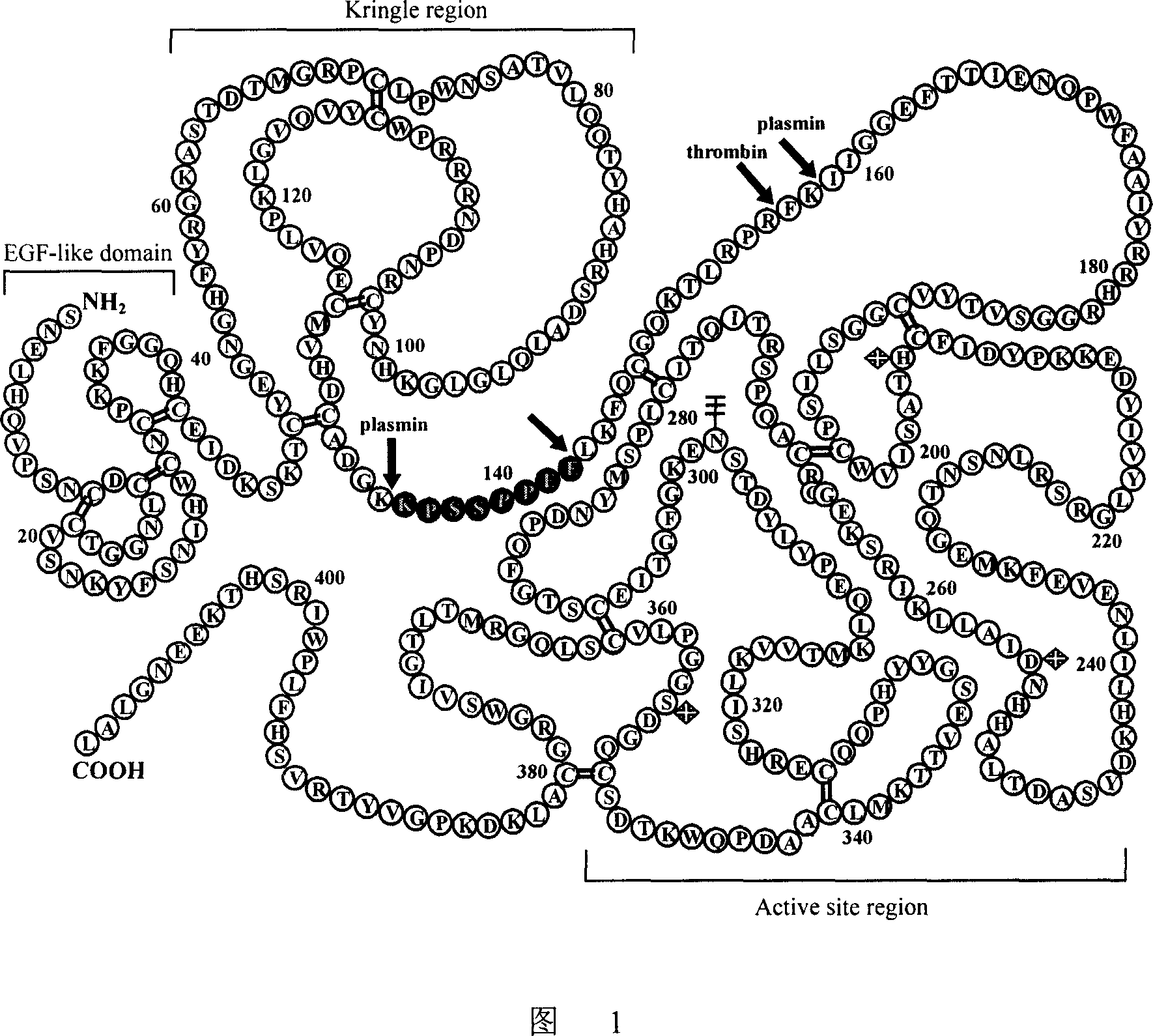
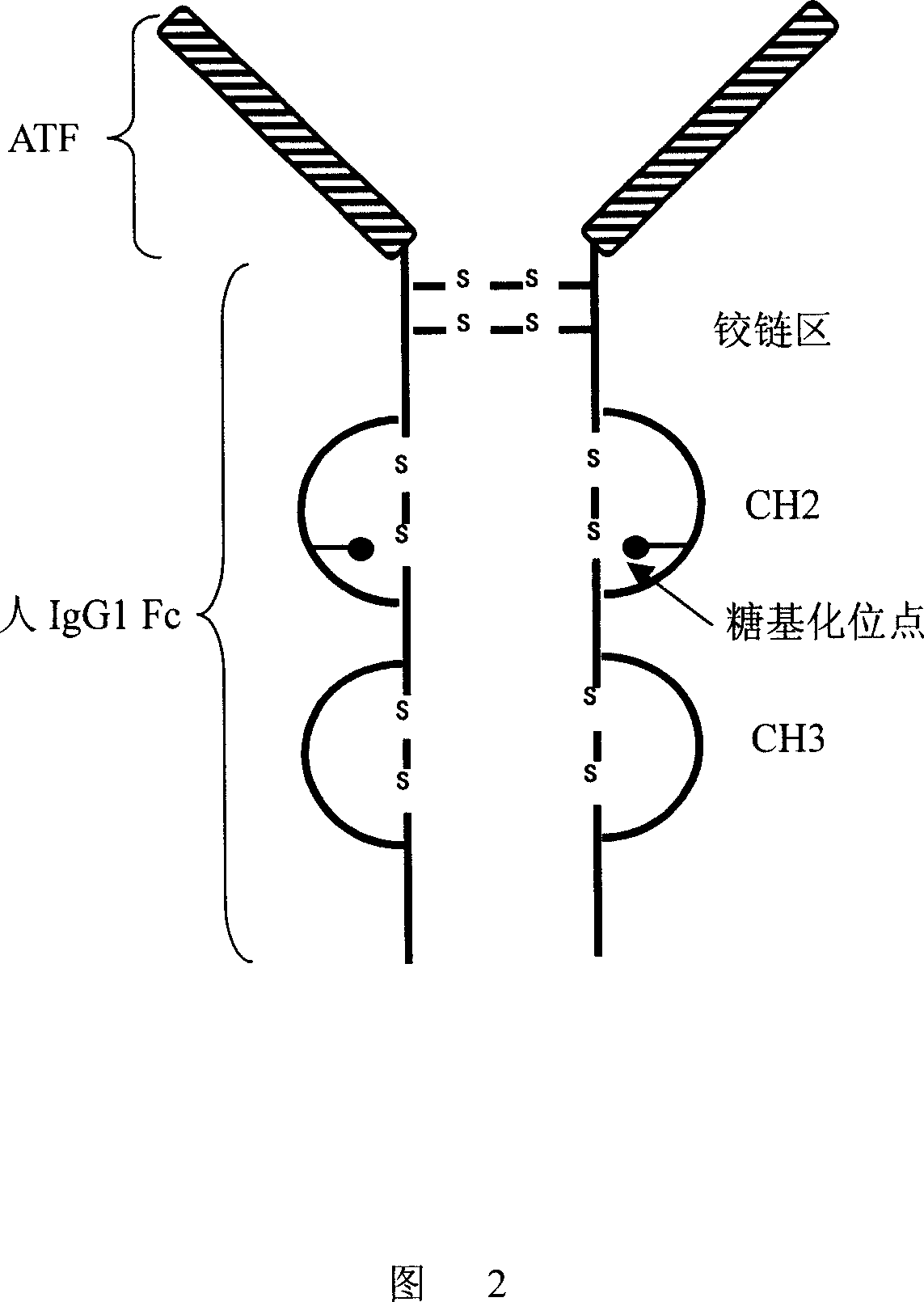
Antibody molecule ATF-Fc fusion albumen of antiurokinase type fibrinolysin activator receptor and use thereof
A technology of fusion proteins and uses, applied in the direction of organic active ingredients, peptide/protein ingredients, medical preparations containing active ingredients, etc., to achieve the effect of long half-life and high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
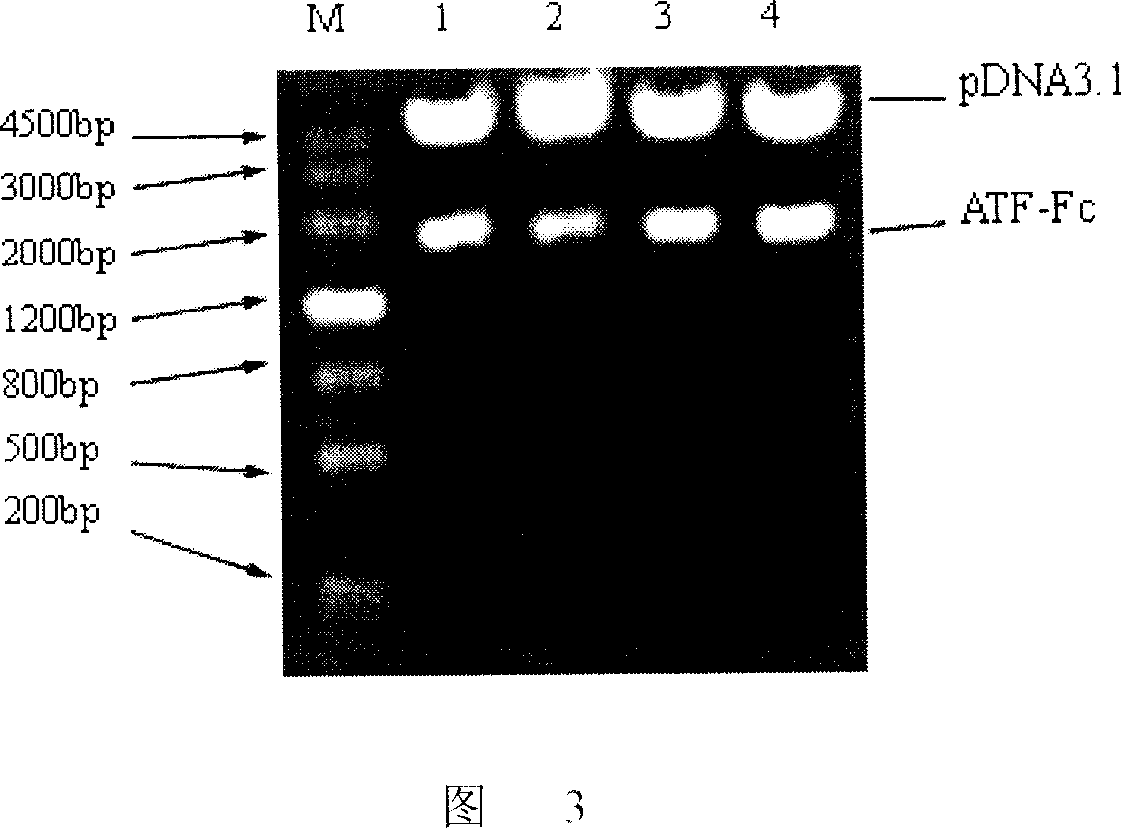
[0051] Cloning of embodiment 1.ATF gene and construction of pcDNA3.1 / ATF-Fc eukaryotic expression vector
[0052] Take the pIRES-dhfr / HMW-ProUK vector as a template (this expression vector is preserved in the General Microorganism Center of China Microbiological Culture Collection Management Committee, the preservation date is February 24, 2006, and the preservation number is CGMCC No.1623, the microorganism of reference The strain is pIRES-dhfr / HMW-ProUK, the proposed classification is named Escherichia coli, and the Chinese invention patent application number is 200610065813.4), using primers ATFs and ATFx as upstream and downstream primers, ATF gene is amplified by PCR, and ATF is recovered Gene.
[0053] ATFs: 5'-TGCGCTAGCCCACCATGAGAGCCCTGCTGGCGCG-3'
[0054] ATFx: 5'-CGCGGATCCACTTACCTGTACTGCCAGATCCGCTTCCATCTGCGCAGTCATGCAC-3'
[0055] The PCR reaction system was: 8 μL dNTP (2.5 mmol / L), 0.5 μL Pyrobest polymerase, 2 μL pIRES-dhfr / HMW-ProUK vector, 10 μL 10×Buffer, 2 μL A...
Embodiment 2
[0057] Example 2. Lipofectamine transfection and cell culture
[0058] Lipofectamine produced by Invitrogen was used for transfection TM2000 cationic liposome transfection kit, operate according to the kit instructions. Transfected CHO-K1 cells were cultured in a 5% CO2, 37°C incubator. After 12 hours of transfection, the DMEM / F12 medium (Hyclone) containing 10% enhanced calf serum (Hyclone) was replaced. After 48 hours, use 750 μg / mL G418 (Sigma) selection medium to pressurize and screen, change the medium every 2-3 days, and after pressurizing for about 12 days, digest the resistant cell clones with 0.25% trypsin, and use the limiting dilution method in Monoclonal culture was carried out in a 96-well microwell cell culture plate (NUNC Company), that is, the cells were diluted to 10 cells / mL with DMEM / F12 medium containing 10% calf serum and 300 μg / mL G418, and each microwell The wells were inoculated with 200 μL of culture medium (each well contained about 2 cells on aver...
Embodiment 3
[0060] Embodiment 3. Purification and SDS-PAGE analysis of expressed protein
[0061] The collected ATF-Fc-H6 serum-free culture supernatant was separated and purified by protein A affinity chromatography column (Pharmacia). Using the specific adsorption of protein A to IgG, the expressed protein was purified by affinity chromatography using nProtein A Sepharose 4 Fast Flow separation medium (Amersham Biosciences). See the product description for the operation method. The bound protein was eluted with pH 2.5, 0.1mol / L glycine-HCl buffer, and there was an obvious elution peak (Figure 4(A)), and the eluted components were eluted with 2mol / L Tris-HCl buffer. The pH of the solution was adjusted to 7. After purification, A was determined by UV spectrophotometer 260 and A 280 Calculate the protein concentration by absorbance, and the formula for protein quantification is: protein content (mg / mL) = 1.45×A 260 -0.74×A 280 . The purity and molecular weight of the protein were de...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com