Etoposide implant
A technology for etoposide and implants, applied in the size, shape, composition, release characteristics and preparation of etoposide implants, etoposide implants and the fields of their preparation, can solve the problem of many prescription components, Complex process and other problems, to achieve the effect of less systemic adverse reactions, simple preparation process and long validity period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1 prepares etoposide implant (A)
[0047]
[0048] Crush the above materials respectively, pass etoposide and polylactic acid through a 120-mesh sieve, and polyethylene glycol pass through a 200-mesh sieve, weigh and mix according to the prescription amount, 5 g in total, mix evenly in a mixer, and oscillate into a glass with an aperture of 0.9 mm. In a 141-hole stainless steel mold, put it in a ZR-II drug pressing machine (developed by Anhui Zhongren Technology Co., Ltd.), press to form at 320Mpa, and demould, to obtain the etoposide implant (A).
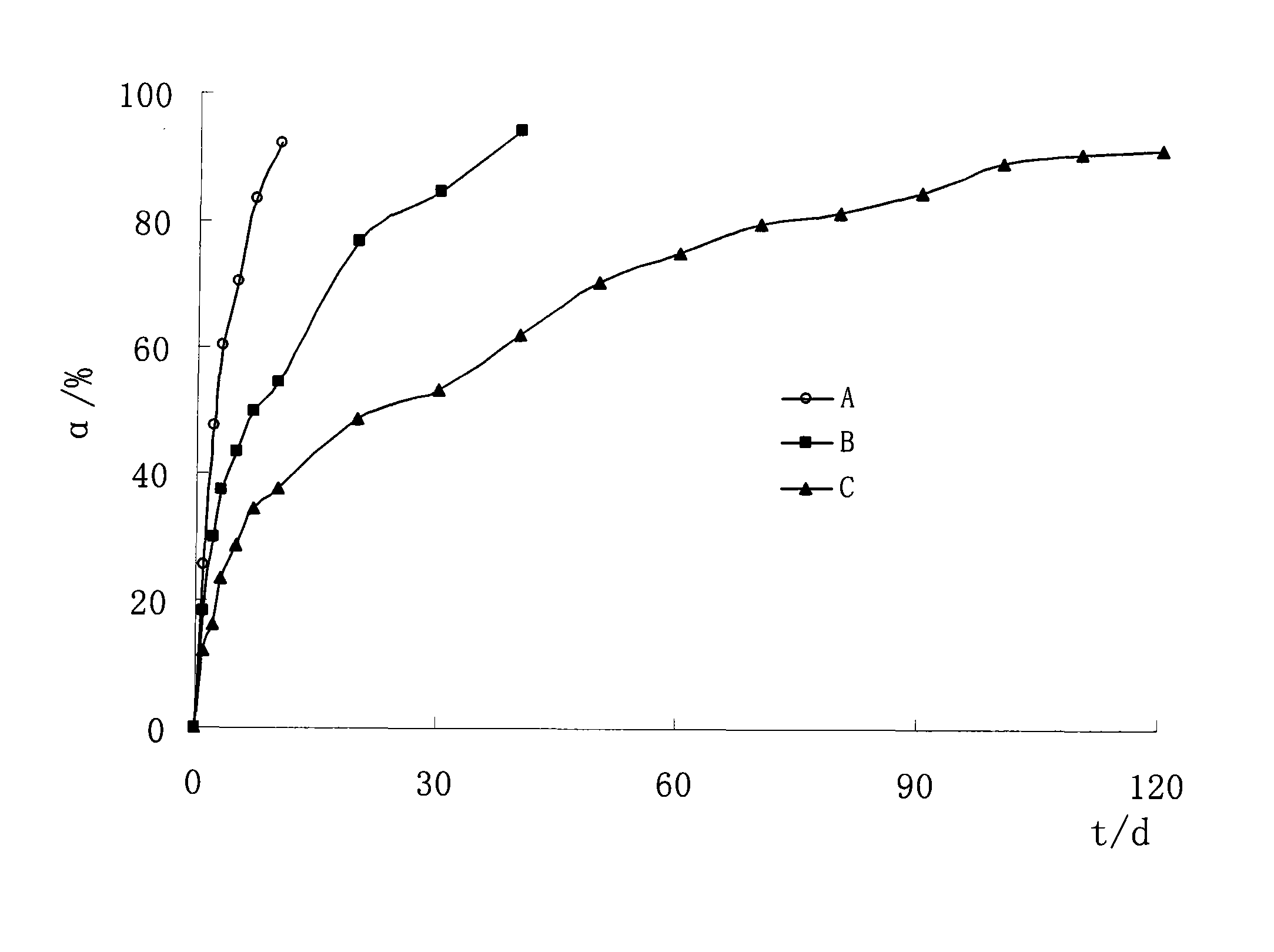
[0049] The implant is easy to demold, has a smooth surface, a diameter of 0.9mm, a length of 1.2mm, a strength of 5.6MPa, and 90% of the drug release time is 10 days, see Example 4 and figure 1 .
Embodiment 2
[0050] Embodiment 2 prepares etoposide implant (B)
[0051]
[0052] Except that the pressure of the medicine pressing machine was 410Mpa, the rest of the preparation process was the same as in Example 1 to obtain the etoposide implant (B).
[0053] The implant is easy to demold, has a smooth surface, a diameter of 0.9mm, a length of 1.9mm, a strength of 9.5MPa, and a 90% release time of 36 days, see Example 4 and figure 1 .
Embodiment 3
[0054] Embodiment 3 prepares etoposide implant (C)
[0055]
[0056] Except that the pressure of the medicine pressing machine was 480Mpa, the rest of the preparation process was the same as in Example 1 to obtain the etoposide implant (C).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com