Type-B encephalitis virus antibody titer standard serum and preparation method thereof
A technology of Japanese encephalitis virus and antibody titer, which is applied in the field of Japanese encephalitis virus antibody titer standard serum and its preparation, can solve problems such as unusability, and achieve the effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 The preparation of Japanese encephalitis virus antibody titer standard serum of the present invention
[0038] Vaccine immunization method: take freeze-dried live attenuated Japanese encephalitis vaccine (Chengdu Institute of Biological Products, China Biotechnology Group, batch number: 200612A095-2, 1 serving each) freeze-dried live attenuated Japanese encephalitis vaccine plus sterile water for injection 0.5 After reconstitution in ml, the skin at the attachment point of the deltoid muscle on the outer side of the upper arm was disinfected with alcohol, and 0.5 ml was injected subcutaneously. Primary immunization and booster immunization The interval between primary immunization and booster immunization is 3 months.
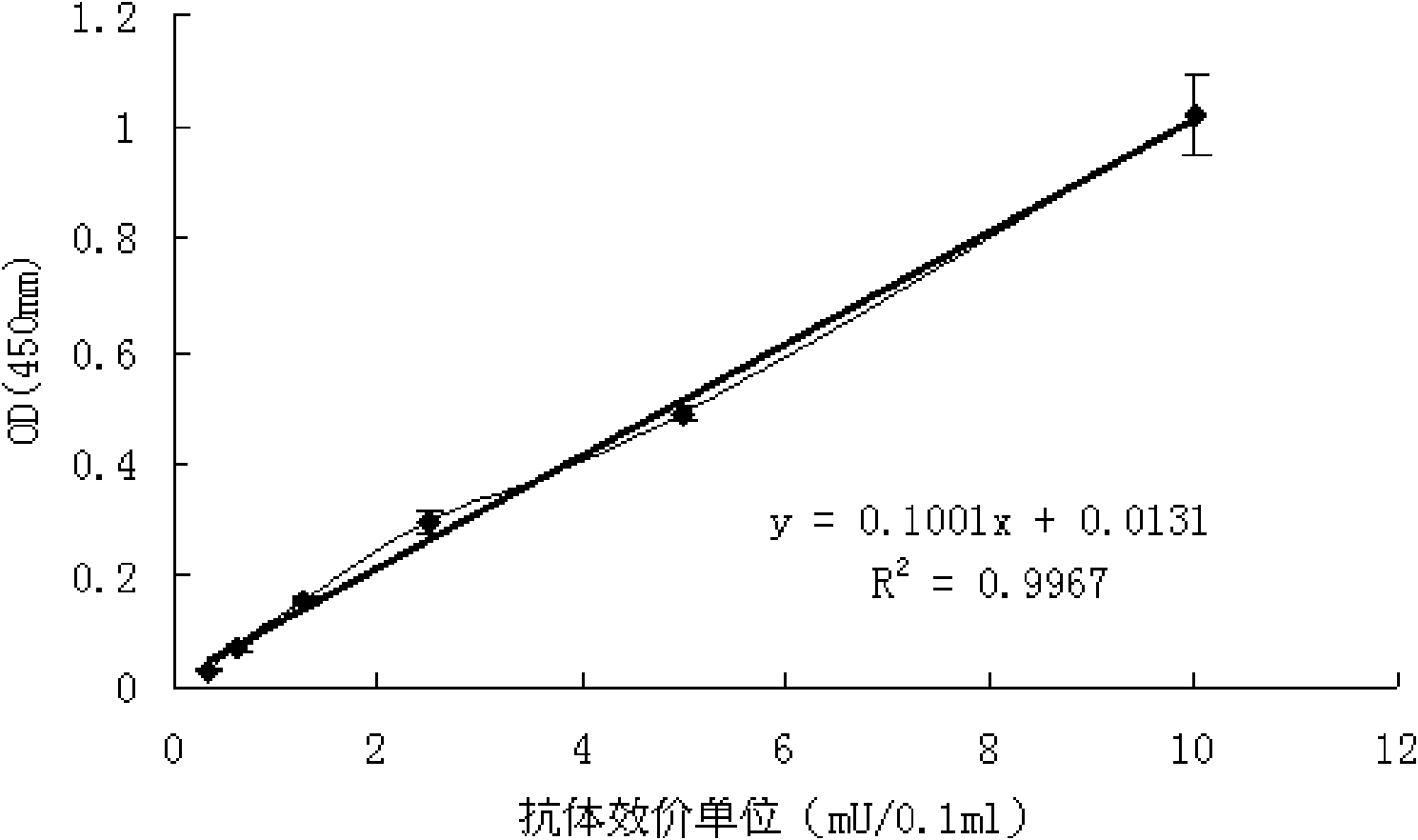
[0039] Standard serum: 14 days after the vaccine booster immunization, venous blood was collected to separate the serum, and the diagnostic kit for IgG antibody to Japanese encephalitis virus was used to detect it. 450nm Value ≥ normal seru...
Embodiment 2
[0042] Example 2 Using the Japanese Encephalitis IgG Antibody Quantitative Diagnostic Kit to Detect Japanese Encephalitis IgG Antibody
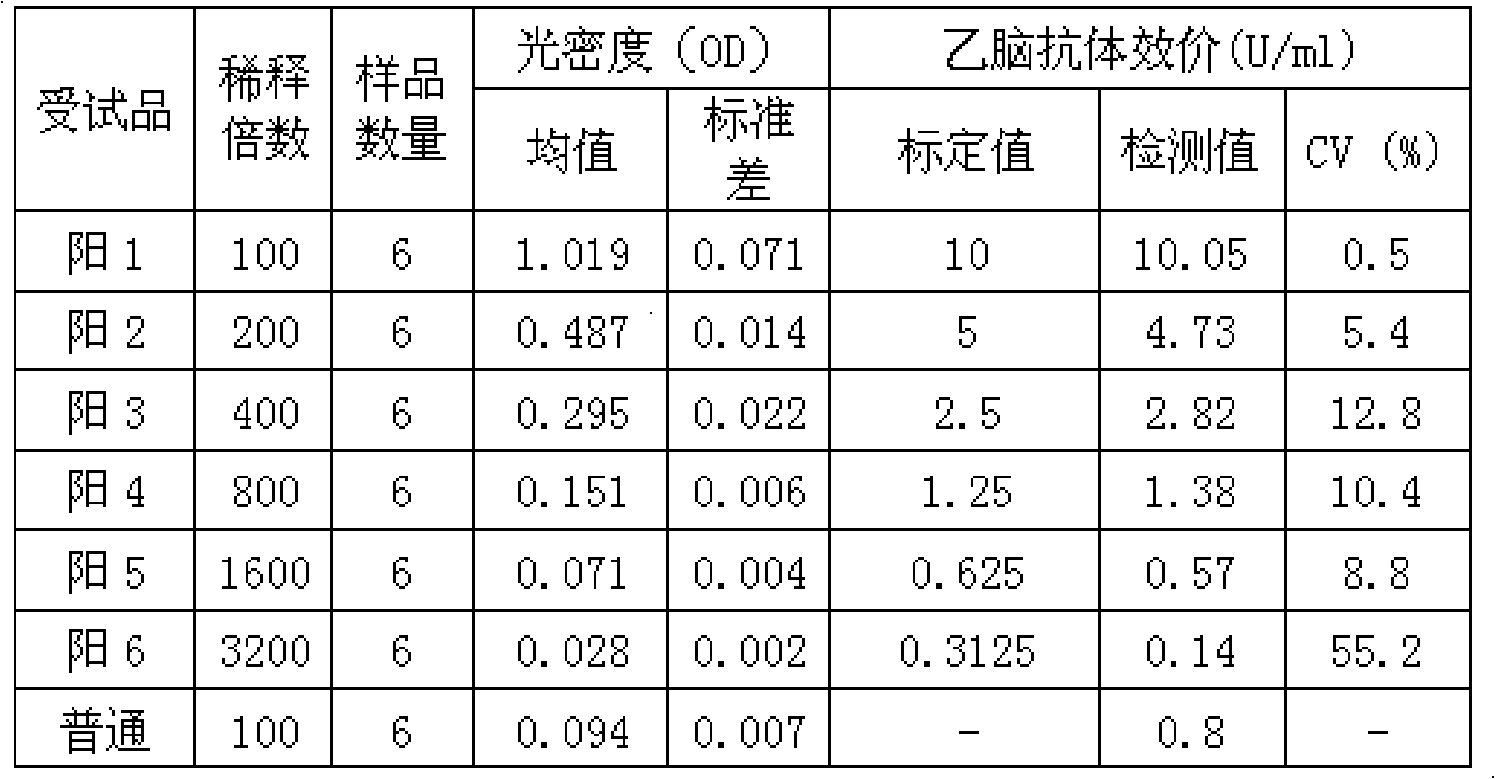
[0043] This example is made by substituting the Japanese encephalitis virus antibody titer standard serum of the present invention for the positive control part in the Japanese encephalitis IgG antibody diagnostic kit with batch number 20080428 produced by Beijing Jinhao Pharmaceutical Co., Ltd. A new Japanese encephalitis IgG antibody quantitative diagnostic kit was developed for detection. The kit consists of: ELISA plate for detecting JE IgG antibody, sample diluent, enzyme conjugate, Japanese encephalitis virus antibody titer standard serum of the present invention as JE IgG antibody positive control, JE IgG antibody negative Control, chromogenic agent A, chromogenic agent B (using TMB chromogenic system).
[0044] The specific detection steps are as follows:
[0045] 1. The sample was diluted 1:100. Reserve a blank well on the enzyme-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com