Method for effectively removing human immune globulin polymer
A human immunoglobulin and multimer technology, applied in the field of biopharmaceuticals, can solve problems such as reducing human immunoglobulin multimers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] 1. After the FII solution is dealcoholized by ultrafiltration, the protein content of the protein solution is adjusted to 4-7g / L, and the pH value of the solution is adjusted to 5.35-5.55.
[0016] 2. Add 1mol / L sodium octanoate to make the final sodium octanoate content reach 6-8mmol / L, add at a speed of 10-15L / h, stir for more than 4 hours after completion, retest the pH value of the solution to 5.35-5.55, otherwise use pH4.0 Acetate buffer was adjusted to this range. The temperature is controlled at 15-25°C.
[0017] 3. Use 7mmol / L sodium octanoate balance solution (containing sodium octanoate 7mmol / L, sodium chloride: 1.25g / L, pH5.35~5.55, temperature 15℃~25.0℃) 400~600L, add diatomaceous earth 4Kg The upper filter plate of the filter press is cleaned cyclically, and after cleaning, it is pressed and filtered, and the outlet temperature is 10°C to 15°C. After pressing the filter, dry the filter plate with pre-cooled clean air for 30-40 minutes. Wash the filter pl...
Embodiment 2
[0022] 1. The pasteurized inactivated protein solution was continuously dialyzed with equal volume of water for injection at 2-8°C to a conductivity of 100μs / cm 2 Below, the protein content is concentrated to 10g / L. Adjust the protein content of the protein solution to 4-7g / L, and adjust the pH value of the solution to 5.35-5.55 with pH4.0 acetate buffer or 0.5mol / L sodium hydroxide.
[0023] 2. The following steps are the same as steps 2, 3, and 4 in Example 1.
[0024] step Example 1 No polymer removal step added Polymer content of protein solution after dealcoholization (%) 9.43% 9.43% Polymer content after press filtration (%) 2.73% / Finished polymer content (%) 2.74% 10.11% Yield of human immunoglobulin (g / ㎏FII) 206 219
Embodiment 3
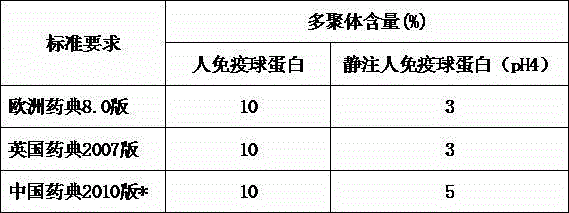
[0026] Place the sample in an environment of 2-8°C, and measure its multimer data. The measurement method adopts "Chinese Pharmacopoeia" Three Volumes 2010 Edition Appendix VI R Human Immunoglobulin Products Monomer Plus Dimer Determination Method, the results are as follows :
[0027] time sample 1 sample 2 0 month 1.63% 2.74% March 1.62% 2.74% June 1.68% 2.76% September 1.71% 2.80% December 1.77% 2.78% 24 months 1.85% 2.79%
[0028] As can be seen from the measurement of the above-mentioned reserved samples, the product obtained by the method of the present invention has stable sample quality and stable multimer content.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com