Immune globulin G4 subtype IgG4 detection kit with consideration to both specificity and sensitivity
A technology for immunoglobulin and detection kits, which is applied in the field of immunoglobulin G4 subtype detection kits, can solve the problems of weakened affinity and insufficient sensitivity, and achieve the effect of simple operation and low false positive rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
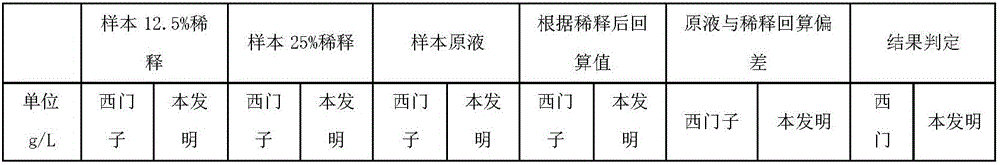
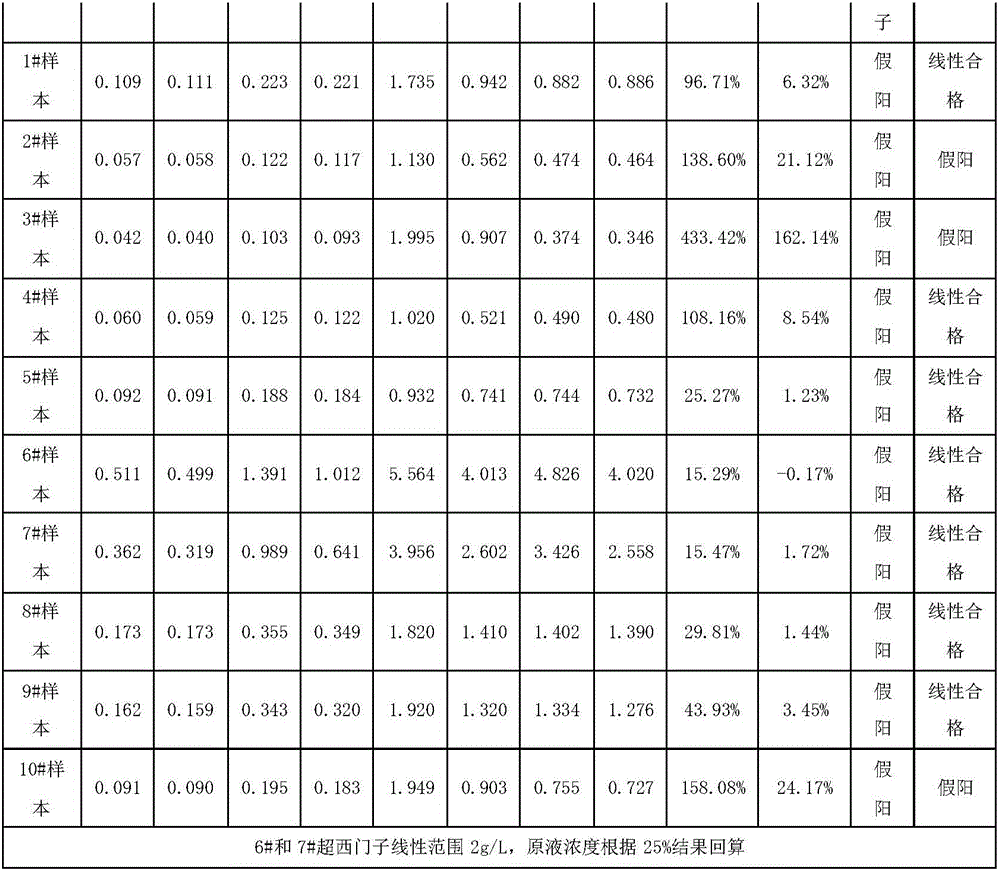
[0035] According to the concentration of each solute, NaCl: 9g / L, glucose: 70g / L, sucrose: 50g / L, sodium azide: 1g / L, use purified water to make a saccharification modification solution, and use the saccharification modification solution to prepare Saquin (product number M9170 , M9124) was diluted to 0.15 mg / mL, stirred at 60 rpm at 25°C for 24 hours; the buffer was replaced, and prepared as a mouse anti-human immunoglobulin G4 (IgG4) monoclonal antibody labeled with glycosylation modifications using conventional chemical coupling Latex microsphere solution was used as the first reagent, and Good's buffer was used as the second reagent. The Siemens IgG4 calibrator was used to draw the standard curve on the automatic biochemical analyzer, and the test was screened by Siemens reagents to obtain false positive interference samples. The results are shown in Table 1 below (Note: According to the requirements of CFDA for in vitro diagnostic kits, most items are considered qualified i...
Embodiment 2
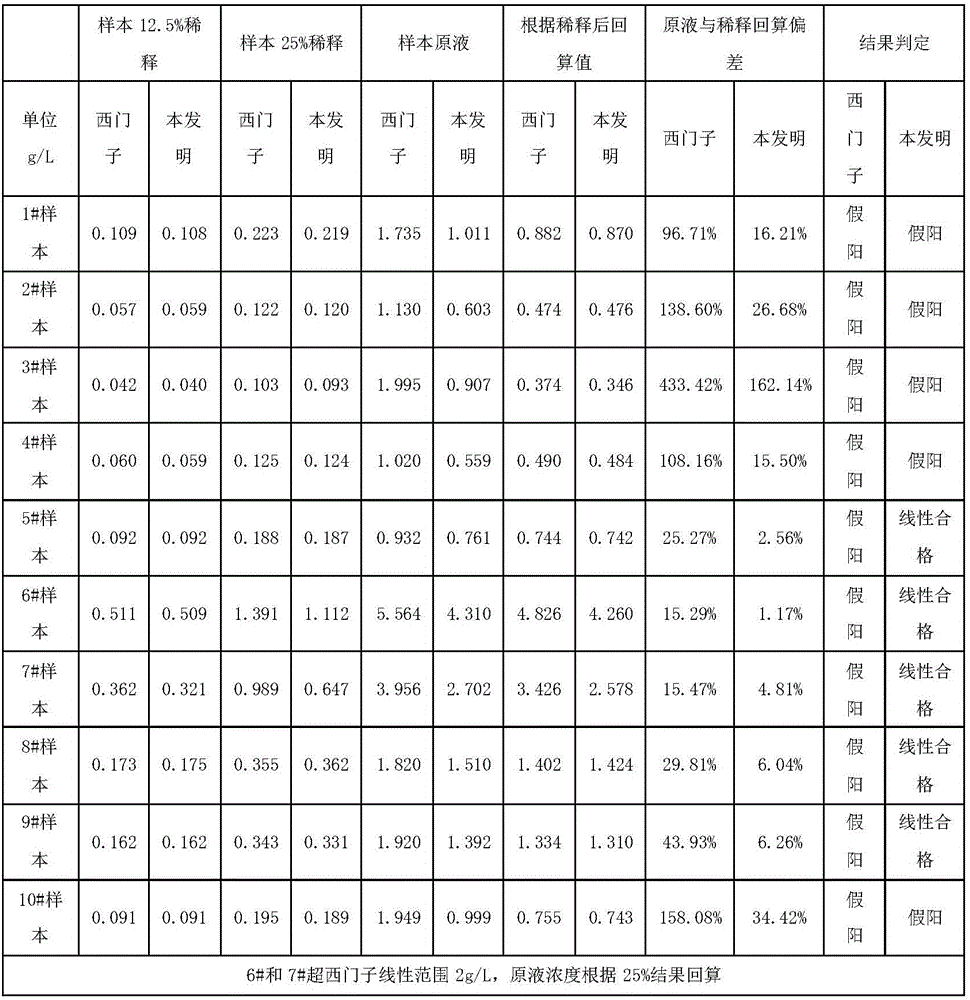
[0041] According to the concentration of each solute NaCl: 9g / L; glucose: 50g / L; sucrose: 30g / L, sodium azide: 1g / L, use purified water to make a saccharification modification solution, and use the saccharification modification solution to prepare Saquin (product number M9170 , M9124) was diluted to 0.40mg / mL, stirred at 30°C and 100rmp for 24 hours; the buffer was replaced, and the mouse anti-human immunoglobulin G4 (IgG4) monoclonal antibody labeled with glycosylation modification was prepared using conventional chemical coupling Latex microsphere solution was used as the first reagent, and phosphate buffer was used as the second reagent. The Siemens IgG4 calibrator was used to draw the standard curve on the automatic biochemical analyzer, and the test was screened by Siemens reagents to obtain false positive interference samples. The results are shown in Table 2 below (Note: According to the requirements of CFDA for in vitro diagnostic kits, most items are qualified if the l...
Embodiment 3
[0046] According to the concentration of each solute NaCl: 9g / L; glucose: 70g / L; sucrose: 50g / L, sodium azide: 1g / L, use purified water to make a saccharification modification solution, and use the saccharification modification solution to prepare Southernbiotech (product number 9190 -01, 9200-01) diluted to 0.15mg / mL, stirred at 100rmp at 25°C for 24 hours; replaced with buffer, prepared as mouse anti-human immunoglobulin G4 (IgG4) labeled with glycosylation modification using conventional chemical coupling The latex microsphere solution of monoclonal antibody was used as the first reagent, glycine buffer was used as the second reagent, and the Siemens IgG4 calibrator was used to draw the standard curve on the automatic biochemical analyzer, and the test was screened by Siemens reagents to obtain false positive interference samples. The results are shown in Table 3 (Note: According to the requirements of CFDA for in vitro diagnostic kits, most items are qualified if the linear...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com