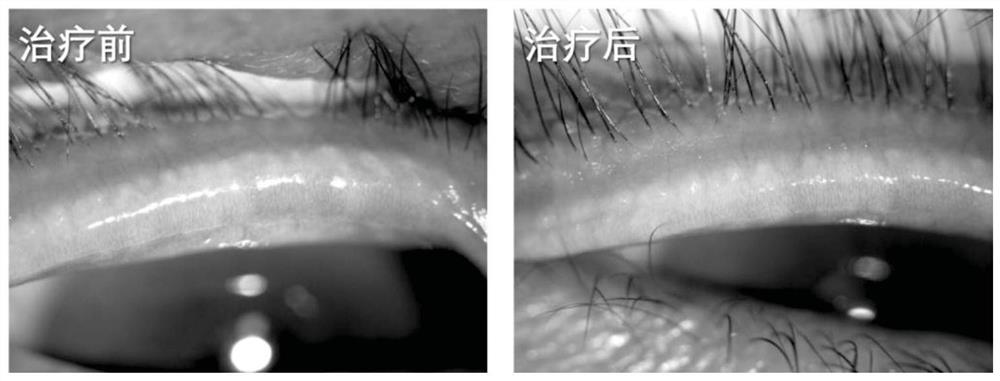
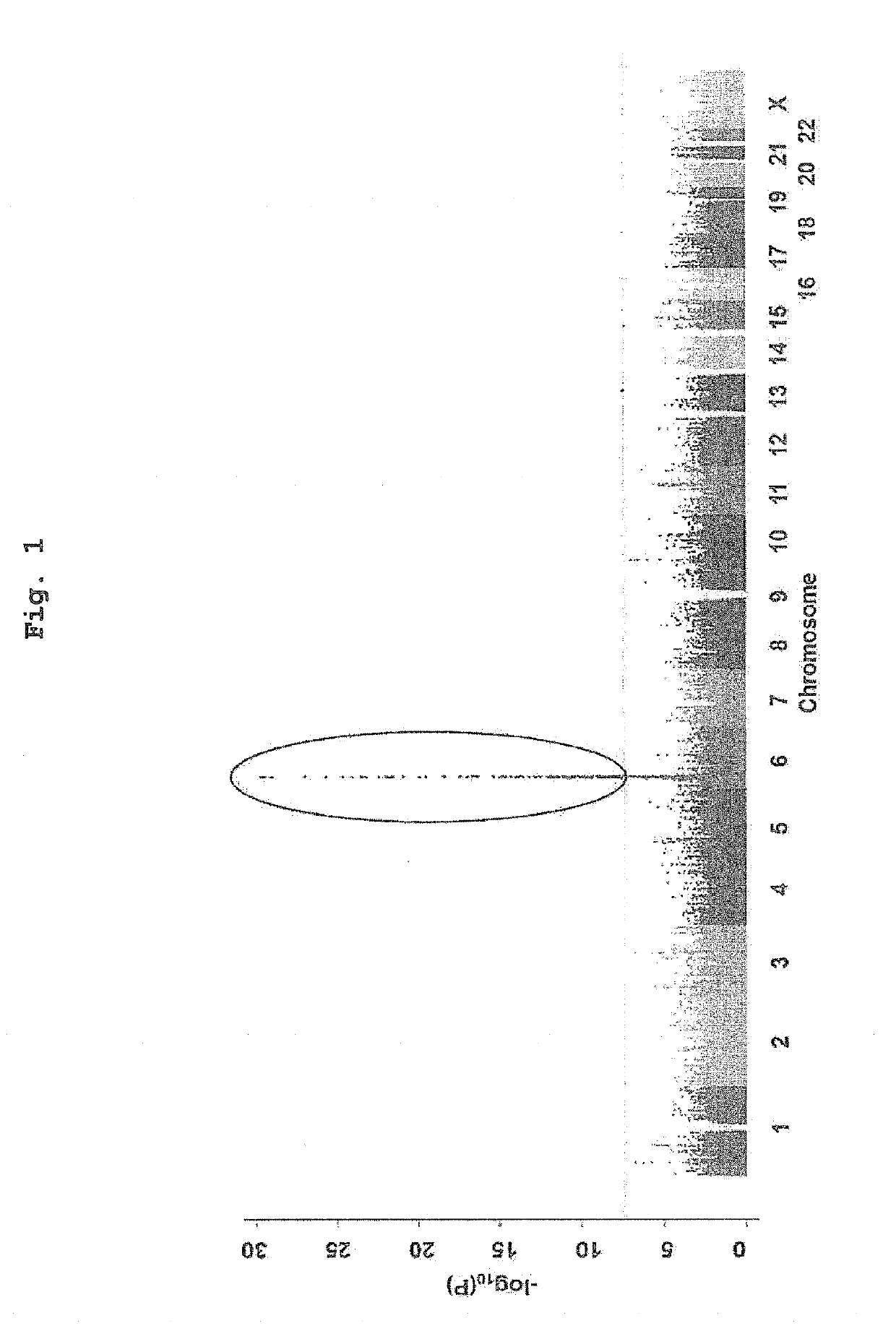
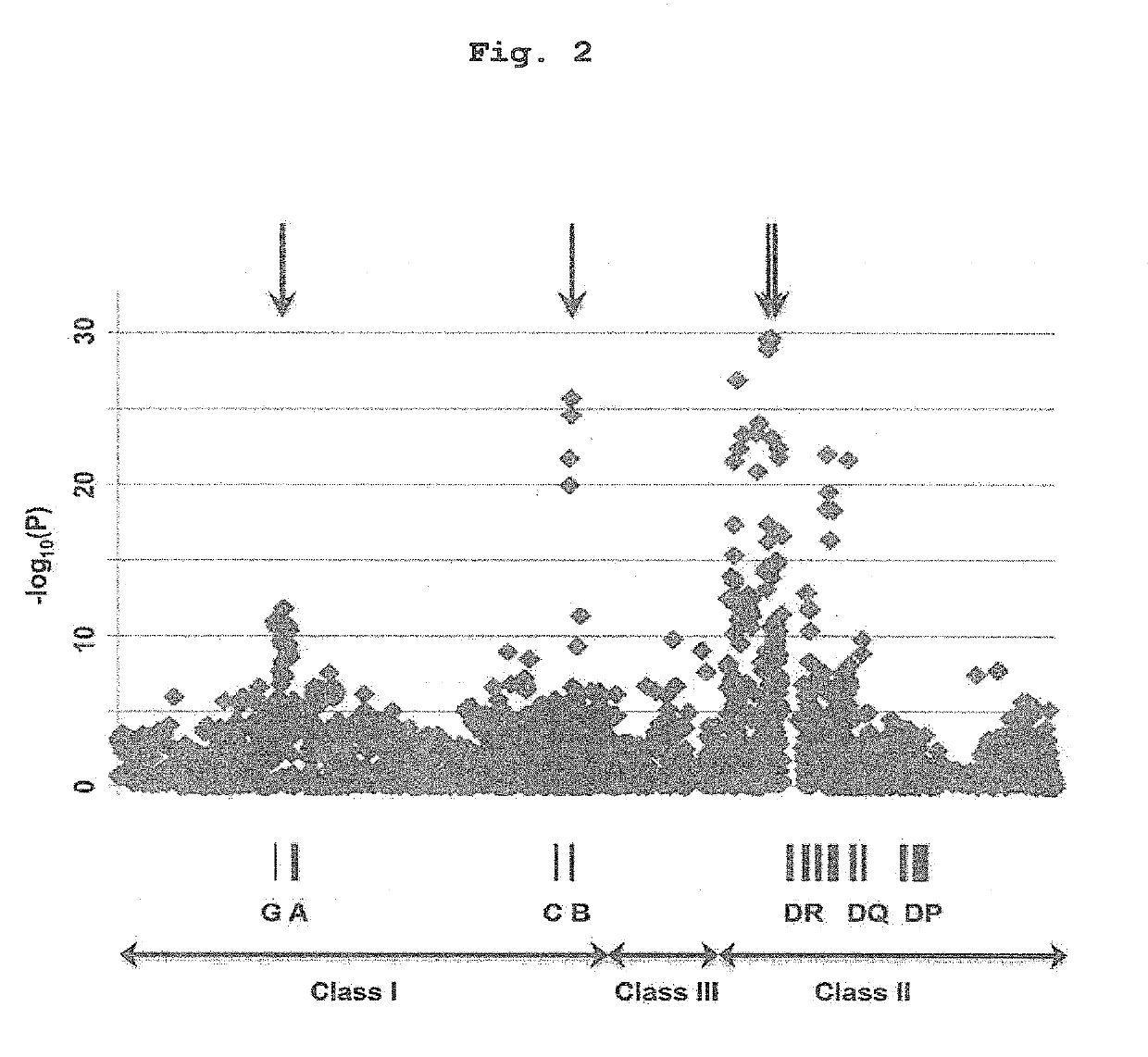
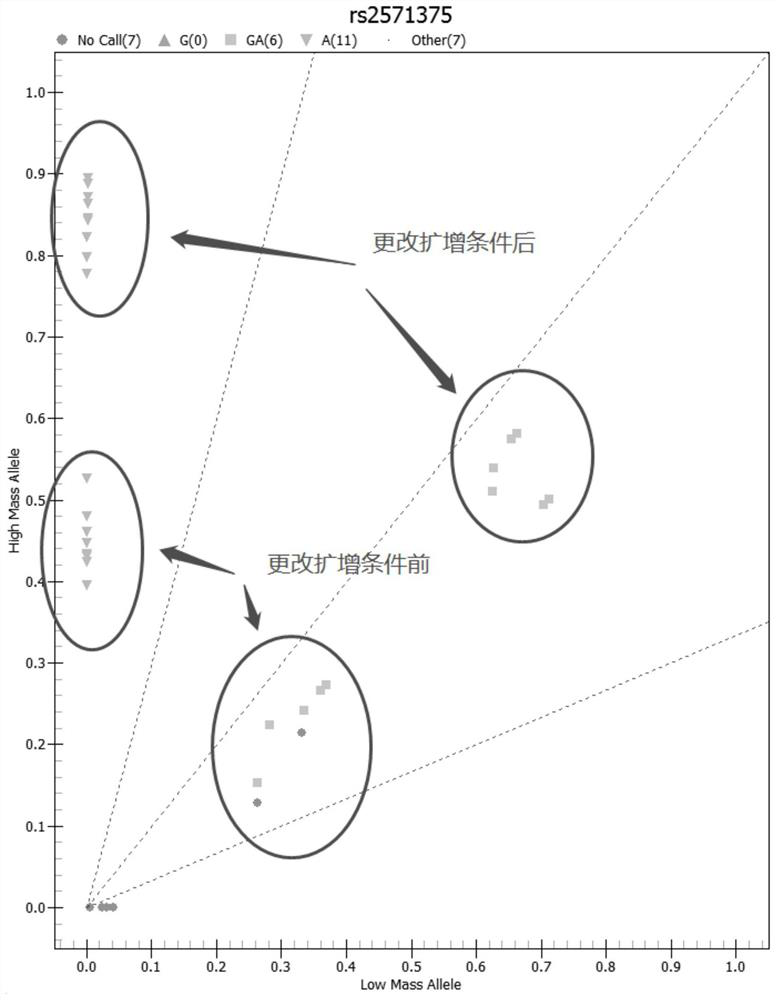
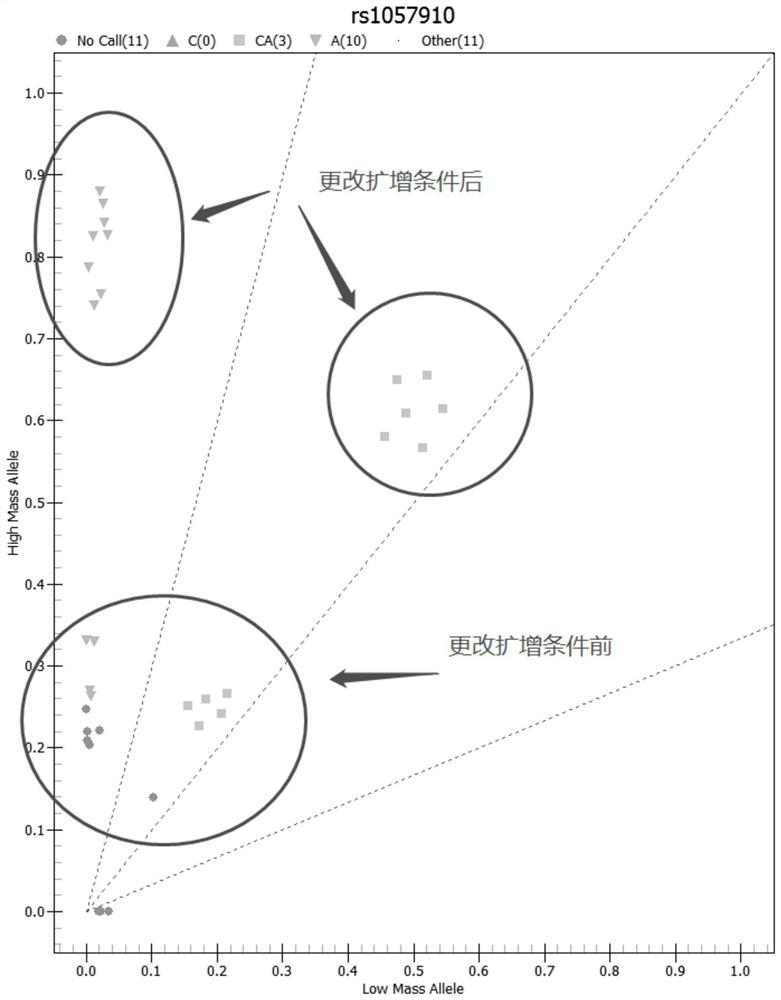
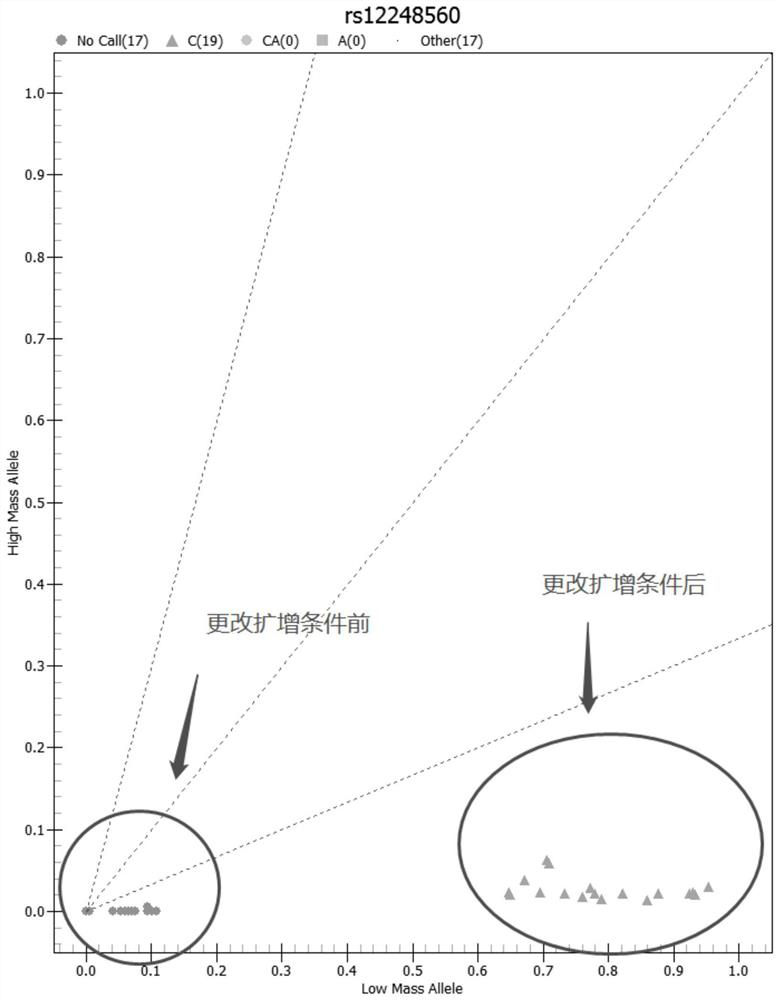
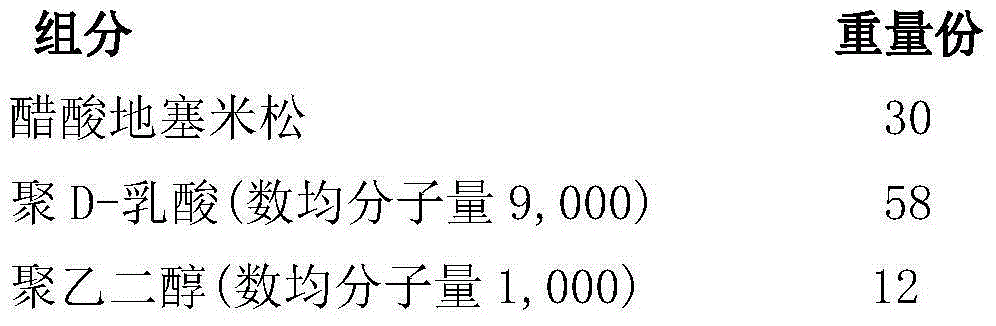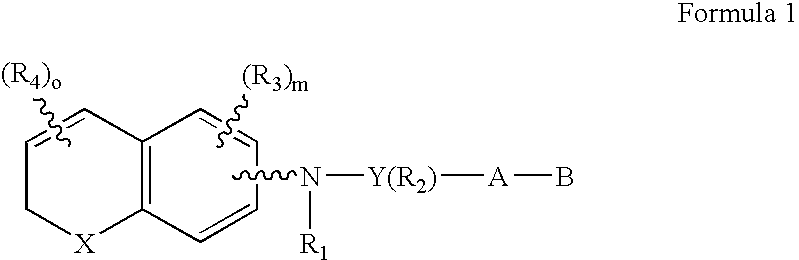Patents
Literature
47results about How to "Avoid serious side effects" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
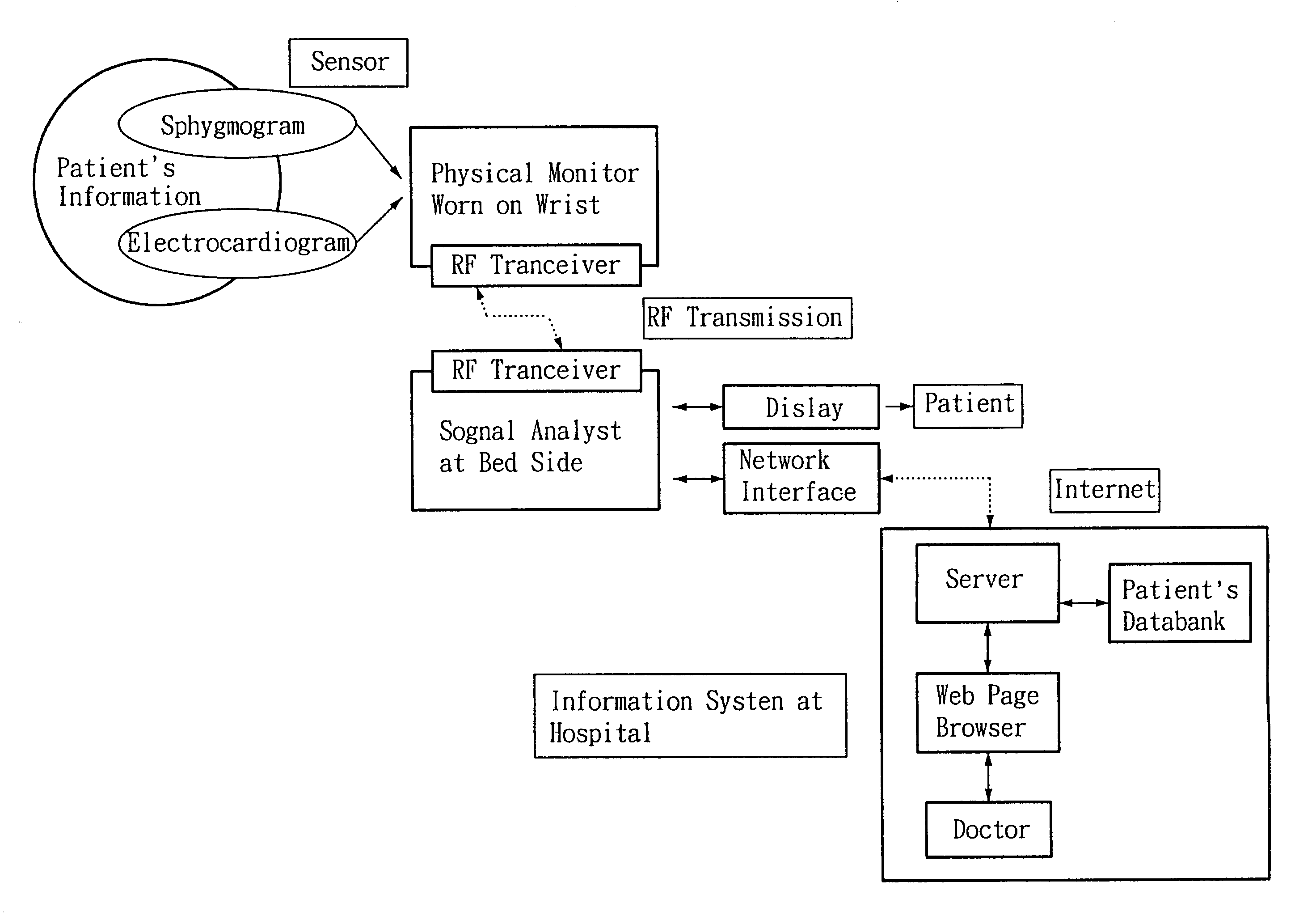
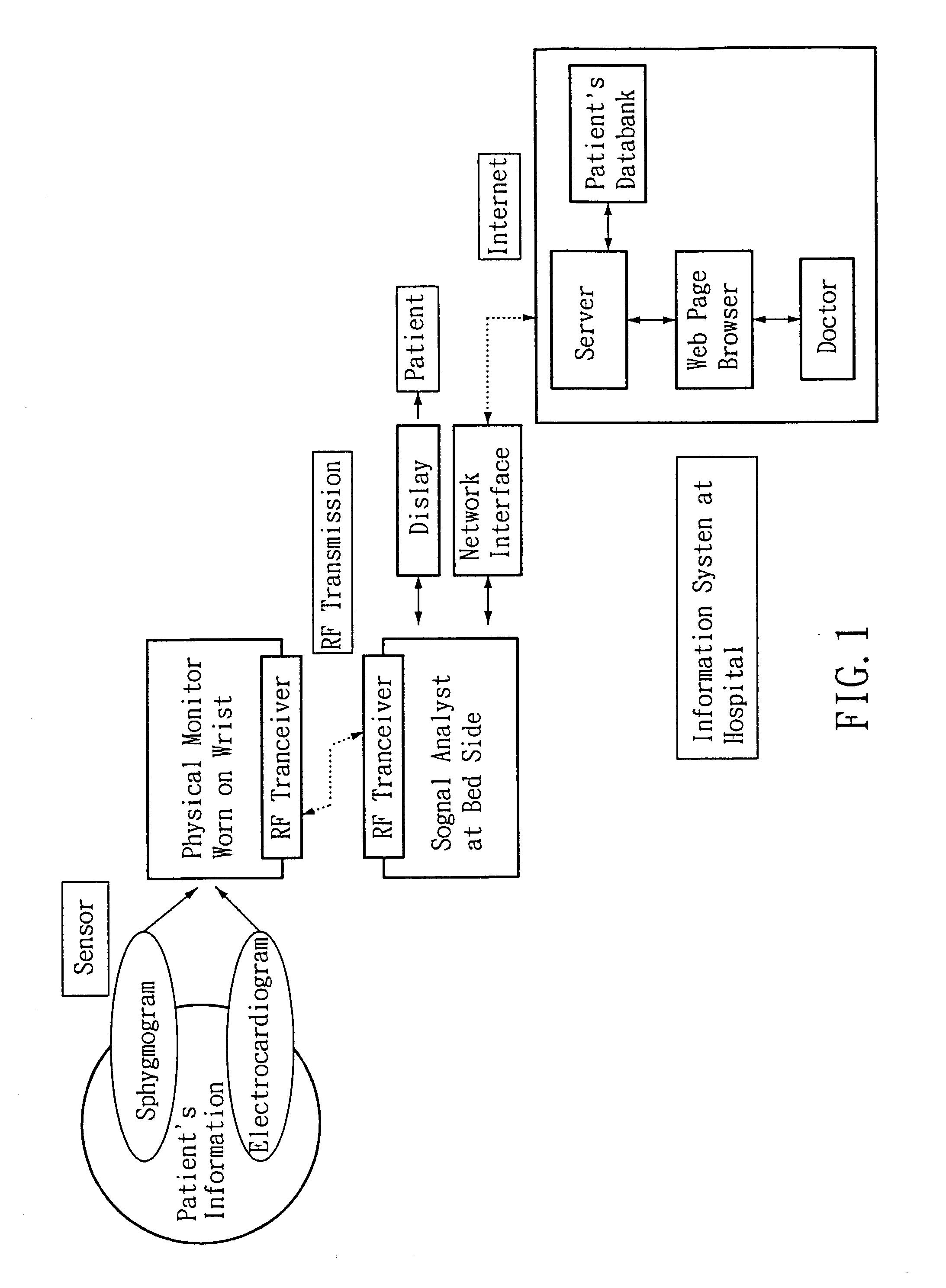
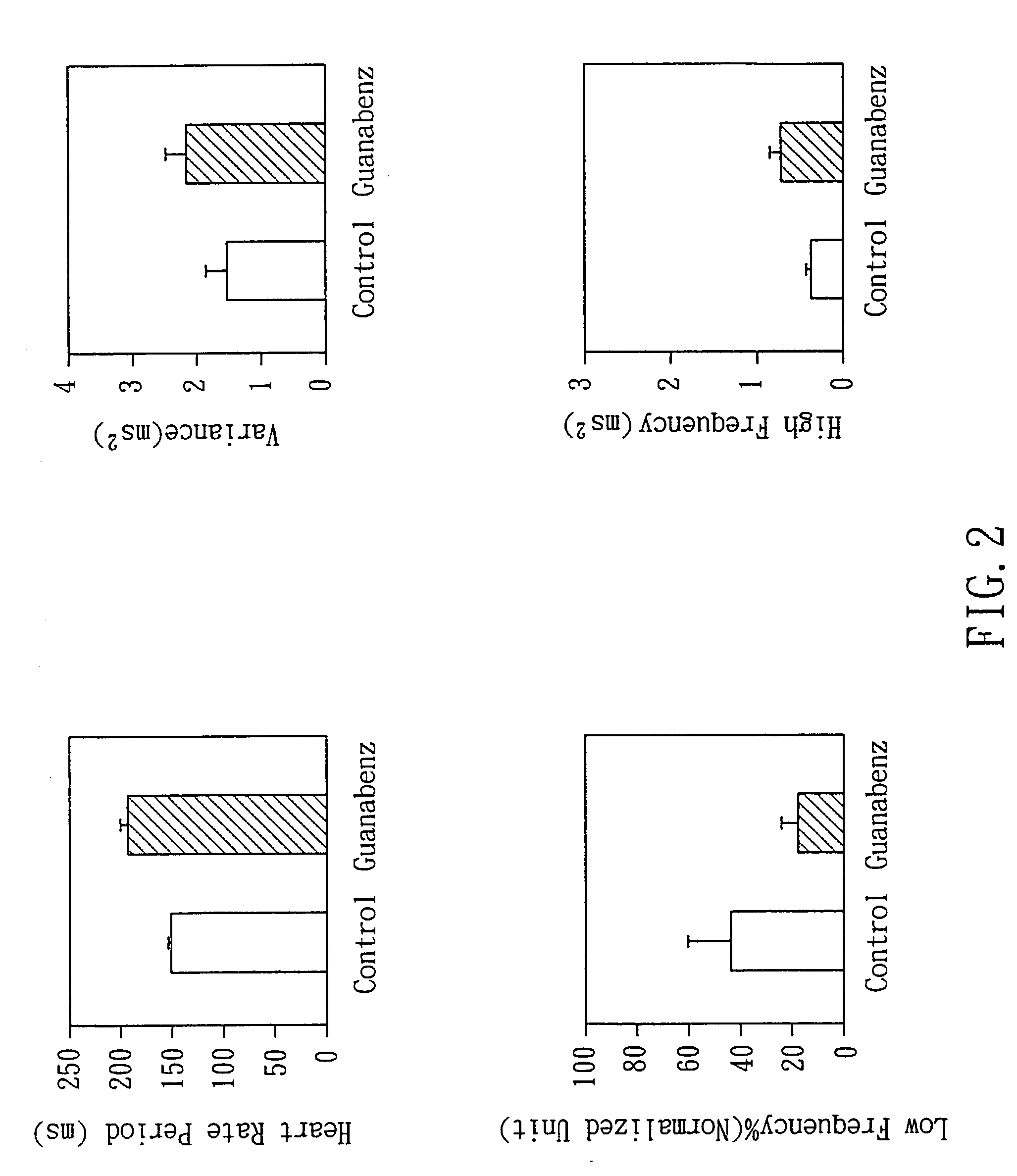
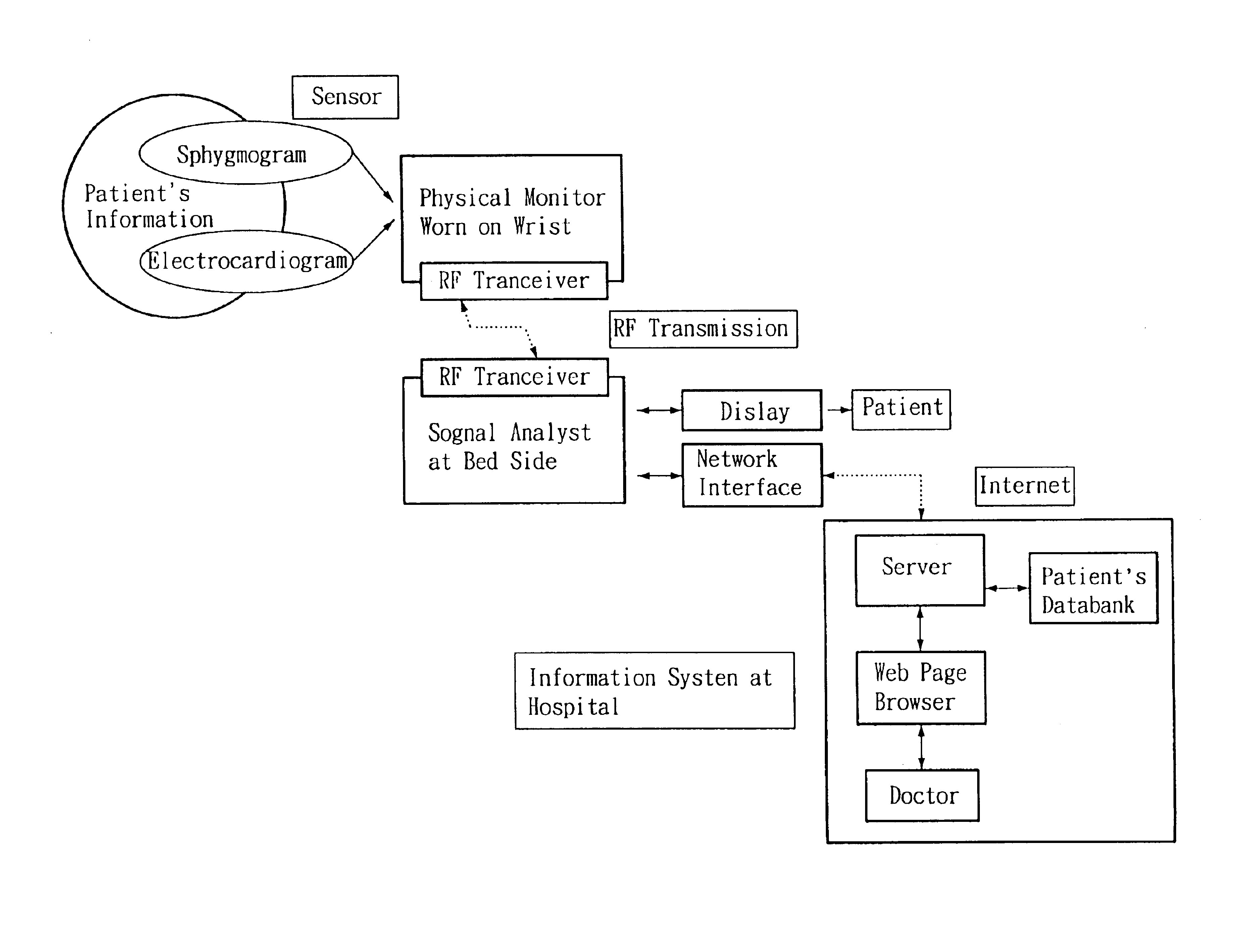
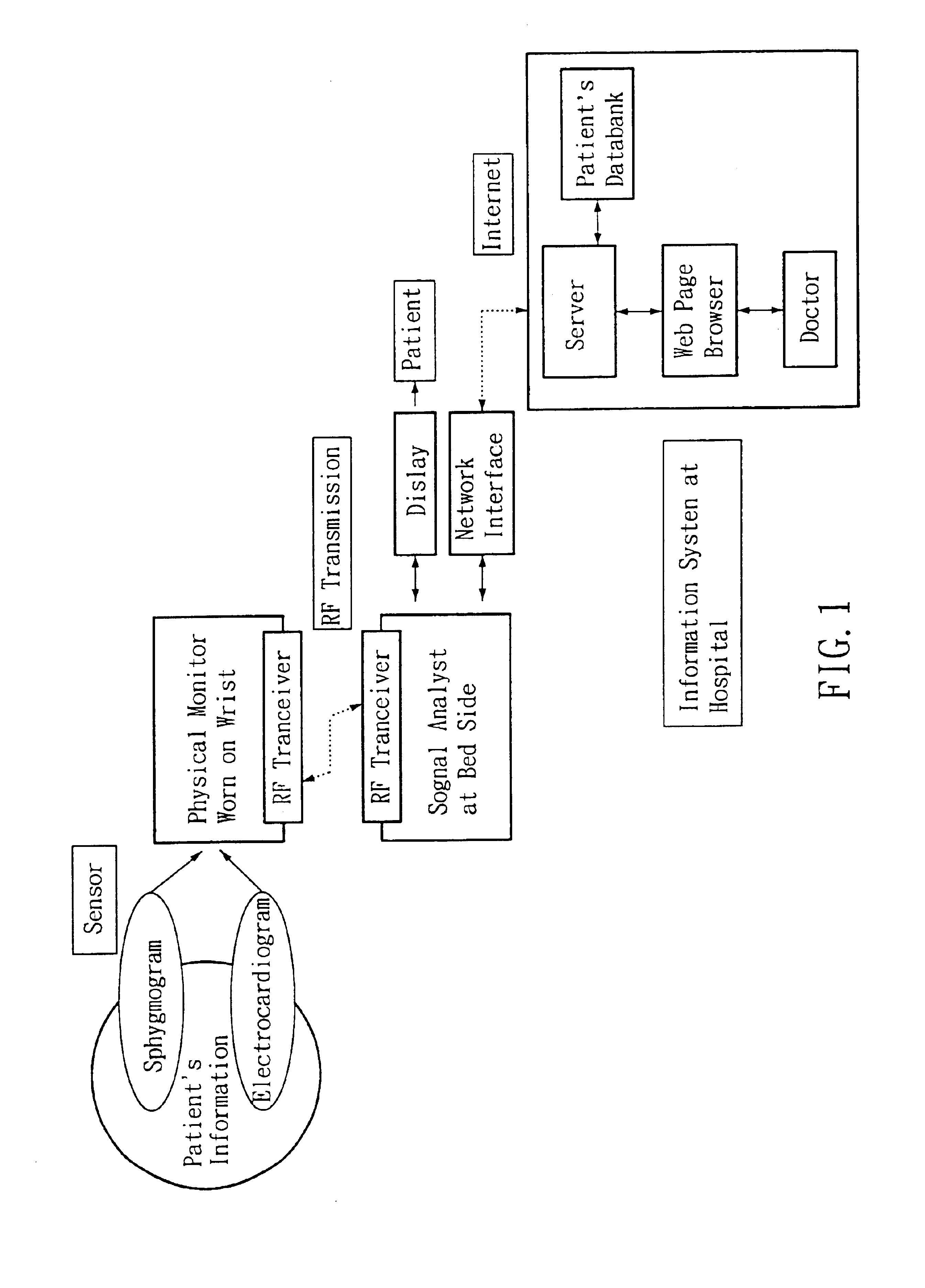
Non-invasive apparatus system for monitoring autonomic nervous system and uses thereof
InactiveUS20040054292A1Avoid serious side effectsDrug and medicationsCatheterTreatment effectSide effect
A non-invasive apparatus system for monitoring autonomic nervous system, and its uses in monitoring autonomic nervous system functional change side effects caused by drugs and monitoring the aging of autonomic nervous system and tracing therapeutic effect thereof.
Owner:IND TECH RES INST +1
Non-invasive apparatus system for monitoring autonomic nervous system and uses thereof
A non-invasive apparatus system for monitoring autonomic nervous system, and its uses in monitoring autonomic nervous system functional change side effects caused by drugs and monitoring the aging of autonomic nervous system and tracing therapeutic effect thereof.
Owner:IND TECH RES INST +1
Propofol medium/long-chain injection and preparation method thereof
ActiveCN102552136AHypocalcemiaAvoid serious side effectsHydroxy compound active ingredientsAnaestheticsGlycerolMedium-chain triglyceride
The invention relates to a propofol medium / long-chain injection and a preparation method thereof. The formula of the propofol medium / long-chain injection is as follows: 10 g to 20 g of propofol, 50 g to 100 g of soybean oil, 50 g to 100 g of medium-chain triglycerides, 10 g to 15 g of lecithin, 0.1 g to 2 g of oleinic acid, 18 g to 25 g of glycerol, an appropriate amount of sodium hydroxide, and water for injection added up to 1000 ml. The propofol medium / long-chain injection is advantageous in reasonable compatibility, less irritation and good stability.
Owner:GUANGDONG JIABO PHARM CO LTD
Composite preparation
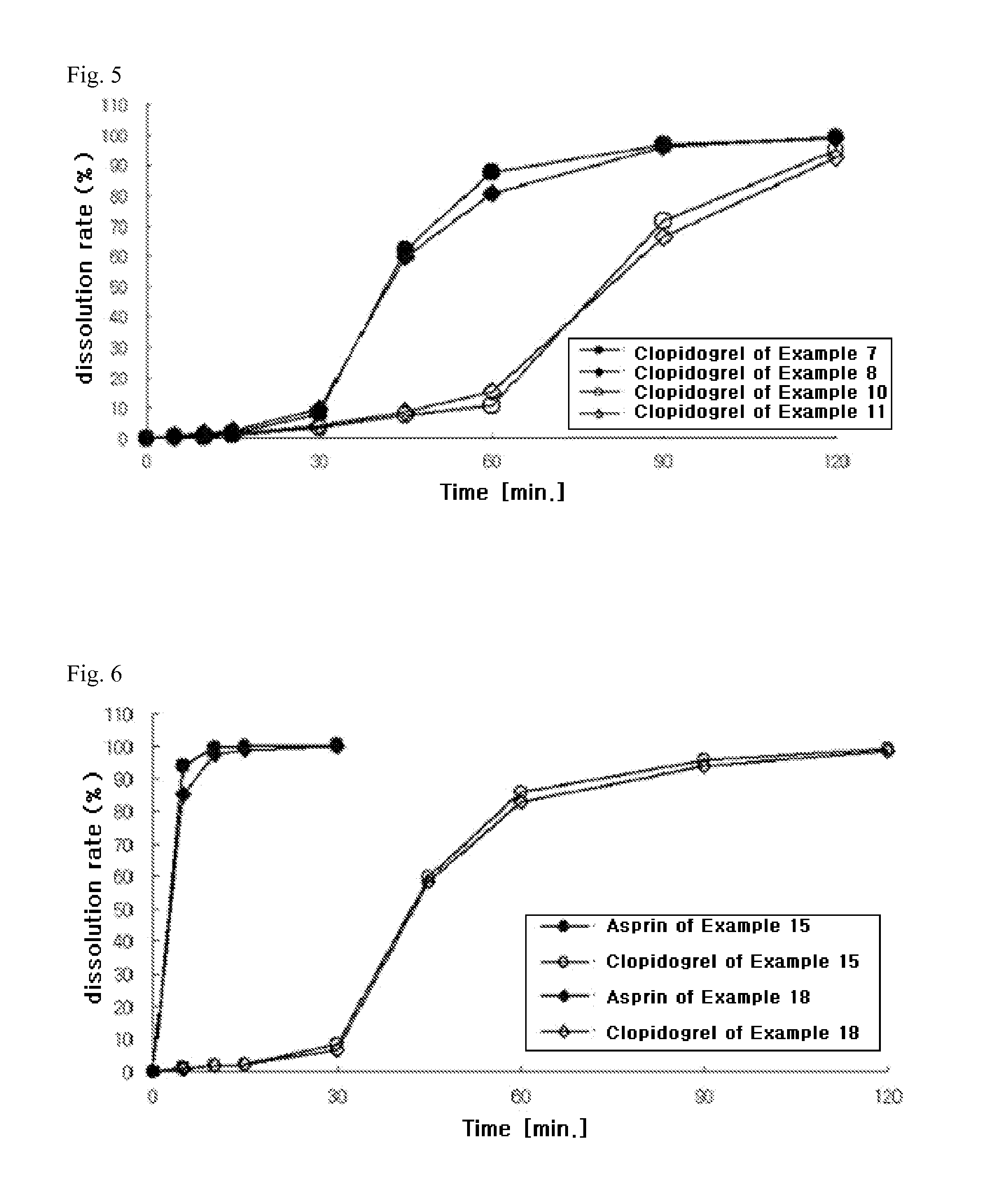
InactiveUS20110038931A1Excellent anti-platelet aggregation effectExcellent in clinical therapeutic effectPowder deliverySalicyclic acid active ingredientsClopidogrel resistanceAdverse drug reaction
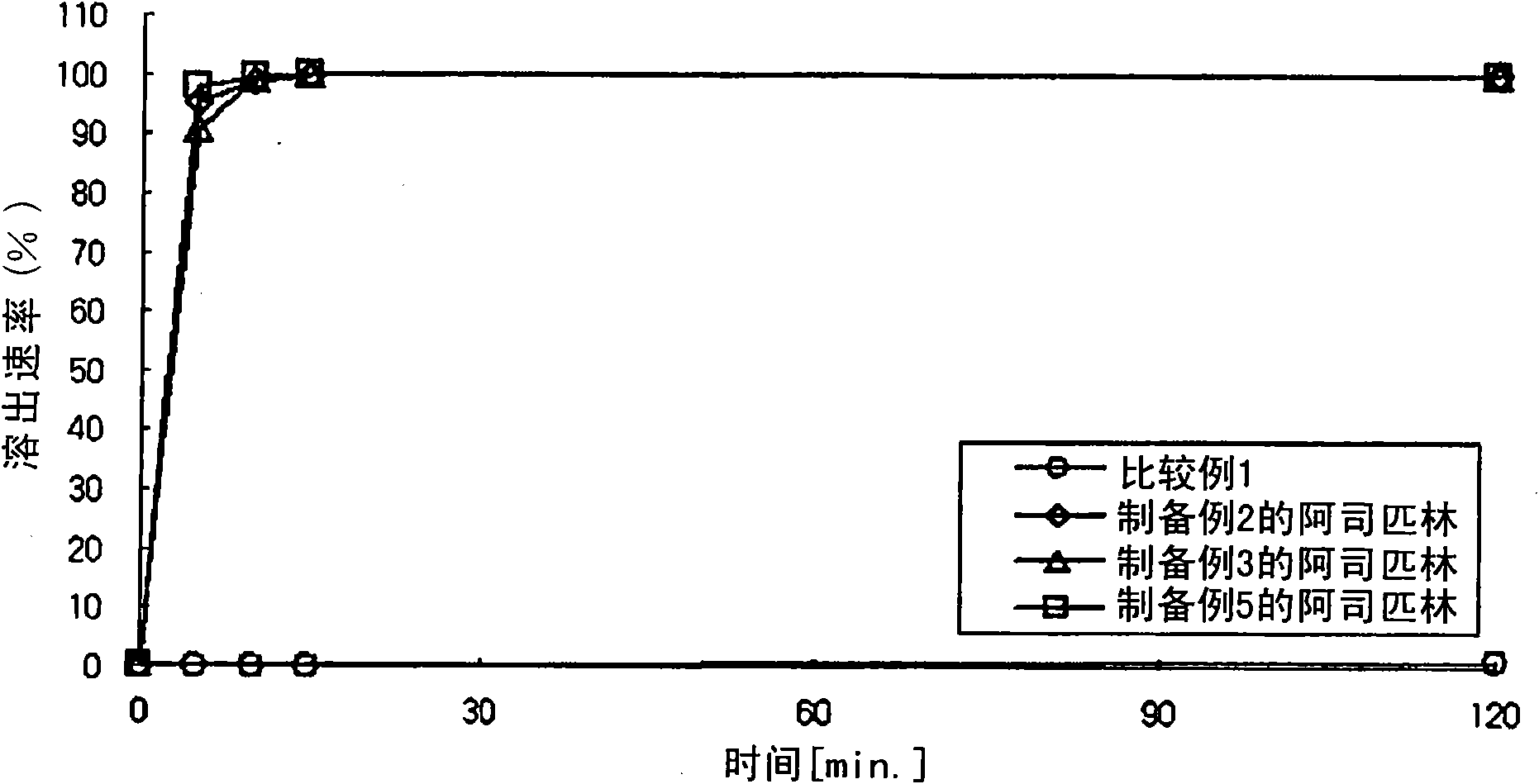
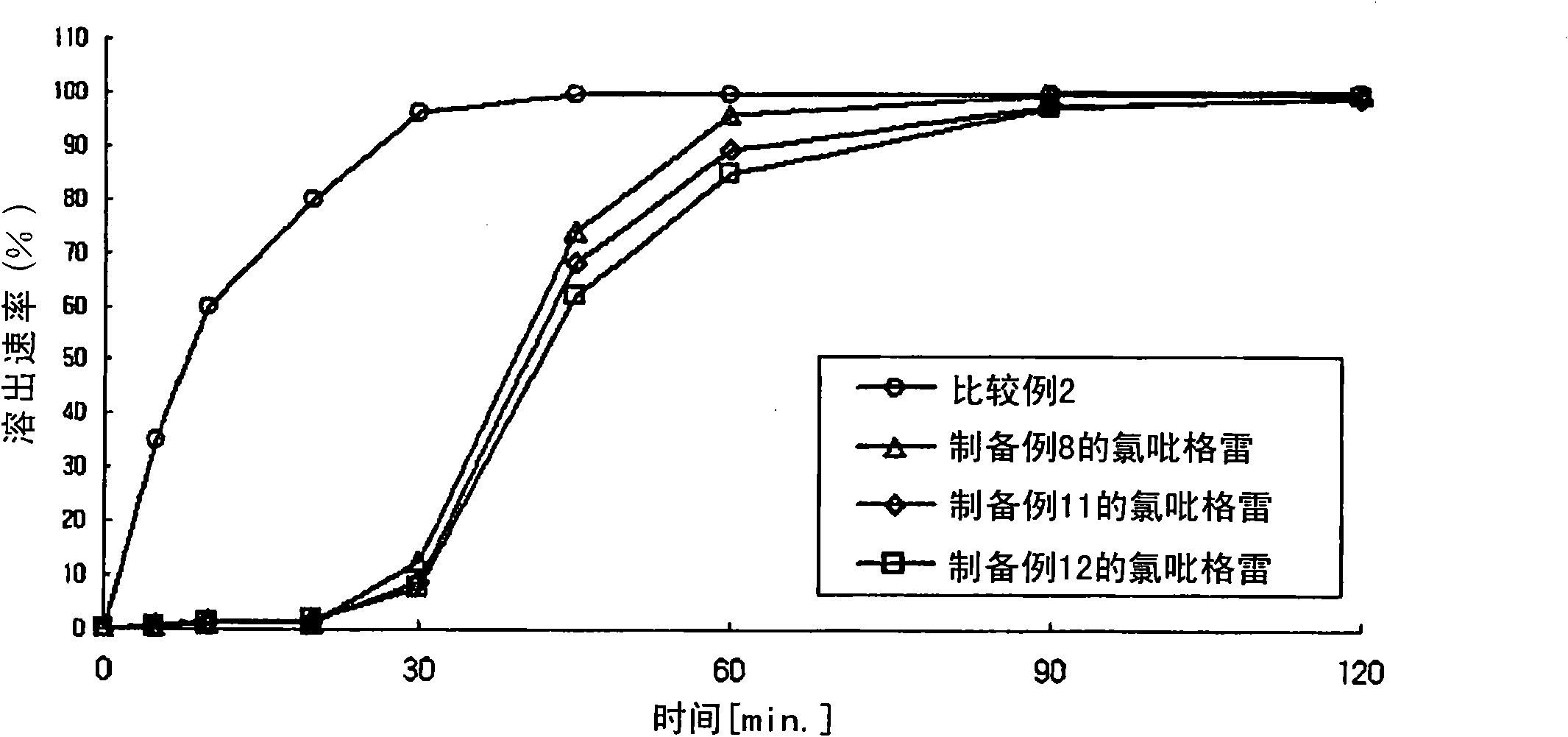
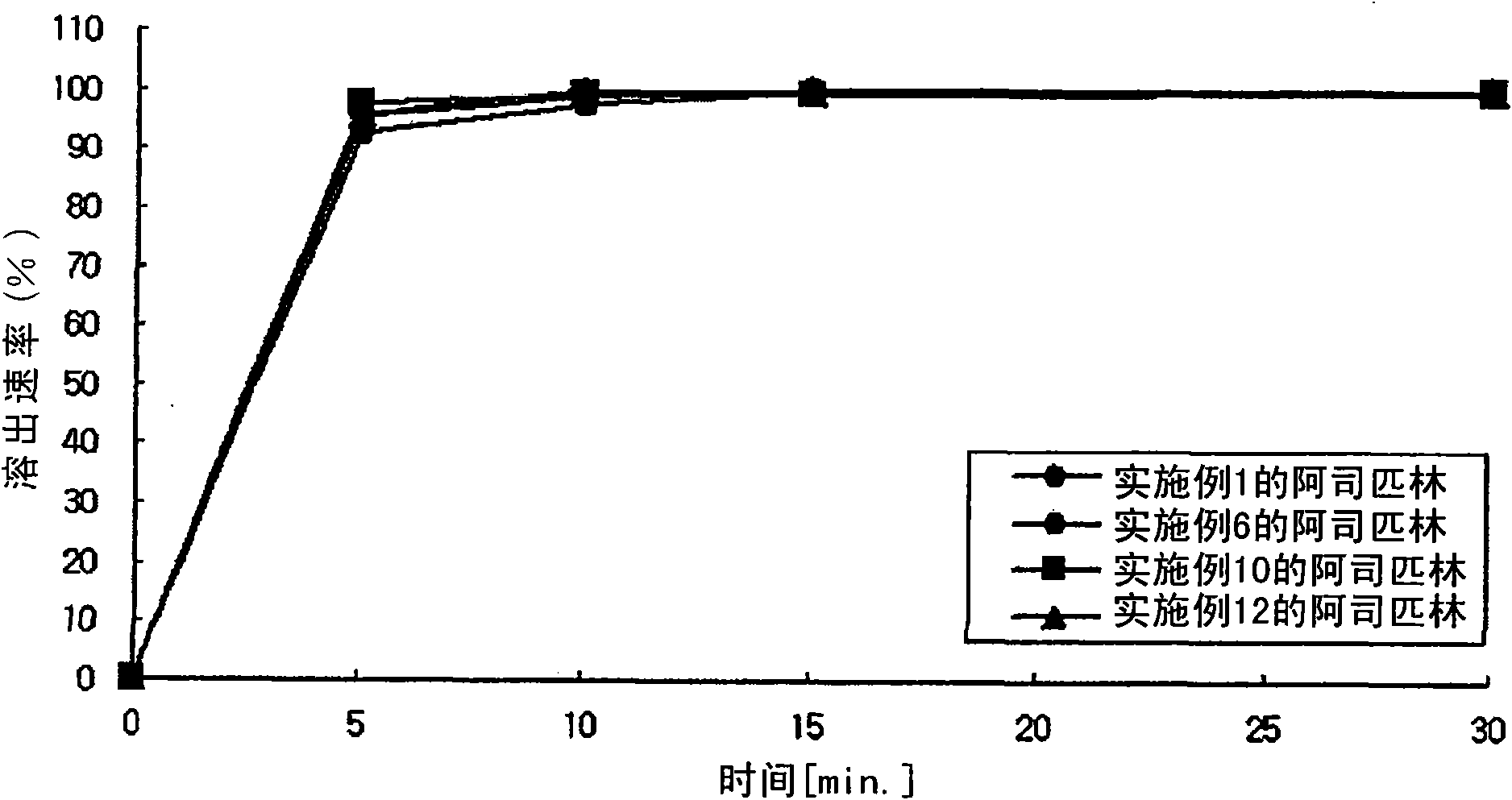
The present invention provides a combination preparation which comprises: a prior-release section comprising aspirin or a pharmaceutically acceptable salt thereof as a pharmacologically active component; and a delayed-release section comprising clopidogrel, an isomer thereof or a pharmaceutically acceptable salt thereof as a pharmacologically active component. The combination preparation of the present invention exhibits a far better effect in preventing platelet aggregation than does simultaneous oral therapy or treatment with the respective single preparations, and not only can it improve the patient's drug-taking compliance by administration once a day but it can also reduce the adverse reactions which follow long-term administration of aspirin. The combination preparation of the present invention is also advantageous in that it exhibits an outstanding effect in inhibiting blood platelet aggregation despite a reduction in the amount of aspirin ingested, and in that it converts clopidogrel resistance into susceptibility and prevents serious adverse reactions caused by clopidogrel resistance and in that it can be stored over the longer term since it is stable under common storage conditions.
Owner:HANALL PHARMA CO LTD
Oxygen, sulfur and nitrogen substituted cyclohexene and cyclohexane derivatives having retinoid-like biological activity
InactiveUS6166244AAvoid serious side effectsAvoid infectionOrganic active ingredientsSilicon organic compoundsPerylene derivativesDrug biological activity
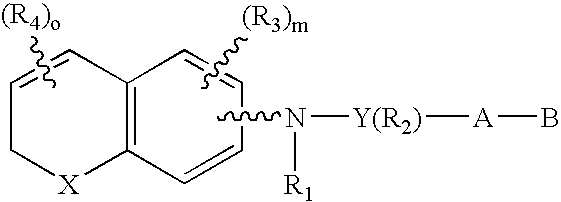
Compounds of the Formula 1, Formula 2, Formula 3 and Formula 4 wherein the symbols have the meaning assigned to them in the disclosure have retinoid-like biological activity. ##STR1##
Owner:ALLERGAN INC
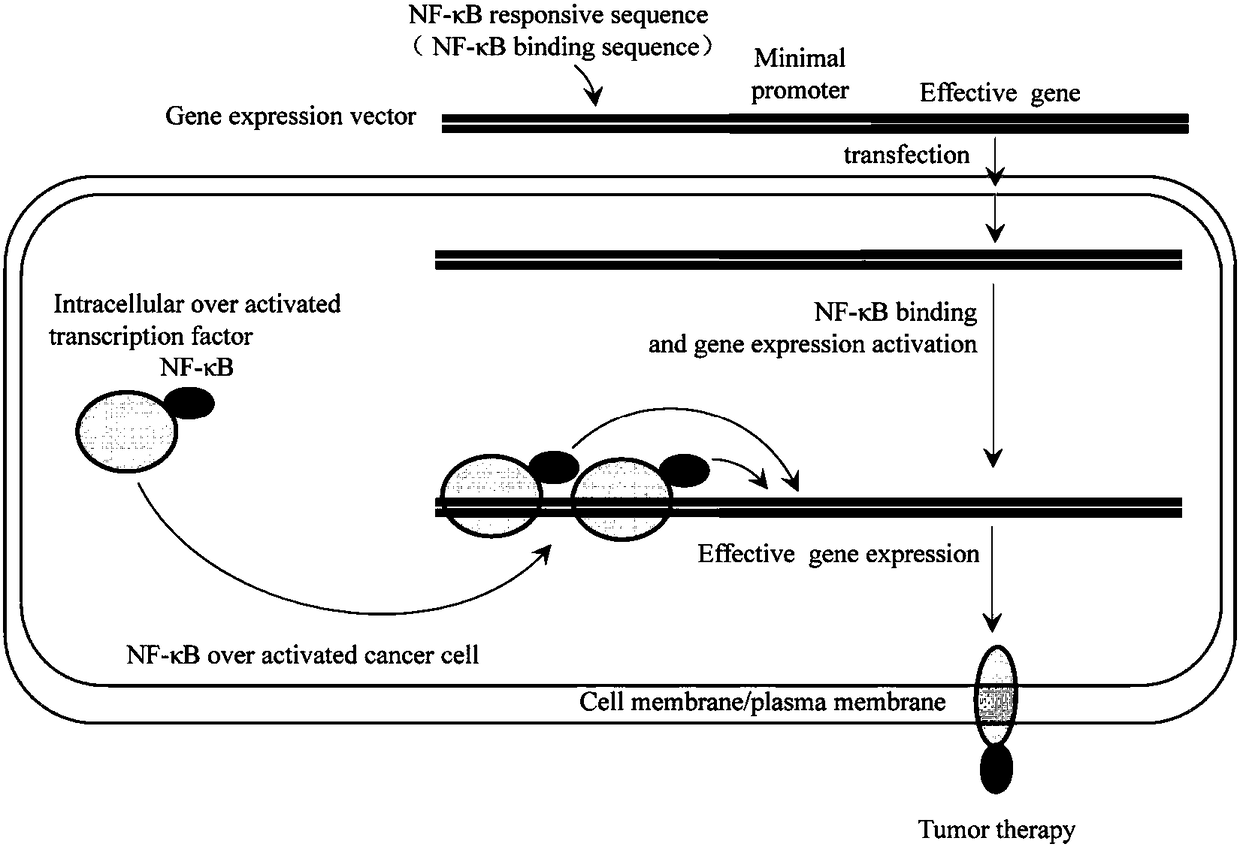
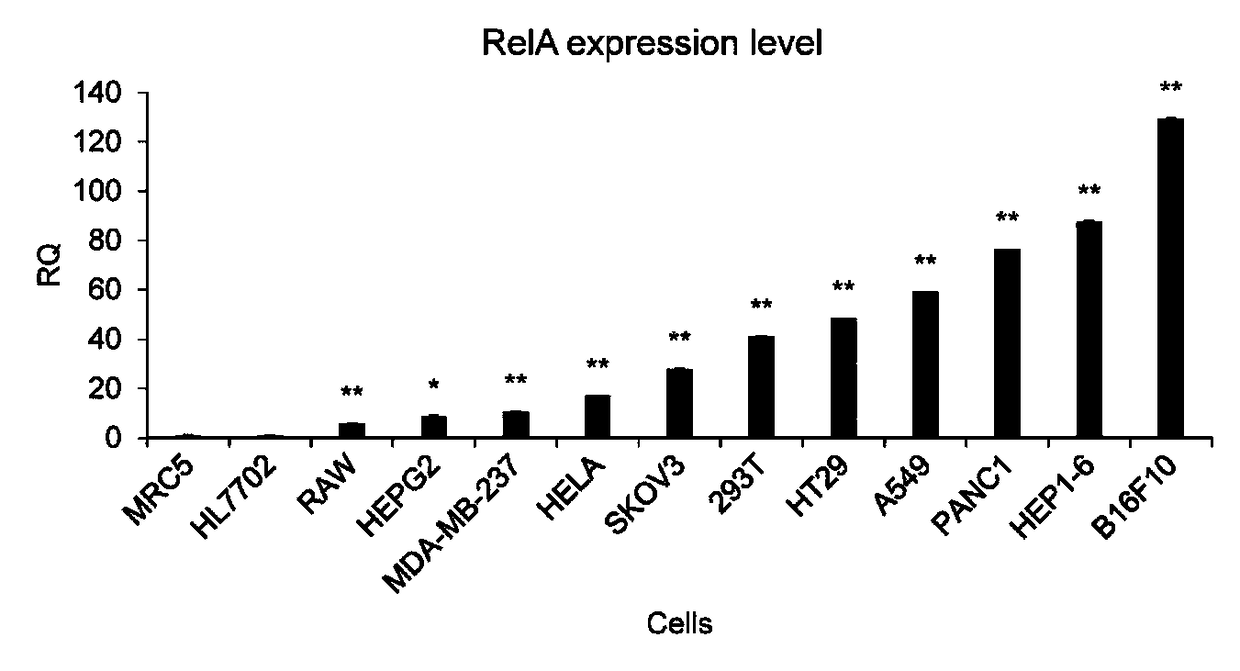
Cancer cell specific effect gene expression vector started by NF-KB and expression product and application thereof
ActiveCN108410893ARevolutionizing the principles of immunotherapyInnovation principleVectorsViral antigen ingredientsAntigenCancer cell
The invention discloses a cancer cell specific effect gene expression vector started by NF-KB and an expression product and an application thereof. The gene expression vector contains two sequence elements which are a promoter sequence for regulating gene expression and an effect gene coding sequence at the downstream of the promoter sequence. The promoter sequence is formed by a section of a NF-KB response sequence and a minimum promoter sequence. While the gene expression vector is guided into a cancer cell, the sequence specificity transcription factor NF-KB in the cell can activate the vector, and an effect gene on the vector is expressed. The effect gene expression product is a polypeptide or a protein of cell surface, the polypeptide or the protein of the cell surface not only can beused as a new antigen substance for exciting an in-vivo immune system to attack the cancer cell, and developing the efficacy of tumor immunity treatment, but also can be used as a cancer cell artificial mark for tumor imaging, diagnosis, cell separation and the like.
Owner:SOUTHEAST UNIV
Composite preparation
ActiveCN101951896AExcellent anti-platelet aggregation effectSignificant clinical effectSalicyclic acid active ingredientsPharmaceutical non-active ingredientsClopidogrel resistanceAdverse drug reaction
The present invention provides a composite preparation which comprises: a prior-release section comprising aspirin or a pharmaceutically acceptable salt thereof as a pharmacologically active component; and a delayed-release section comprising clopidogrel, an isomer thereof or a pharmaceutically acceptable salt thereof as a pharmacologically active component. The composite preparation of the present invention exhibits a far better effect in preventing platelet aggregation than does simultaneous oral therapy or treatment with the respective single preparations, and not only can it improve the patient's drug-taking compliance by administration once a day but it can also reduce the adverse reactions which follow long-term administration of aspirin. The composite preparation of the present invention is also advantageous in that it exhibits an outstanding effect in inhibiting blood platelet aggregation despite a reduction in the amount of aspirin ingested, and in that it converts clopidogrel resistance into susceptibility and prevents serious adverse reactions caused by clopidogrel resistance and in that it can be stored over the longer term since it is stable under common storage conditions.
Owner:HANALL PHARMA CO LTD
Substituted 1,3-dioxanes useful as PPAR modulators
ActiveUS20100168169A1Sufficient potencyDecrease, prevent, ameliorate, or treat the disease affecting the subjectBiocideSenses disorderDiabetes mellitusMedicine
Specifically useful stereoisomers of 1,3-dioxane derivatives are described and their use in the treatment of a disease or condition dependent on PPAR modulation, such as diabetes, cancer, inflammation, neurodegenerative disorders and infections as well as their use in the treatment of a disease related to TP, such as cardiovascular diseases.
Owner:EVOLVA SA
External Preparation
InactiveUS20080108700A1Improve light resistancePoor photostabilityBiocidePeptide/protein ingredientsMast cellPharmacology
It is an object of the present invention to provide an external preparation having enhanced transdermal penetration of a mast cell degranulation inhibitor. It is also an object of the present invention to provide a method for improving the photostability of a preparation containing a mast cell degranulation inhibitor. The present invention provides an external preparation containing a mast cell degranulation inhibitor and a topical anesthetic. Further, the method for enhancing the photostability of a preparation containing a mast cell degranulation inhibitor according to the present invention includes adding a topical anesthetic thereto.
Owner:MEDRX CO LTD
Propofol medium/long-chain injection and preparation method thereof
ActiveCN102552136BHypocalcemiaAvoid serious side effectsHydroxy compound active ingredientsAnaestheticsGlycerolMedium-chain triglyceride
Owner:GUANGDONG JIABO PHARM CO LTD
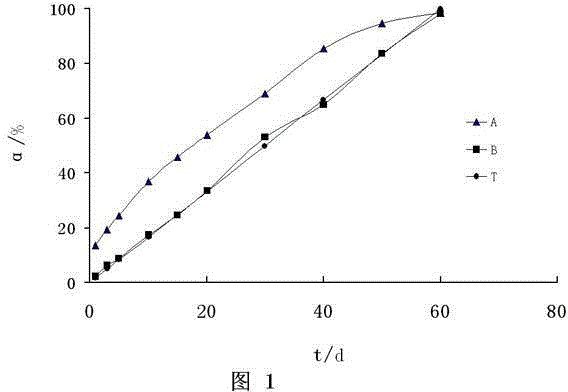
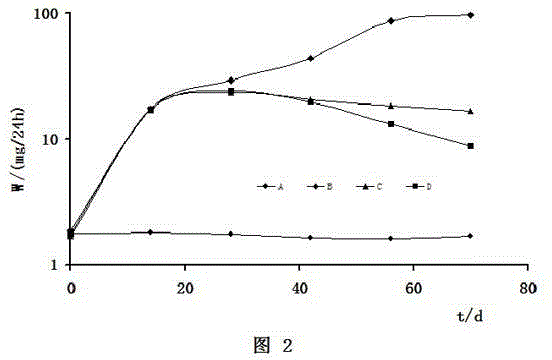
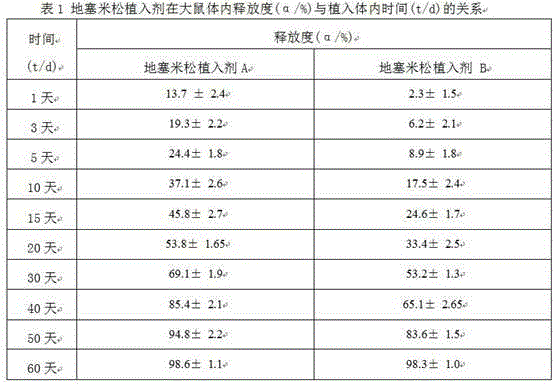
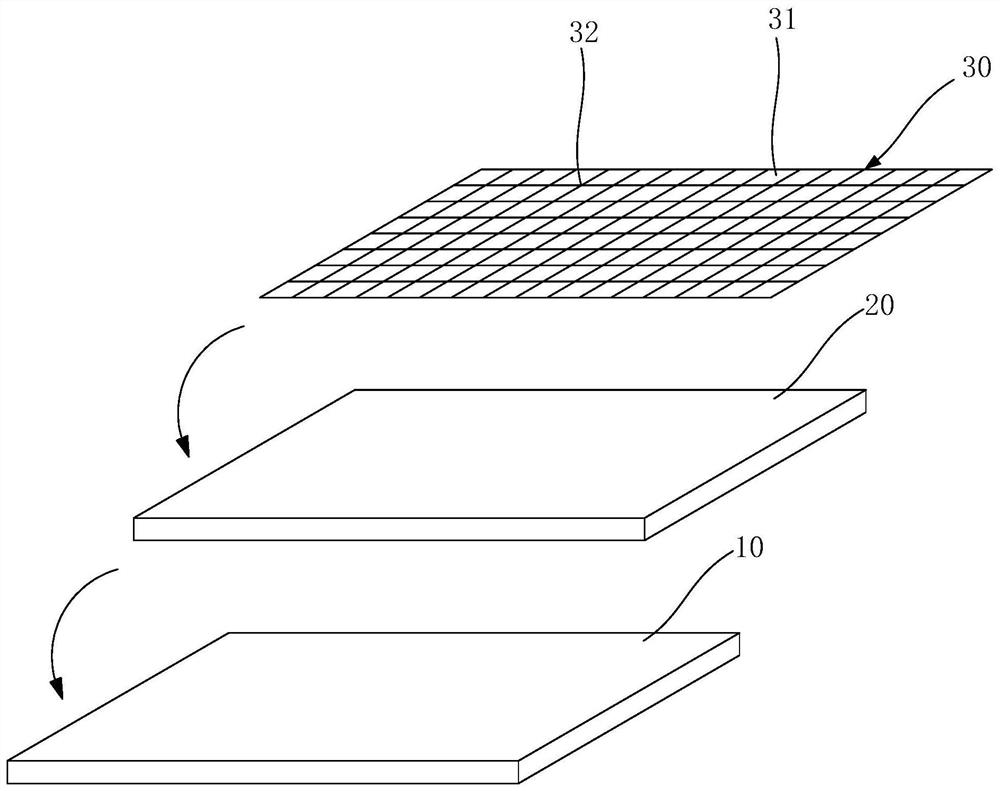
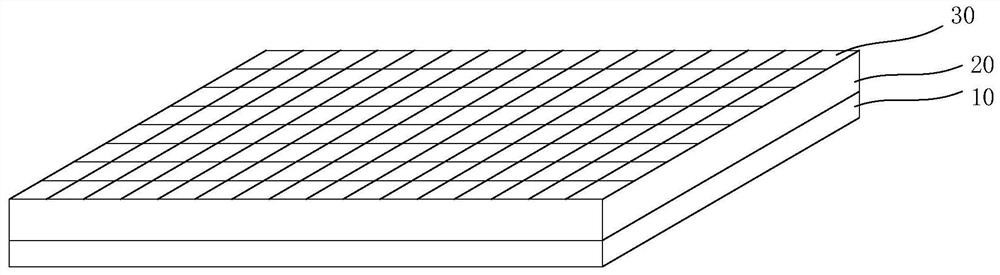
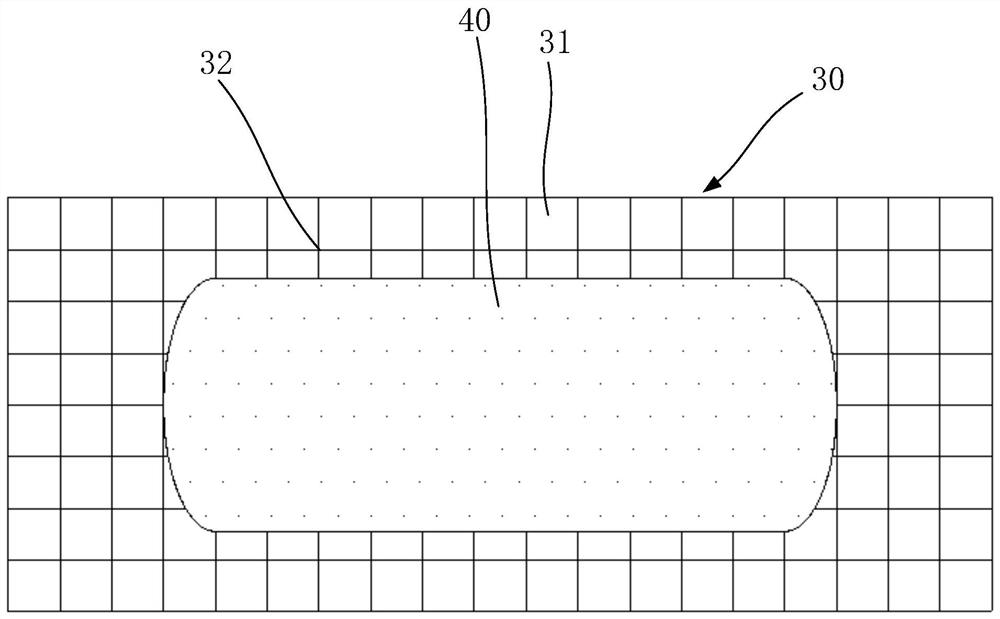
Preparation method of dexamethasone implant for kidney
ActiveCN105560161APromote meltingEasy extrusionOrganic active ingredientsPharmaceutical delivery mechanismDexamethasone acetateEngineering
The present invention relates to a preparation method, uses and a use method of a dexamethasone implant for kidney, wherein the dexamethasone implant comprises dexamethasone or dexamethasone acetate, a degradable polymer material, and a water soluble auxiliary material. The preparation method comprises: crushing various materials, mixing, carrying out micro-spheroidization, carrying out mold pressing molding, and heating for a certain time at a proper temperature to prepare the cylindrical implant with a diameter of 0.2-0.9 mm and a length of 0.8-4 mm. According to the present invention, the implant has characteristics of smooth surface and uniform drug release in vivo, wherein the time for releasing 90% of the drug is 1 month to 1 year; and the implant can be implanted into the renal sac through a drug implanting needle so as to treat nephrotic syndrome, nephritis and other chronic kidney diseases.
Owner:ANHUI ZHONGREN TECH +2
Medicine composition for preventing and treating AIDS viral infection
InactiveCN101391103AGood treatment effectExtension of timePeptide/protein ingredientsSuppositories deliveryStimulantWhite blood cell
The invention relates to pharmaceutical composition for preventing and curing the infection of AIDS virus. The components of the composition comprise at least an immunomodulator-immunologic stimulant, at lest a mucosa sorbefacient, human seralbumin and phenylcarbinol, wherein the immunomodulator-immunologic stimulant is recombined human interleukin, interferon, thymin or Toll sample receptor irritant agent, and the mucosa sorbefacient is surface active agent. The pharmaceutical composition of the invention is prepared to aqua or suppository, alleviates the pain caused by the injection of the immunomodulator-immunologic stimulant; greatly improves the security and validity of the use through nontraumatic administration through the mucosa of rectum, has the treating effect exceeding the subcutaneous injection and muscle injection, and has wide applying and generalizing foreground.
Owner:张可 +1
Anticancer Chinese medicine compound nanometer emulsion and its preparation method
InactiveCN100998867AGood curative effectSmall toxicityOrganic active ingredientsEmulsion deliveryGlycerolPharmaceutical drug
An anticancer Chinese medicine in the form of nano-class emulsion for treating cancers is proportionally prepared from taxol or polyene taxol or amarin or hydroxy camptothecine, brucea fruit oil, soybean phosphatide or lecithin for injection, poloxamer, glycerine for injection and the water for injection. Its preparing process is also disclosed.
Owner:TAIJI MEDICINE INST CHONGQING
Photosensitizing ointment
InactiveUS20030083324A1Avoid serious side effectsEffective penetrationAntibacterial agentsBiocideRepeated treatmentPhototoxicity
A system and a method using photodynamic therapy for treatment of epithelial diseases are provided, wherein the photosensitizers used have enhanced selectivity for the affected region so that the treatment has reduced or no side effects. Selectivity is achieved by avoiding systemic application of the photosensitizer and by topically applying the photosensitizers with certain carriers. Compositions of hydrophilic medical or cosmetic carriers like ointments, creams or lotions can be used. Hydrophobic photosensitizers such as bacteriopheophorbide and its derivatives are preferred because of their ability to penetrate tissue and to distribute evenly, as well as their low threshold of phototoxicity. After the phototoxic sensitizer has been administered to the afflicted tissue, the tissue is irradiated with an appropriate radiation source, such as sunlight or a radiation source emitting a defined wavelength like a diode laser. Deeper penetration of the radiation may be achieved with longer wavelengths (700-800 nm), which are in the red part of the spectrum. The photosensitizing agent can be topically applied easily and repeatedly, and thus the system is especially useful for treating diseases like psoriasis, where frequent and repeated treatments may be necessary. A method of photodynamic therapy for epithelial diseases is also provided, which comprises the steps of: (a) applying topically a therapeutically effective amount of the photosensitizer like bacteriopheophorbide or a bacteriopheophorbide derivative to an area afflicted by an epithelial disease or an infection, and (b) exposing the treated area to radiation to photoactivate the photosensitizer to produce a cytotoxic response in the afflicted area.
Owner:CERAMOPTEC IND INC
Preparation method and application of total gingerol external preparation
InactiveCN112138131ALow extraction rateReduced odor componentsAntibacterial agentsAntimycoticsGel preparationUse medication
The invention discloses a preparation method of a total gingerol external preparation. The preparation method comprises the steps of supercritical extraction, macroporous resin column chromatography,HPLC purification and preparation of a gel preparation, wherein the gel preparation is prepared from 100 parts of refined gingerol, 5-15g of carbomer, 20-30g of glycerin and 0.1-0.2g of polysorbate, and distilled water. According to the preparation method, the external preparation is prepared from total gingerol, so that the medicinal effect of total gingerol, such as removing heat, relieving painand the like can be utilized, and the serious effect on the digestive tract caused by taking oral medicines for a long time can be avoided.
Owner:成都克洛玛生物科技有限公司
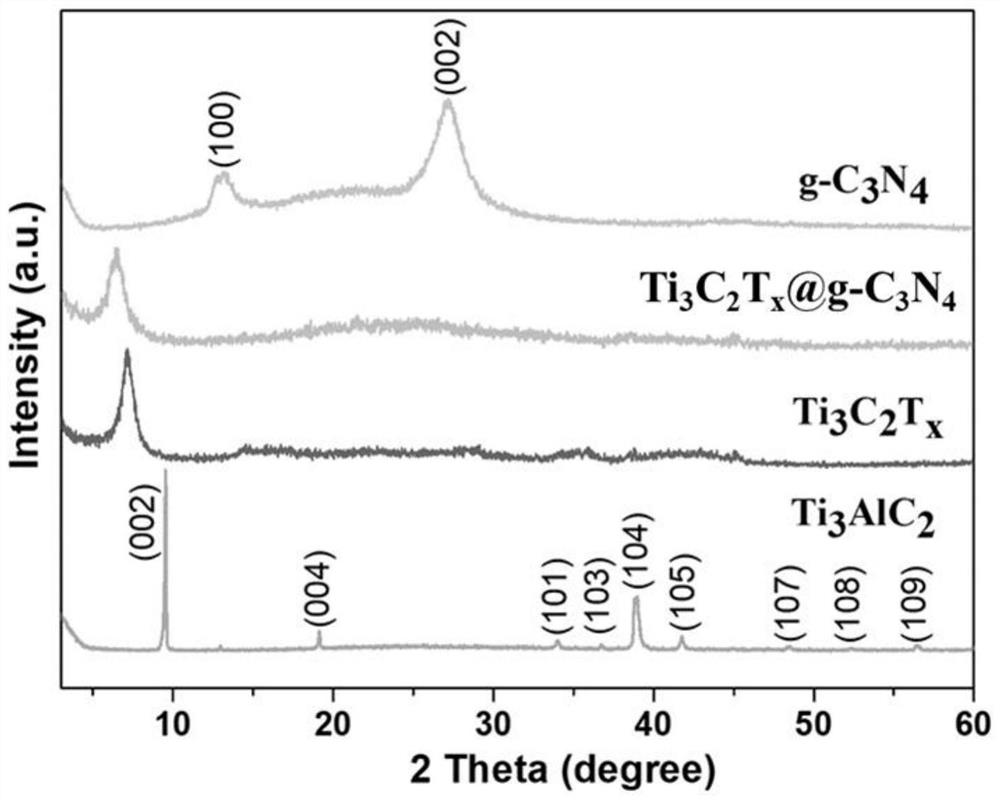
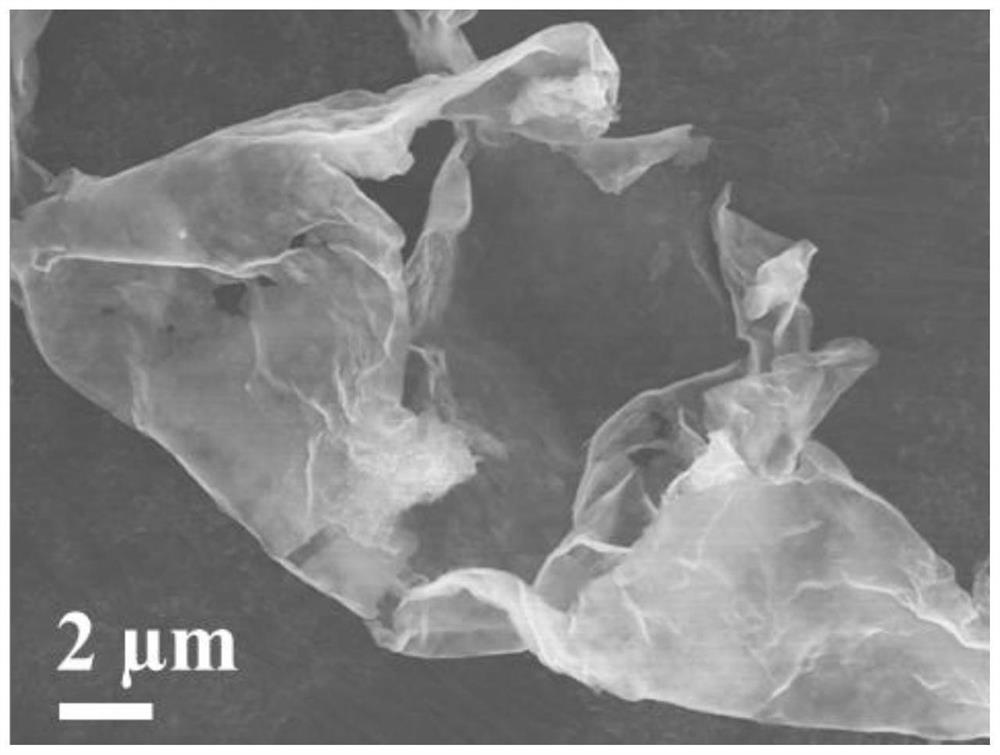
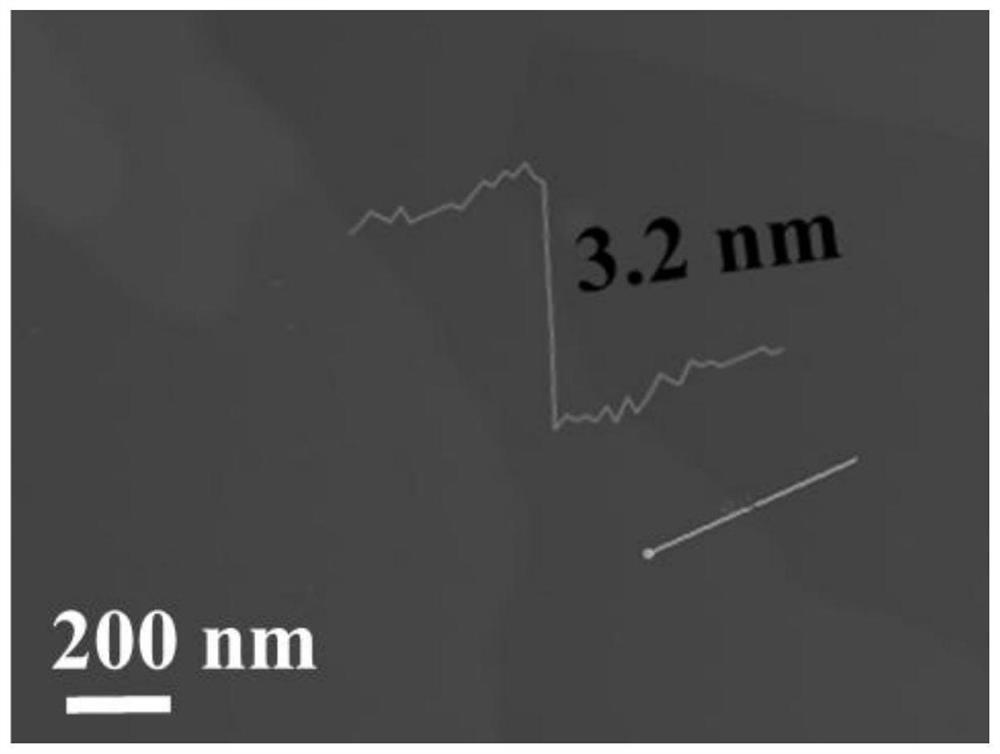
Ti3C2Tx-coated g-C3N4 composite material as well as preparation method and application thereof
PendingCN114335458AImprove Interface StabilityImprove electrochemical performanceMaterial nanotechnologyCell electrodesLithium metalElectrochemistry
The invention provides a Ti < 3 > C < 2 > T < x >-g-C < 3 > N < 4 > composite material as well as a preparation method and application thereof, and relates to the technical field of composite materials. The Ti < 3 > C < 2 > T < x > (at) g-C3N4 composite material provided by the invention has excellent cycle stability, when the Ti < 3 > C < 2 > T < x > (at) g-C3N4 composite material provided by the invention is applied to a lithium metal negative electrode, a three-dimensional Ti < 3 > C < 2 > T < x > framework is used as a lithium metal deposition substrate of a three-dimensional structure, and a g-C3N4 layer on the surface is used as a uniform artificial solid electrolyte interface (SEI), so that the interface stability of the lithium metal negative electrode can be improved, and the lithium metal negative electrode can be applied to a lithium battery. The electrochemical performance is improved.
Owner:BEIHANG UNIV
A kind of preparation method of dexamethasone implant for kidney
ActiveCN105560161BPromote meltingEasy extrusionOrganic active ingredientsPharmaceutical delivery mechanismDexamethasone acetateDrug release
The present invention relates to a preparation method, uses and a use method of a dexamethasone implant for kidney, wherein the dexamethasone implant comprises dexamethasone or dexamethasone acetate, a degradable polymer material, and a water soluble auxiliary material. The preparation method comprises: crushing various materials, mixing, carrying out micro-spheroidization, carrying out mold pressing molding, and heating for a certain time at a proper temperature to prepare the cylindrical implant with a diameter of 0.2-0.9 mm and a length of 0.8-4 mm. According to the present invention, the implant has characteristics of smooth surface and uniform drug release in vivo, wherein the time for releasing 90% of the drug is 1 month to 1 year; and the implant can be implanted into the renal sac through a drug implanting needle so as to treat nephrotic syndrome, nephritis and other chronic kidney diseases.
Owner:ANHUI ZHONGREN TECH +2
Apple polysaccharide, its preparation method and application in preventing and treating tumor metastasis medicine
InactiveCN101167831AAbundant resourcesEasy to prepareOrganic active ingredientsFermentationLymphatic SpreadFiltration
The invention relates to the technical field of drugs for preventing and inhibiting tumor metastasis, in particular to an apple polysaccharide, its preparation method and its application in drugs for preventing and inhibiting tumor metastasis. One of the objects of the present invention is to provide a modified apple polysaccharide (MAP) used as a drug for preventing and inhibiting tumor metastasis. The second object is to provide a preparation method for the above-mentioned apple polysaccharide. And the application of anti-tumor metastasis drugs to fill the serious shortage of anti-tumor metastasis drugs. Technical solution provided by the present invention: an apple polysaccharide prepared by the following method, comprising the following steps: (1) obtaining apple crude polysaccharide; (2) reducing the degree of esterification so that the degree of esterification is less than 20% Then through gel chromatography or molecular weight cutoff, the apple polysaccharide with a molecular weight of 5-15 kDa is obtained.
Owner:刘莉 +1
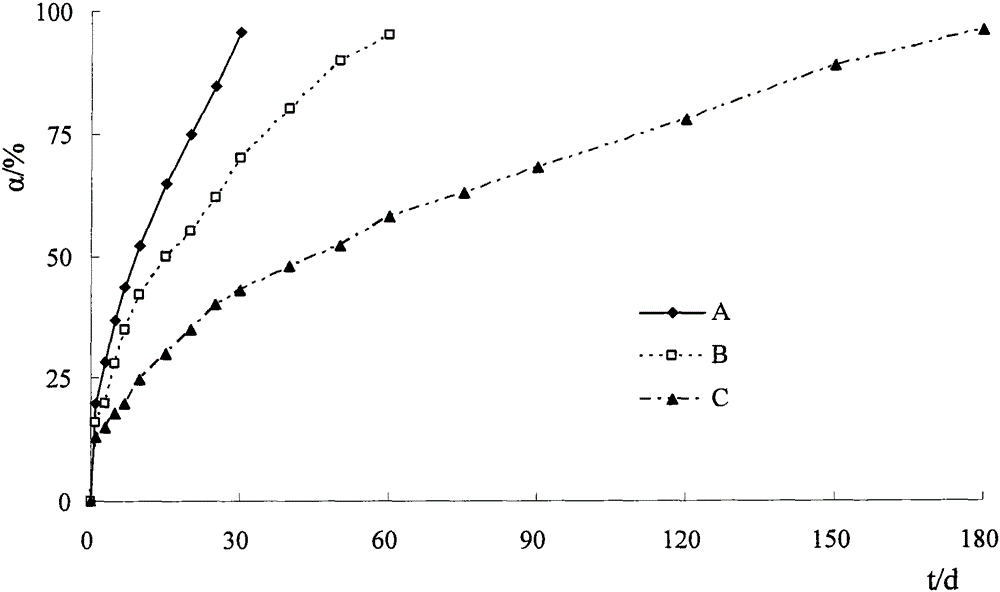
Etoposide implant
ActiveCN103417468AHigh mechanical strengthImprove stabilityOrganic active ingredientsPharmaceutical non-active ingredientsPolyethylene glycolBiomedical engineering
The invention relates to an etoposide implant, and a preparation method, purpose and an application method thereof. The implant is a circular column with diameter of 0.2-1.2 mm and length of 1-5 mm; and the implant is prepared from etoposide, polylactic acid and polyethylene glycol through compacting. It takes the implant 6-120 days to release 90% of the medicine in human body; and the implant can be implanted into a tumor focus through medicine implant needle percutaneous puncture or surgery, or implanted into positions requiring long-time etoposide medication treatment for regional continuous long-time medicament treatment.
Owner:合肥中人科技有限责任公司
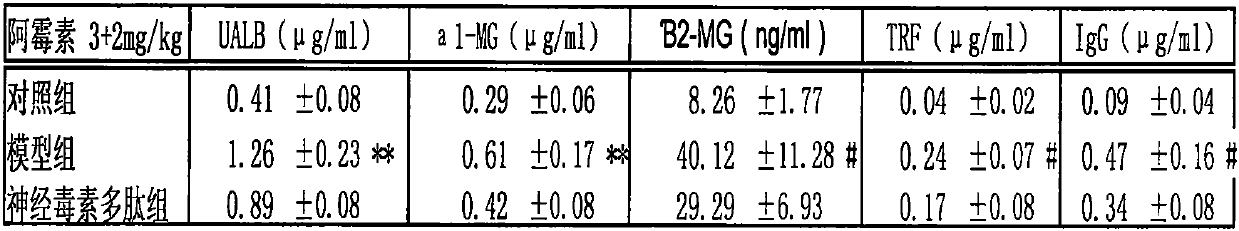
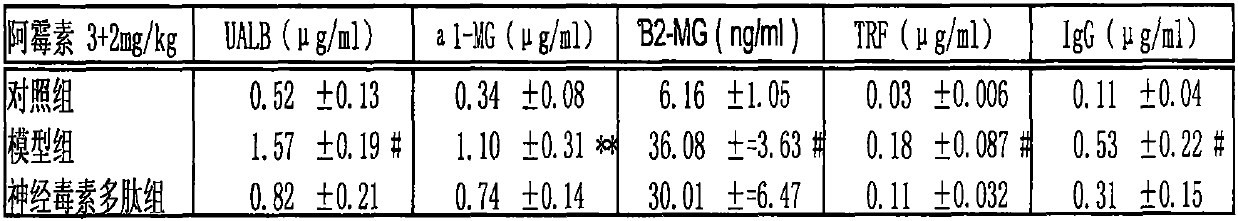
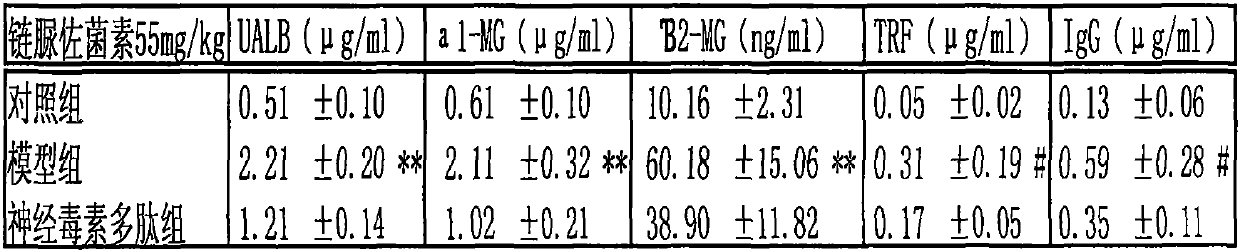
Application of cobra neurotoxin polypeptide molecules to treatment of nephritis albuminuria
PendingCN110755603AReduced autoimmune responseAlleviate pathological changesPeptide/protein ingredientsUrinary disorderDisease courseAutoimmunity
After a nephritis patient suffers from nephritis for several years, albumin in a little blood begins to leak into urine, at the moment, albumin leakage is easy to neglect because clinical symptoms arenot significant or the albumin is confused with other albuminuria caused by hypertension and hyperlipaemia, so that the illness state is worsened. But when it is found that other indexes reflecting renal functions, such as [beta]2 microglobulin, [alpha]1 microglobulin, transferrin and immunoglobulin are also obviously increased in urine, the situation confirms that the renal functions already have pathology changes. At present, no other effective methods exist except hormone on treatment, but long-term hormone treatment can cause many side reactions to the patient. If illness course freely develops, more albumin leaks into urine, and kidney damage can be further worsened. Cobra neurotoxin polypeptide molecules can effectively reduce leak of various albumin in urine, control and delay kidney pathologic progress, improve renal functions and have important significance in treatment of the nephritis through restraining autoimmunity inflammatory reactions of the kidney.
Owner:祁展楷
A kind of dexamethasone implant
ActiveCN103417467BMeet treatment requirementsIncrease concentrationAntibacterial agentsOrganic active ingredientsDexamethasoneMedicine
The invention relates to a dexamethasone implant, and a preparation method, purpose and an application method thereof. The implant is a circular column with diameter of 0.2-1.2 mm and length of 1-5 mm; and the implant is prepared from dexamethasone medicine, polylactic acid and polyethylene glycol through compacting. It takes the implant 7-180 days to release 90% of the medicine in human body; and the implant can be implanted into saccus renalis through medicine implant needle percutaneous puncture for treating chronic nephritis, or implanted into positions requiring long-time dexamethasone medication treatment for regional continuous long-time medicament treatment.
Owner:合肥中人科技有限责任公司
Dentifrice compositions comprising a stable low water, phase comprising polyphosphate and ionic active ingredients
InactiveCN1286448CAvoid serious side effectsCosmetic preparationsToilet preparationsBULK ACTIVE INGREDIENTNuclear chemistry
Disclosed are dentifrice compositions comprising in a single phase: (a) from about 0.1 % to about 30 % of one or more linear polyphosphates having an average chain length of about 4 or more; (b) an ionic active ingredient selected from the group consisting of a fluoride ion source, a stannous ion source, a zinc ion source, a copper ion source and mixtures thereof, wherein the ionic active ingredient is present as a solid dispersion in the composition and delivers an effective amount of ionic active when solubilized; (c) a binder system comprised of (i) from about 0.05 % to about 3 % of a thickening agent selected from the group consisting of polysaccharides, carbomers, poloxamers, modified celluloses, and mixtures thereof; and (ii) from about 0.1 % to about 70 % of at least one humectant; wherein the dentifrice composition has a total water content of less than about 10 %. Further disclosed are methods for stabilizing dentifrice compositions by providing such a binder system.
Owner:THE PROCTER & GAMBLE COMPANY
Pharmaceutical preparation, system containing pharmaceutical preparation and preparation method and application of pharmaceutical preparation
InactiveCN111821257AObtain continuous and safe analgesic effectConvenient room temperature storageNervous disorderAerosol deliveryProcainePharmaceutical formulation
The invention discloses a pharmaceutical preparation, a system containing the pharmaceutical preparation and a preparation method and an application of the pharmaceutical preparation. The pharmaceutical preparation comprises water, a pH buffer solution and local anesthetics, wherein the local anesthetics in the pharmaceutical preparation comprise dissolved and undissolved local anesthetics, wherein the mass percentage of the local anesthetic in the pharmaceutical preparation is 2% or above; the pH buffer solution enables the mass percentage of the dissolved local anesthetic in the pharmaceutical preparation to be 1% or below; the local anesthetic is lidocaine, tetracaine, bupivacaine, articaine, cinchocaine, dibucaine, etidocaine, levobupivacaine, mepivacaine, prilocaine, ropivacaine, trimecaine, benzocaine, or procaine; and the sum of the mass percentages of the components in the medicinal preparation is 100%. In many analgesic applications, the pharmaceutical formulation is capable of obtaining a continuous analgesic effect of 6, 12, 18, 24, 36, or even 48 hours or more.
Owner:HUZHOU INNOVATION PHARMACEUTICAL CO LTD
Agents for measuring pyrimidine metabolizability
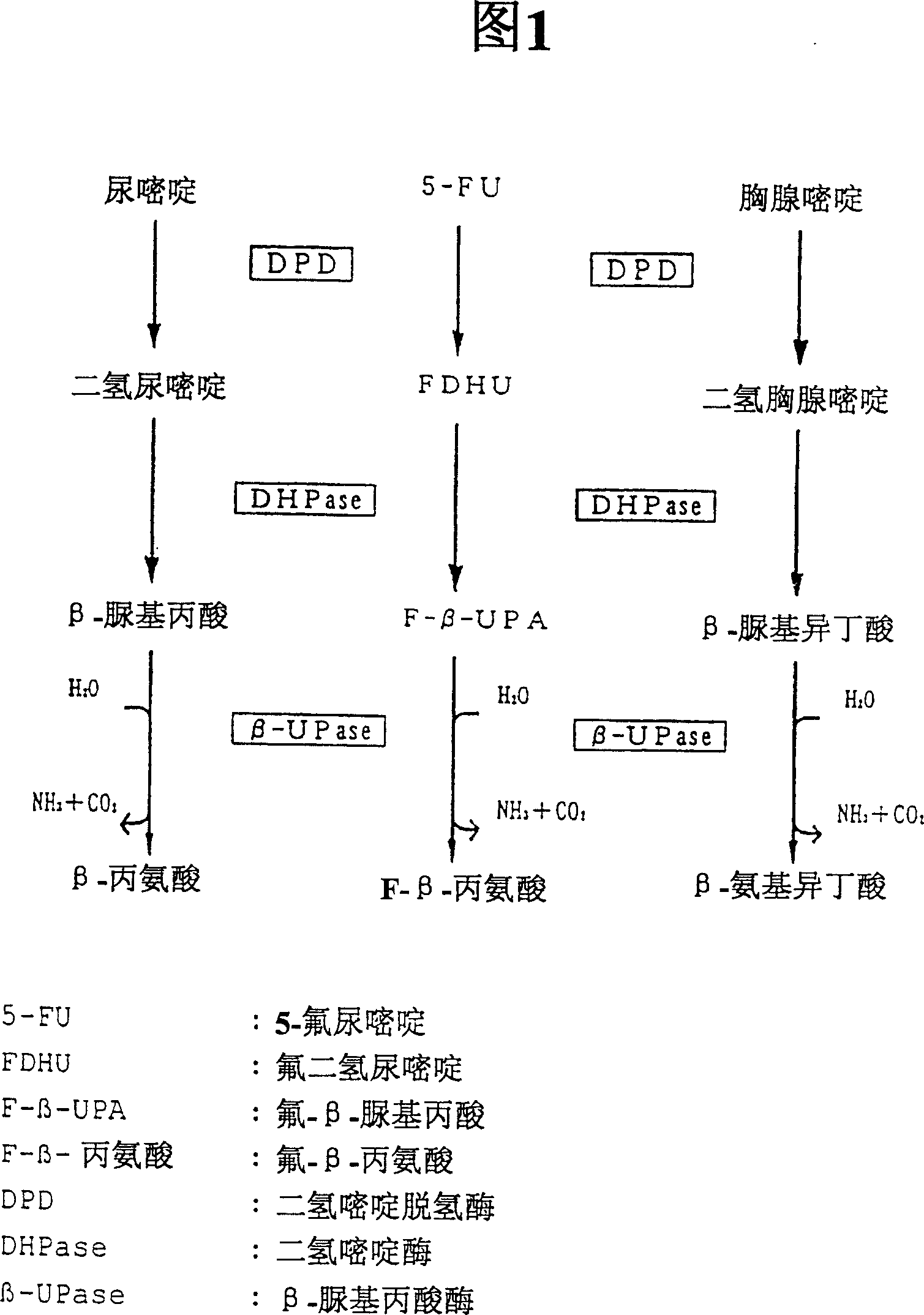
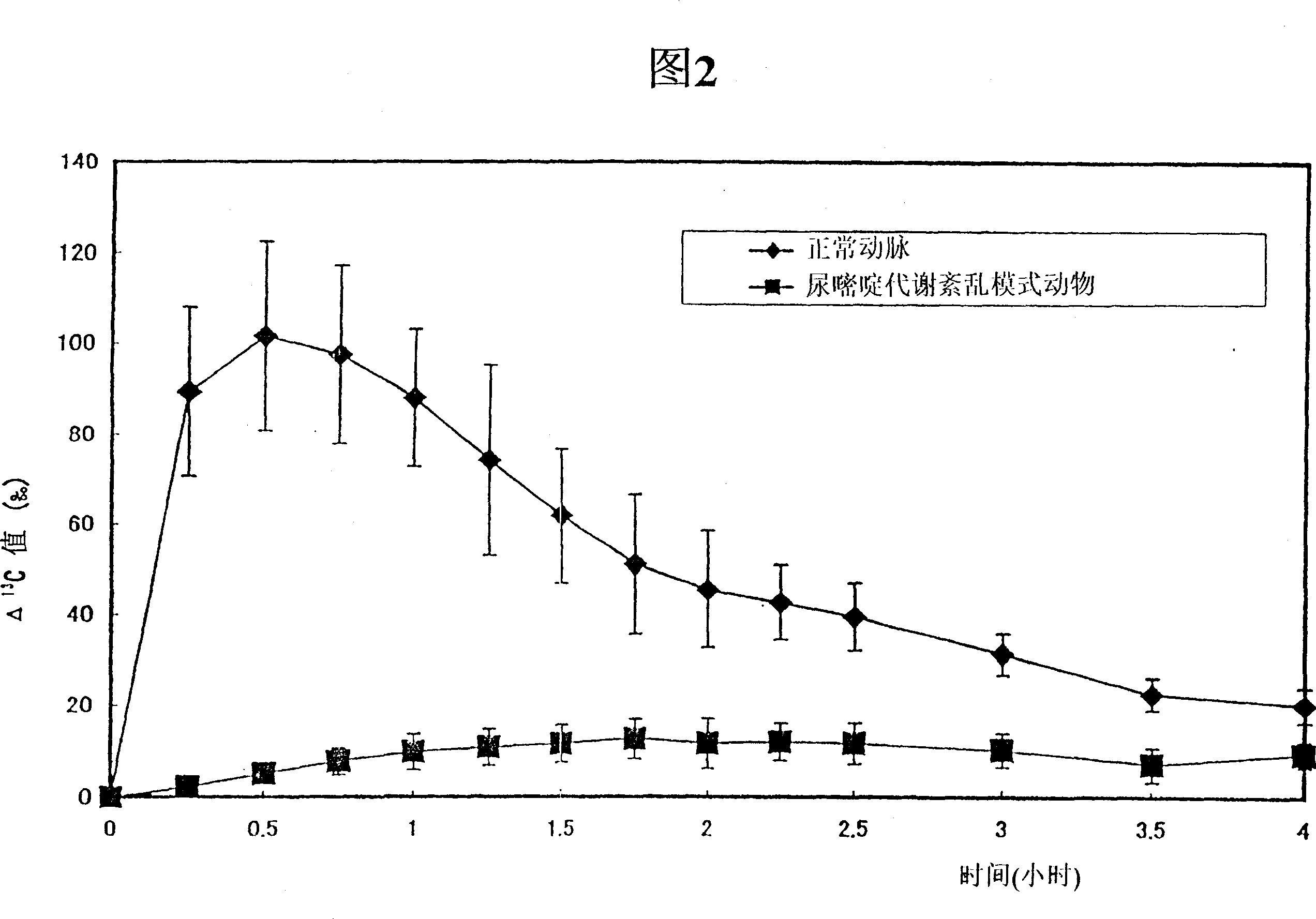
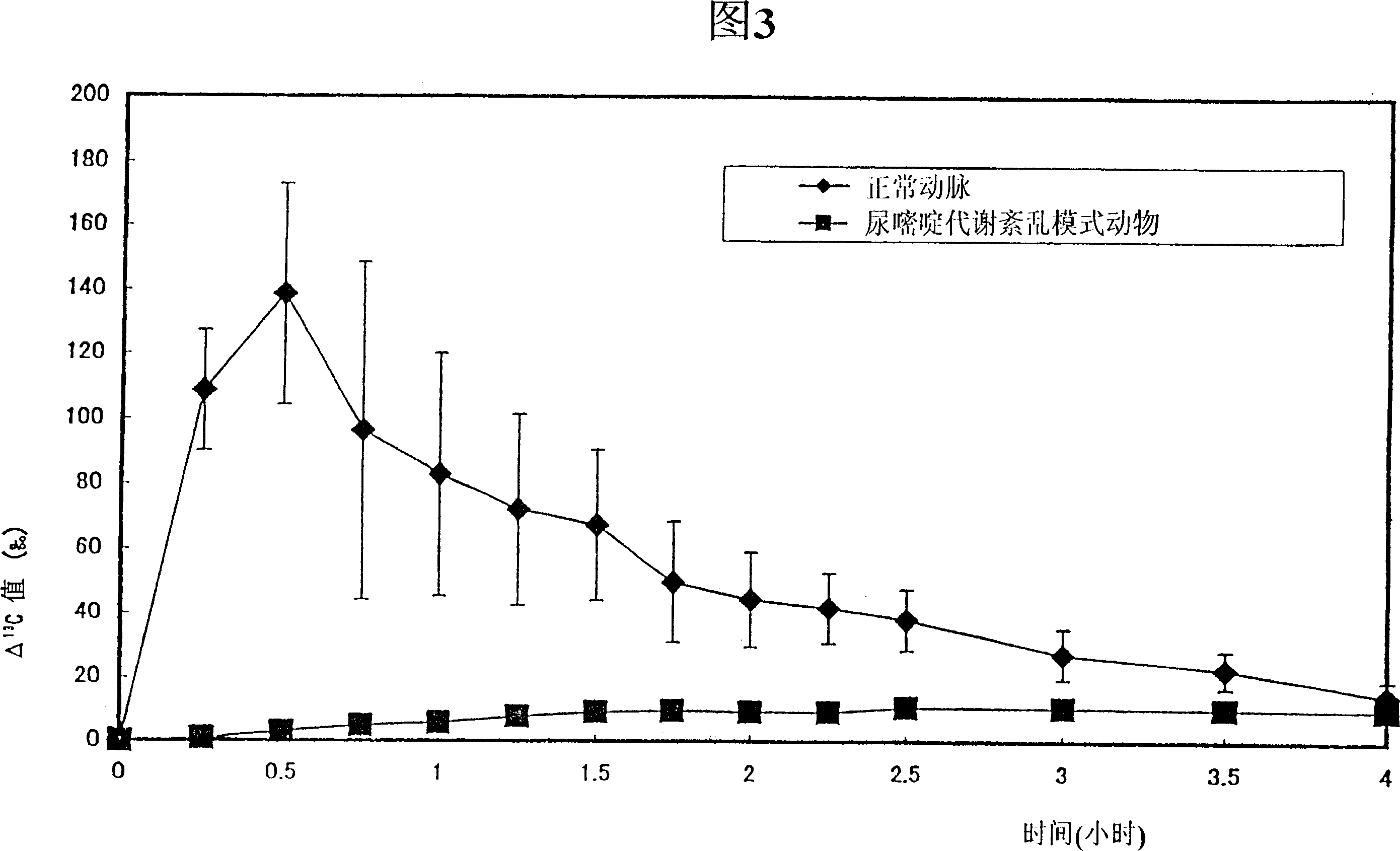
InactiveCN100341580CAvoid serious side effectsTreatment safetyCompounds screening/testingDispersion deliveryMetaboliteIsotope
The present invention provide a method for assessing the sensitivity to a pyrimidine drug such as 5-FU which is degraded in the pyrimidine metabolic pathway, specifically in vivo pyrimidine metabolizing activity, of an individual subject; and a preparation useful for the assessment. The present invention can be carried out by: administering a preparation comprising as an active ingredient a pyrimidine compound or its metabolite which acts as a substrate for a pyrimidine metabolizing enzyme, in which compound or metabolite at least one of C, O and N is labeled with an isotope; and assessing in vivo pyrimidine metabolizing activity based on the amount of excreted metabolite.
Owner:OTSUKA PHARM CO LTD
Uses of imidazolidine and composition comprising imidazolidine
ActiveCN112220749AAvoid serious side effectsOrganic active ingredientsSenses disorderImidazolidineOphthalmology
The invention relates to uses of imidazolidine and a composition containing the imidazolidine, and particularly provides uses of the imidazolidine and the composition containing the imidazolidine to treatment and prevention of corneal neovascularization, corneal fibrosis, pterygium, meibomian gland dysfunction and visual fatigue.
Owner:EYE MEDICAL XIAMEN BIOTECHNOLOGY CO LTD
Test method for evaluating the risk of Anti-thyroid drug-induced agranulocytosis, and evaluation kit
InactiveUS20190316199A1Safe and secure and accurate treatmentAvoid serious side effectsMicrobiological testing/measurementDNA/RNA fragmentationAntithyroid drugsAgranulocytoses
The present invention provides a test method and an evaluation kit for determining the risk of antithyroid drug-induced agranulocytosis. More particularly, it provides a test method for determining the risk of antithyroid drug-induced agranulocytosis, including testing susceptibility polymorphism to antithyroid drug-induced agranulocytosis, and determining the risk of antithyroid drug-induced agranulocytosis, and an evaluation kit for the risk of antithyroid drug-induced agranulocytosis, containing a polynucleotide capable of detecting susceptibility polymorphism to antithyroid drug-induced agranulocytosis.
Owner:GENOCONCIERGE KYOTO INC
Amines substituted with a dihydronaphthalenyl, chromenyl, or thiochromenyl group, a pyridyl group and an alkyl group, having retinoid-like biological activity
InactiveUS7026487B2Increased circulating levelFunction improperlyOrganic active ingredientsSenses disorderHormoneMolecular biology
Compounds of the formula where the symbols are as defined in the specification, have retinoid agonist, antagonist or negative hormone-like biological activity.
Owner:ALLERGAN INC
Primer set, application, product and method for detecting SNP sites related to children's drug metabolism
ActiveCN110511993BImprove accuracyShort detection cycleMicrobiological testing/measurementDNA/RNA fragmentationPsychosis drugIMMUNE SUPPRESSANTS
Owner:JIANGSU SIMCERE MEDICAL DEVICE CO LTD +2
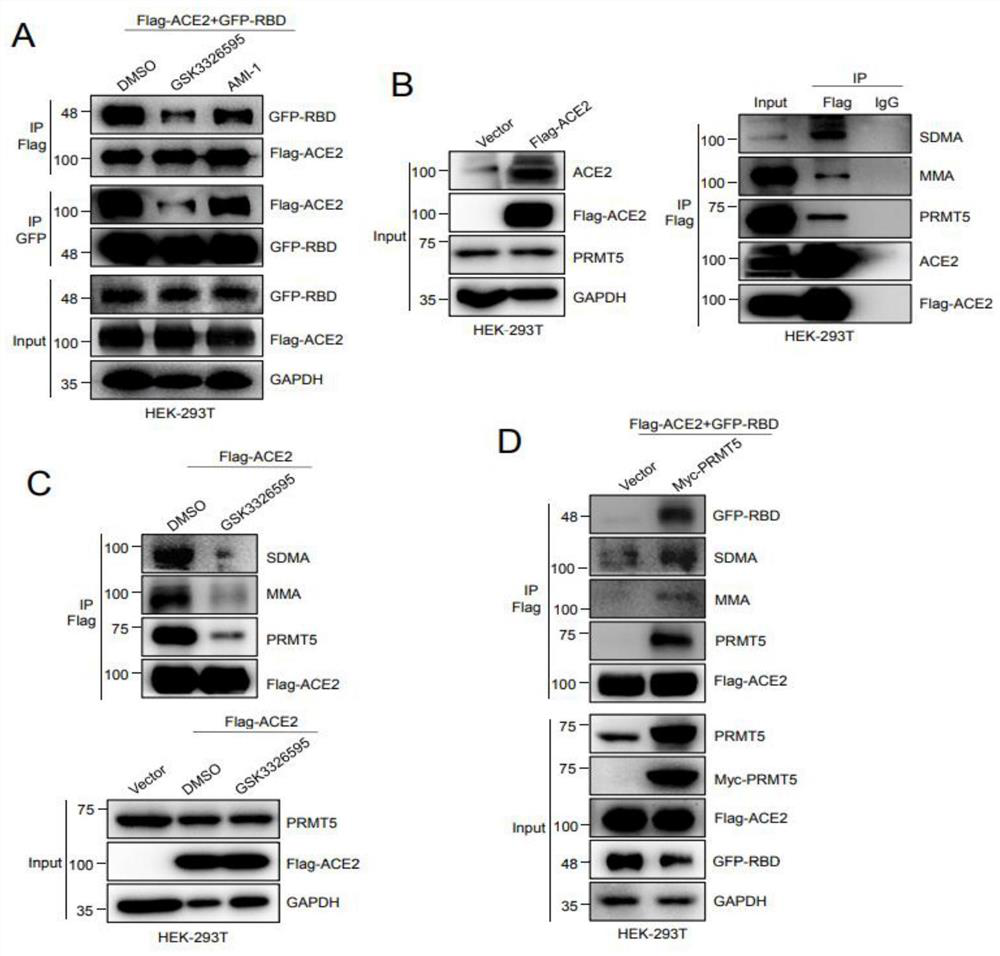
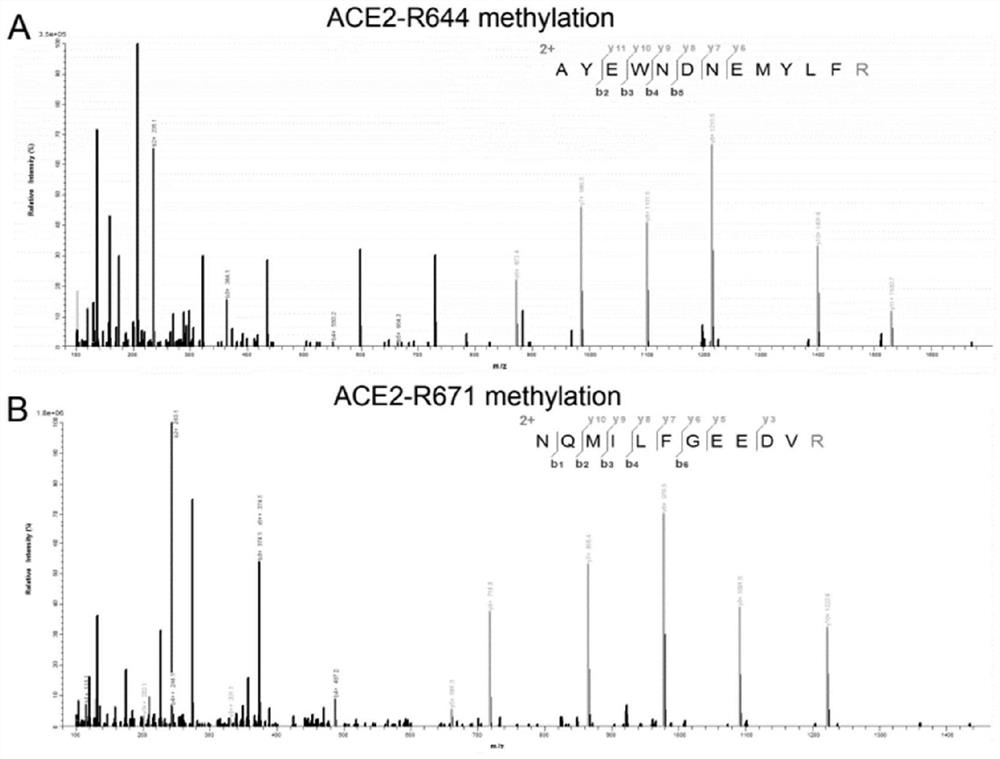
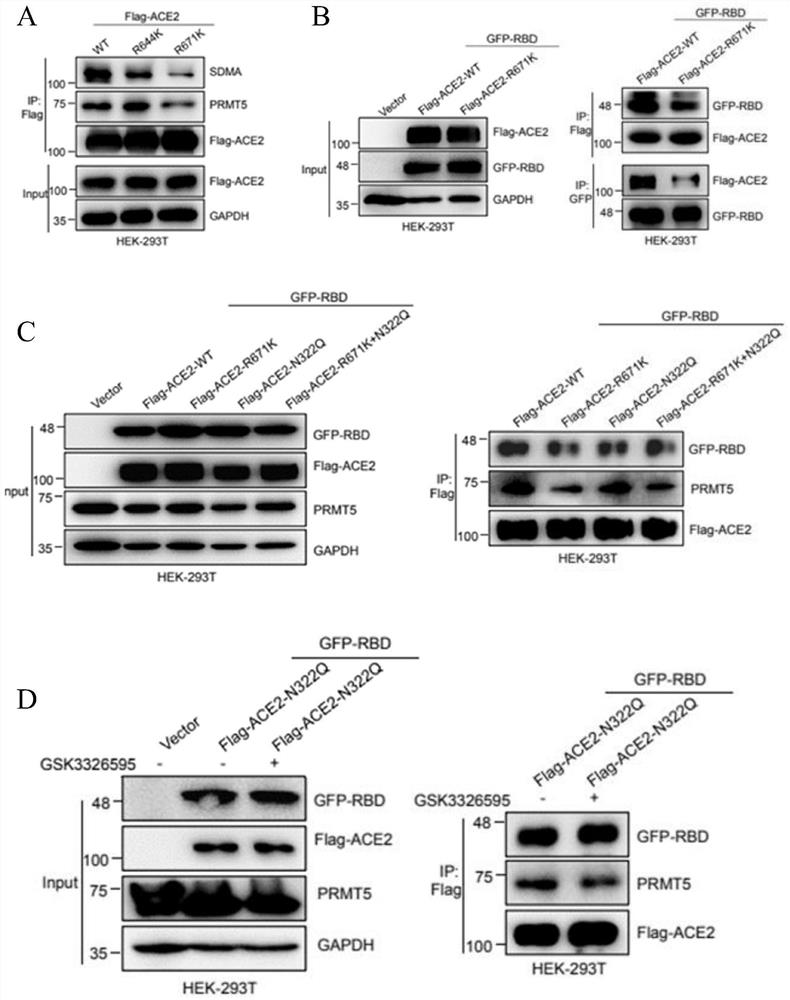
Application of PRMT5 specific inhibitor in prevention of coronavirus infection
ActiveCN113546173AAvoid serious side effectsOrganic active ingredientsAntiviralsPharmaceutical drugBiomedicine
The invention relates to the field of biotechnology and biomedicine, in particular to application of a PRMT5 specific inhibitor in prevention of coronavirus infection. More specifically, the invention provides a gene editing reagent of a PRMT5 inhibitor and / or ACE2 protein and application of a pharmaceutical composition composed of the gene editing reagent in preparation of products for preventing coronavirus infection / treating diseases caused by coronavirus infection. Preferably, the coronavirus comprises a novel 2019 coronavirus.
Owner:XUZHOU MEDICAL UNIV
Artificial recombination selection replication type adenovirus and application
InactiveCN101016537AAvoid serious side effectsGrowth inhibitionGenetic material ingredientsAntiviralsTumor specificCell immunity
The invention discloses an artificial reconstructing selection replicative form adenovirus, which is characterized by the following: possessing nucleic acid sequence of SEQ ID NO.1; duplicating in virus selective infection tumour cell; carrying TNFalpha gene of Midkine starting; generating TNFalpha in Midkine high expression tumor cell; causing extinction of tumor cell; generating lethalling action for tumor cell with duplication of virus, expressing TNFalpha and antiviral cell immunity. This invention does not express Midkine, which can be used to preparing medicine for treat specificity lethal adenovirus and provide new method to treat tumor.
Owner:ZHEJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com