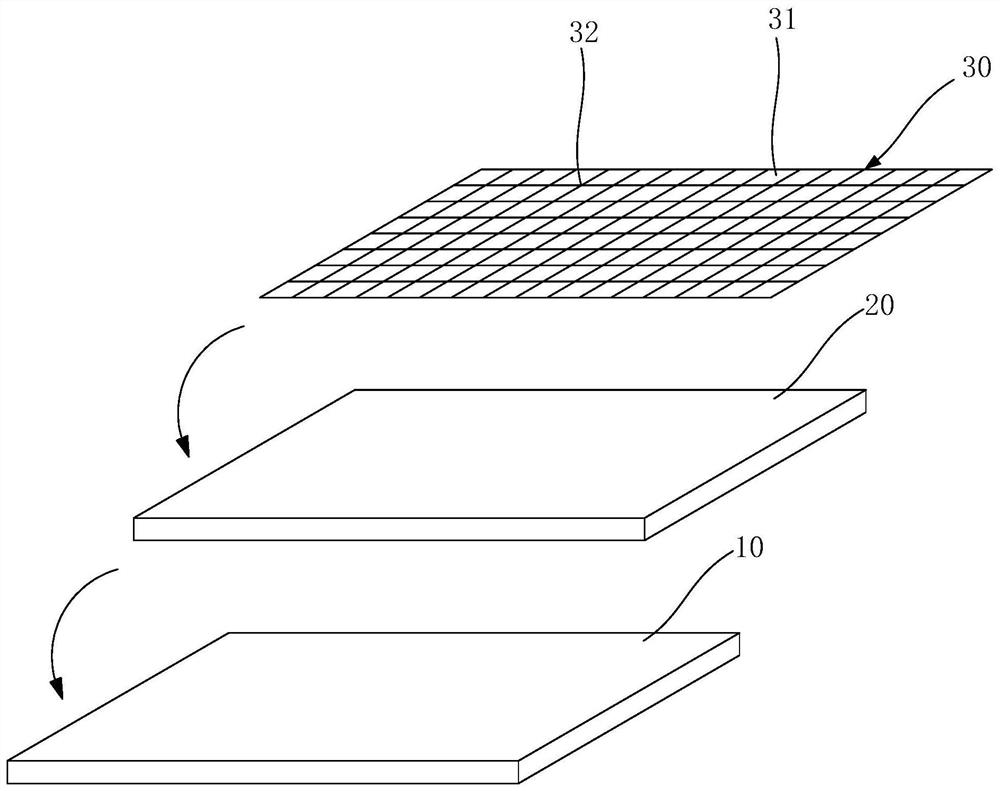
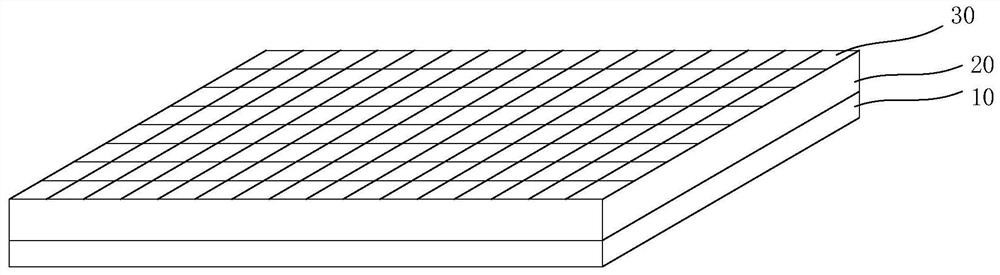
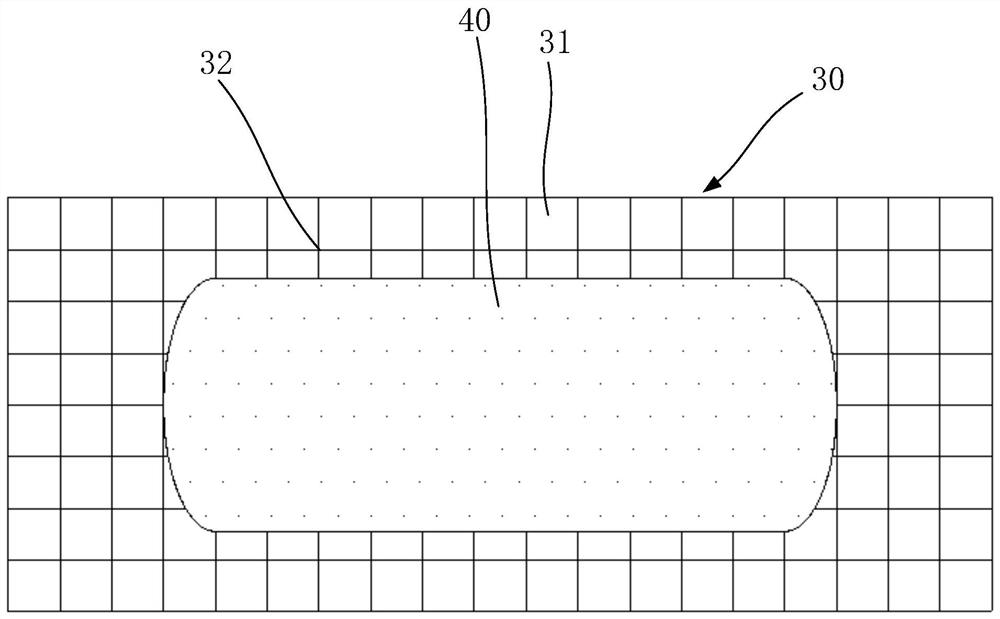
Pharmaceutical preparation, system containing pharmaceutical preparation and preparation method and application of pharmaceutical preparation
A pharmaceutical preparation and preparation technology, applied in the field of pharmaceutical preparations, can solve problems such as the inability to achieve long-term continuous and safe analgesic effects, achieve continuous and safe analgesic effects, avoid serious side effects, and be convenient to use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] A pharmaceutical preparation, the composition of which is shown in the table below:
[0111] Element mass percent or molar concentration Lidocaine 5% Disodium phosphate 0.094mol / L Sodium dihydrogen phosphate 0.006mol / L xanthan gum 4% sodium hydroxide 0.08% water Top up to 100%
[0112] The preparation method of described pharmaceutical preparation is as follows:
[0113] Add sodium hydroxide, disodium hydrogen phosphate, sodium dihydrogen phosphate and xanthan gum to the water and stir until a homogeneous viscous liquid is obtained. Then, add lidocaine and heat to 80° C., stir rapidly, and cool to room temperature to obtain the product.
[0114] In this pharmaceutical preparation, the pH buffer pair is composed of disodium hydrogen phosphate and sodium dihydrogen phosphate; xanthan gum is a thickener and a suspending agent.
[0115] Effect data: the obtained pharmaceutical preparation is recorded as pharmaceutical...
Embodiment 2
[0117] A pharmaceutical preparation, the composition of which is shown in the table below:
[0118]
[0119]
[0120] The preparation method of described pharmaceutical preparation is as follows:
[0121] Sodium hydroxide, disodium hydrogen phosphate, sodium dihydrogen phosphate, Pemulen TR-2, and hydroxyethylcellulose were added to the water and stirred until a homogeneous viscous liquid was obtained. Then, add lidocaine and glycerin and heat to 80° C., stir rapidly, and cool to room temperature to obtain the obtained product.
[0122] In this pharmaceutical preparation, the pH buffer pair is composed of disodium hydrogen phosphate and sodium dihydrogen phosphate; sodium hydroxide is used to adjust the pH value and participate in the pH buffer pair; Pemulen TR2 is a suspending agent; suspending agent.
[0123] Effect data: The obtained pharmaceutical preparation is recorded as pharmaceutical preparation B, and the obtained pharmaceutical preparation is a viscous solut...
Embodiment 3
[0125] A pharmaceutical preparation, the composition of which is shown in the table below:
[0126]
[0127]
[0128] The preparation method of described pharmaceutical preparation is as follows:
[0129] Sodium hydroxide, disodium hydrogen phosphate, sodium dihydrogen phosphate, Pemulen TR-2, and hydroxyethylcellulose were added to the water and stirred until a homogeneous viscous liquid was obtained. Then, add lidocaine and glycerin and heat to 80° C., stir rapidly, and cool to room temperature to obtain the obtained product.
[0130] In this pharmaceutical preparation, the pH buffer pair is composed of disodium hydrogen phosphate and sodium dihydrogen phosphate; sodium hydroxide is used to adjust the pH value and participate in the pH buffer pair; Pemulen TR2 is a suspending agent; suspending agent.
[0131] Effect data: the obtained pharmaceutical preparation is recorded as pharmaceutical preparation C, and the obtained pharmaceutical preparation is a viscous solut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com