Composite preparation
A compound preparation and disintegrating agent technology, which is applied in the direction of pill delivery, drug combination, medical preparations of non-active ingredients, etc., can solve the problem that the compound preparation of clopidogrel and aspirin for cardiovascular diseases has not yet been developed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
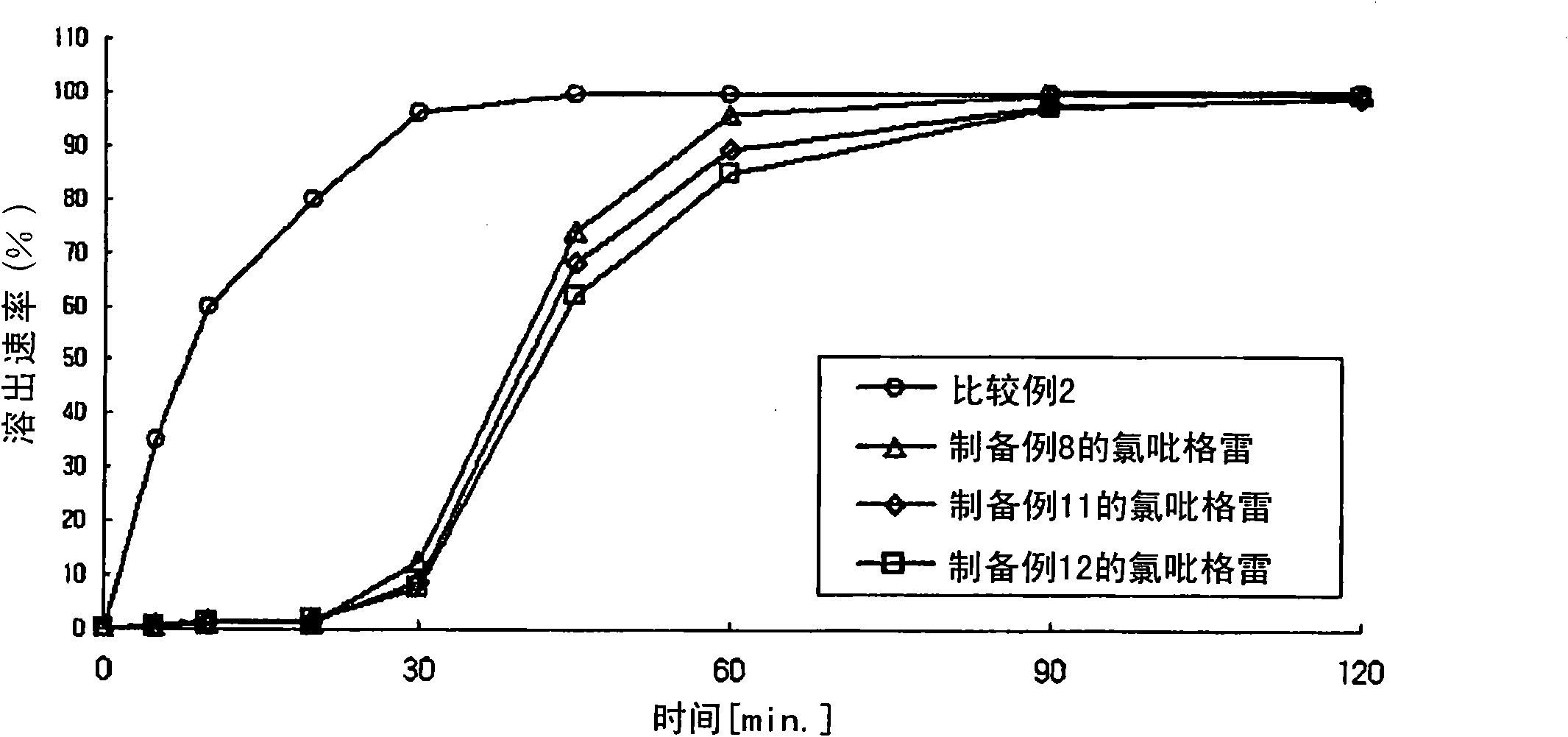
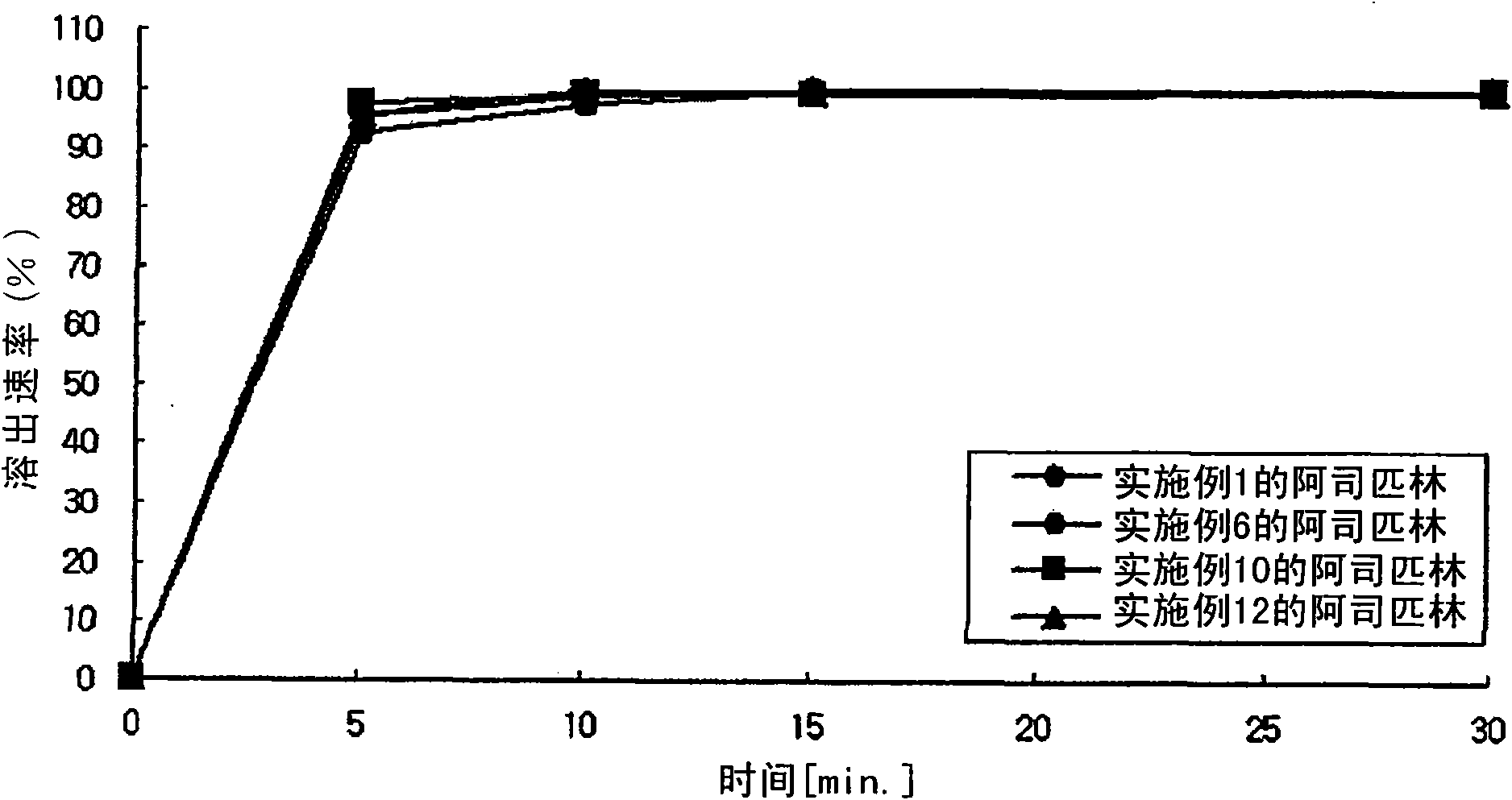
Examples
preparation example 1
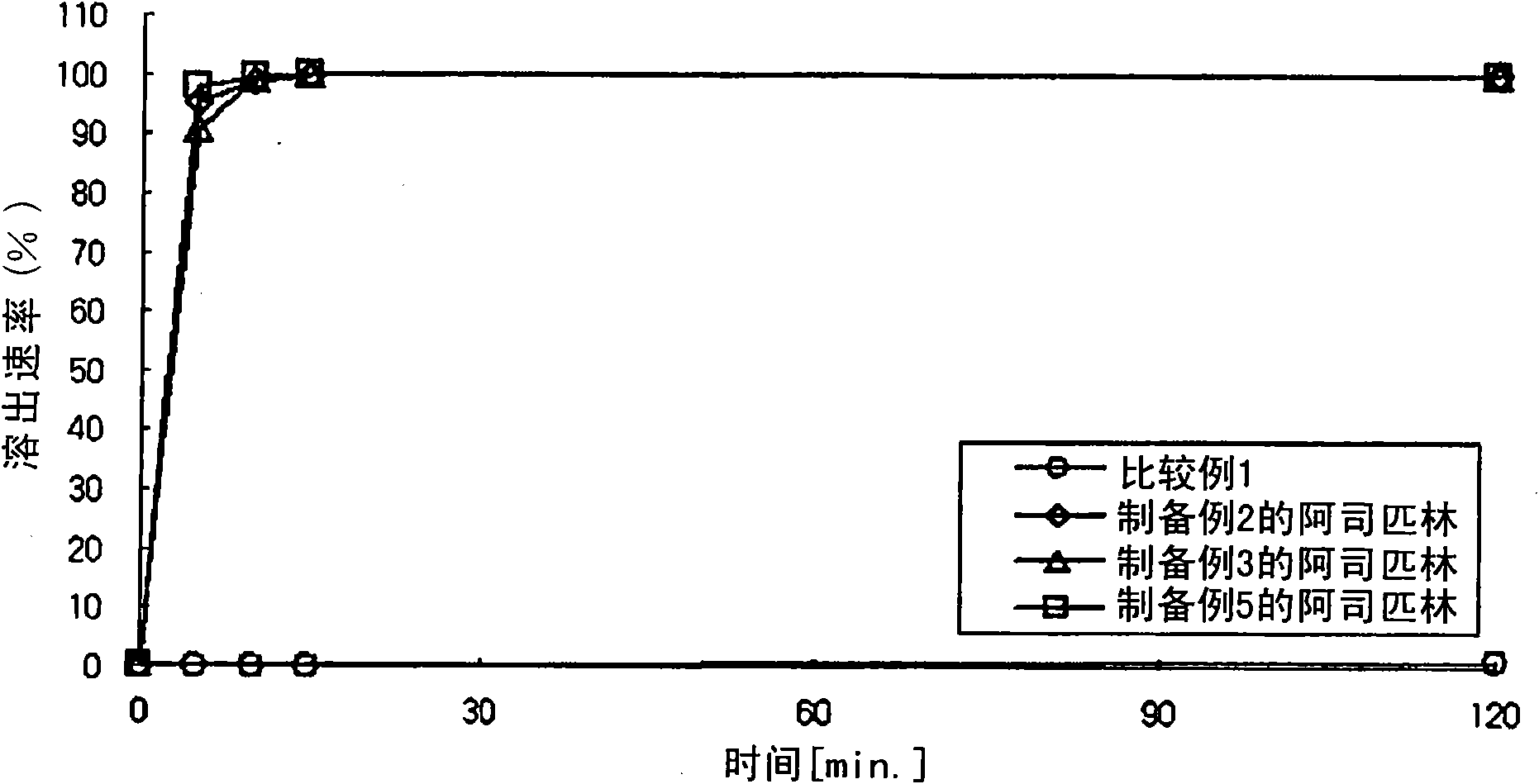
[0131] Preparation Example 1: Preparation of Aspirin-Containing Prior Release Compartment
[0132] According to the composition and content shown in Table 1 below, aspirin, microcrystalline cellulose (Avicel PH101, FMC Biopolymer, USA), pregelatinized starch (Starch 1500G, Colorcon, USA) and colloidal silicon dioxide (Aerosil 200, Degussa , Germany) were mixed in a double cone mixer for 20 minutes to prepare a mixture. Meanwhile, stearic acid (Whawon Pharm. Co. Ltd., South Korea) was sieved through a No. 35 sieve, and then mixed with the above mixture for 4 minutes to obtain aspirin-containing immediate-release granules. The granules were then compressed into tablets using a tablet press (MRC-30, Sejong Machinery Co., Ltd., South Korea). Meanwhile, hydroxypropylmethylcellulose (Shin-Etsu Chemical Co., Ltd., Japan), polyethylene glycol 400 (Duksan Pure Chemical Co., Ltd., South Korea), talc (Whawon Pharm.Co. Ltd., South Korea) and titanium oxide (Whawon Pharm. Co. Ltd., South...
preparation example 2
[0133] Preparation Example 2: Preparation of Aspirin-Containing Prior Release Compartment
[0134] According to the ingredients and contents shown in Table 1 below, aspirin, lactose (DMV, Germany), povidone granules (trade name: Ludipress, BASF, Germany), sodium bicarbonate (Duksan Pure Chemical Co., Ltd., South Korea) and citric acid (Duksan Pure Chemical Co., Ltd., South Korea) were mixed in a double cone mixer for 20 minutes. Meanwhile, stearic acid was sieved through a No. 35 sieve, and then mixed with the above mixture for 4 minutes to obtain aspirin-containing immediate-release granules. The granules were then compressed into tablets using a tablet press (MRC-30, Sejong Machinery Co., Ltd., South Korea). Meanwhile, hydroxypropylmethylcellulose (Shin-Etsu Chemical Co., Ltd., Japan), polyethylene glycol 400, talc, and titanium oxide were dissolved in an ethanol / methylene chloride mixture to prepare a coating solution. The compressed tablets were placed in a coating machi...
preparation example 3
[0135] Preparation Example 3: Preparation of Aspirin-Containing Prior Release Compartment
[0136] Aspirin, magnesium oxide, magnesium carbonate, calcium carbonate and pregelatinized starch were mixed in a double cone mixer for 20 minutes according to the ingredients and contents shown in Table 1 below. Meanwhile, stearic acid was sieved through a No. 35 sieve, and then mixed with the above mixture for 4 minutes to obtain aspirin-containing immediate-release granules. The granules were then compressed into tablets using a tablet press (MRC-30, Sejong Machinery Co., Ltd., South Korea). Meanwhile, hydroxypropylmethylcellulose, polyethylene glycol 400, talc, and titanium oxide were dissolved in an ethanol / dichloromethane mixture to prepare a coating solution. The compressed tablets were placed in a coating machine (SFC-30, Sejong Machinery Co., Ltd., South Korea), and the coating solution was sprayed thereon to prepare film-coated tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com