NGF-Fc fusion protein and preparation method thereof
A fusion protein and immunoglobulin technology, which is applied in the field of highly efficient expression of NGF-Fc fusion protein, can solve the problems of prolonging plasma half-life and failing to enhance protein stability, and achieve the effects of druggability, prolonging half-life and improving expression level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
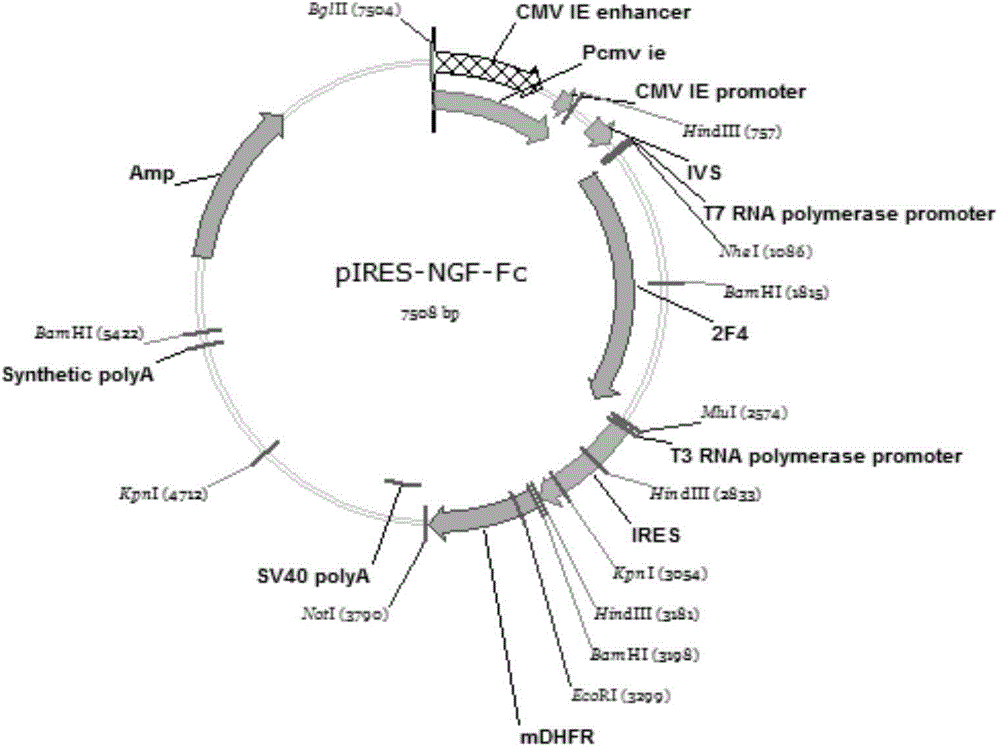
[0038] Example 1 Preparation of NGF-Fc fusion gene
[0039] Preparation of DNA sequence encoding NGF peptide: artificially synthesized DNA sequence encoding NGF peptide, and introduced NheI and BamHI restriction sites at both ends of the DNA sequence. Use NheI and BamHI double enzyme digestion system to carry out enzyme digestion and purification to obtain the NGF fragment with NheI-BamHI sticky end, and carry out double enzyme digestion and purification with NheI and BamHI on the transition plasmid pUC-Fc containing the DNA sequence encoding the Fc peptide. Obtain the DNA carrier fragment with NheI-BamHI cohesive end, connect the NGF fragment and the vector fragment, transform Escherichia coli, obtain the positive clone pUc-NGF-Fc, and confirm it by enzyme digestion and sequencing.
Embodiment 2
[0040] Example 2 Preparation of NGF-Fc fusion gene expression vector
[0041] pUc-NGF-Fc was double digested with NdeI and NotI, the NGF-Fc fragment was recovered from gel sugar, the expression vector was double digested with NdeI and NotI, the large fragment (vector fragment) was recovered from gel sugar, and ligated with T4DNA ligase Carrier DNA fragments and NdeI and NotI fragments, and the ligated products were transformed into Escherichia coli, and positive clones were obtained by selection and identification, and the enzyme digestion identification was correct.
Embodiment 3
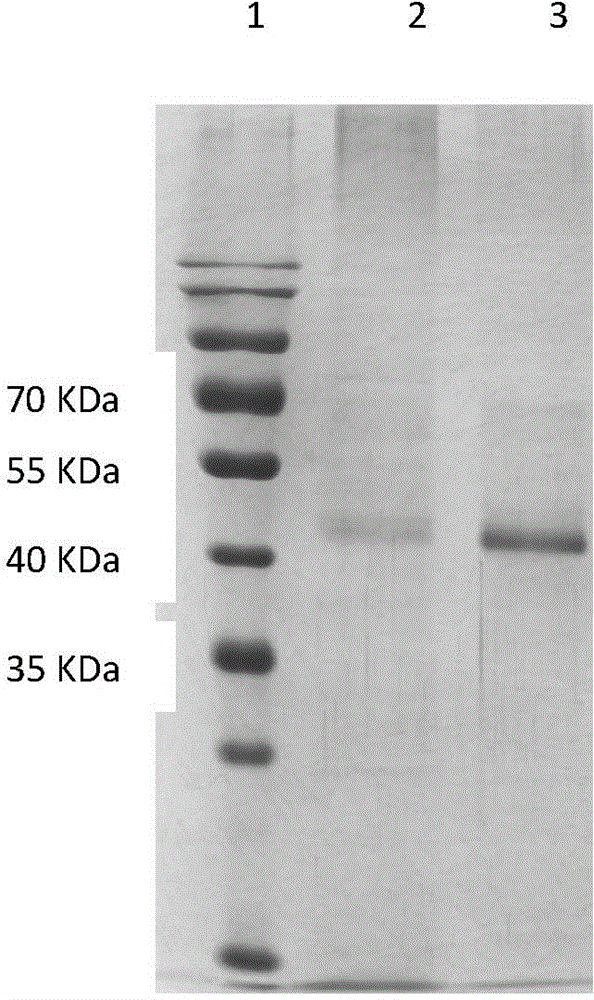
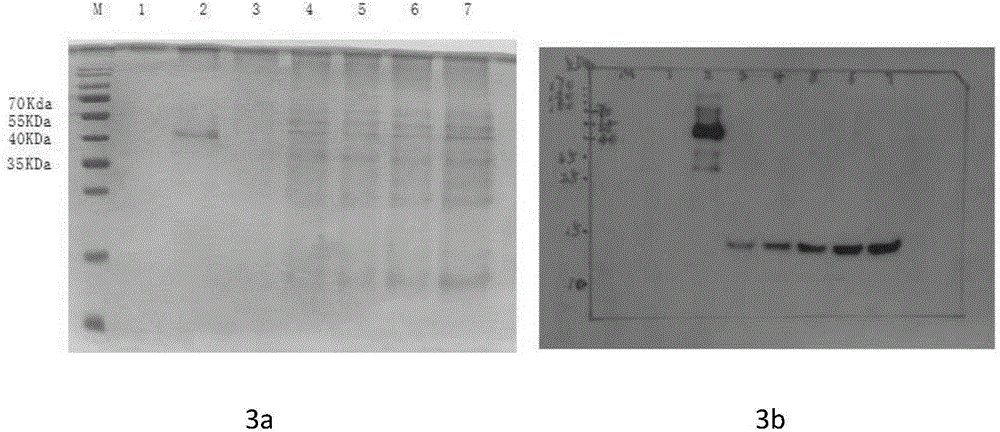
[0042] Embodiment 3 NGF-Fc fusion protein expression and purification
[0043] Transfection of NGF-Fc Fusion Expression Vector into CHO Cells Lipofectamine-mediated CHO Cell Transfection: Culture CHO cells to 40%-60% confluence. Prepare transfection solution: Solution A: Dilute 10 μg DNA to 100 μl with serum-free DMEM medium; Solution B: Dilute 15 μl Lipofectamine with serum-free DMEM medium to 100 μl; Gently mix A and B two solutions, leave at room temperature for 15 minutes, and slowly add In the cell culture dish, shake well, place in a CO2 incubator at 37°C for 24 hours, aspirate the supernatant, and replace with complete medium to continue culturing;
[0044] Screening of positive cells: Aspirate the culture medium, replace it with the screening medium ((10% calf serum DMEM complete medium containing 800 μg PmlG418 containing 800 μg PmlG418) for screening high-expression monoclonal cell lines, and replace it with the screening medium after most of the cells die Maintenan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com