Method for producing intravenous injection human immune globulin
A human immunoglobulin, intravenous injection technology, applied in the field of preparation of human plasma protein, can solve the problems of slow separation speed, difficult operating temperature, low product purity, etc., achieve high recovery rate, high production safety, and fast separation speed Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
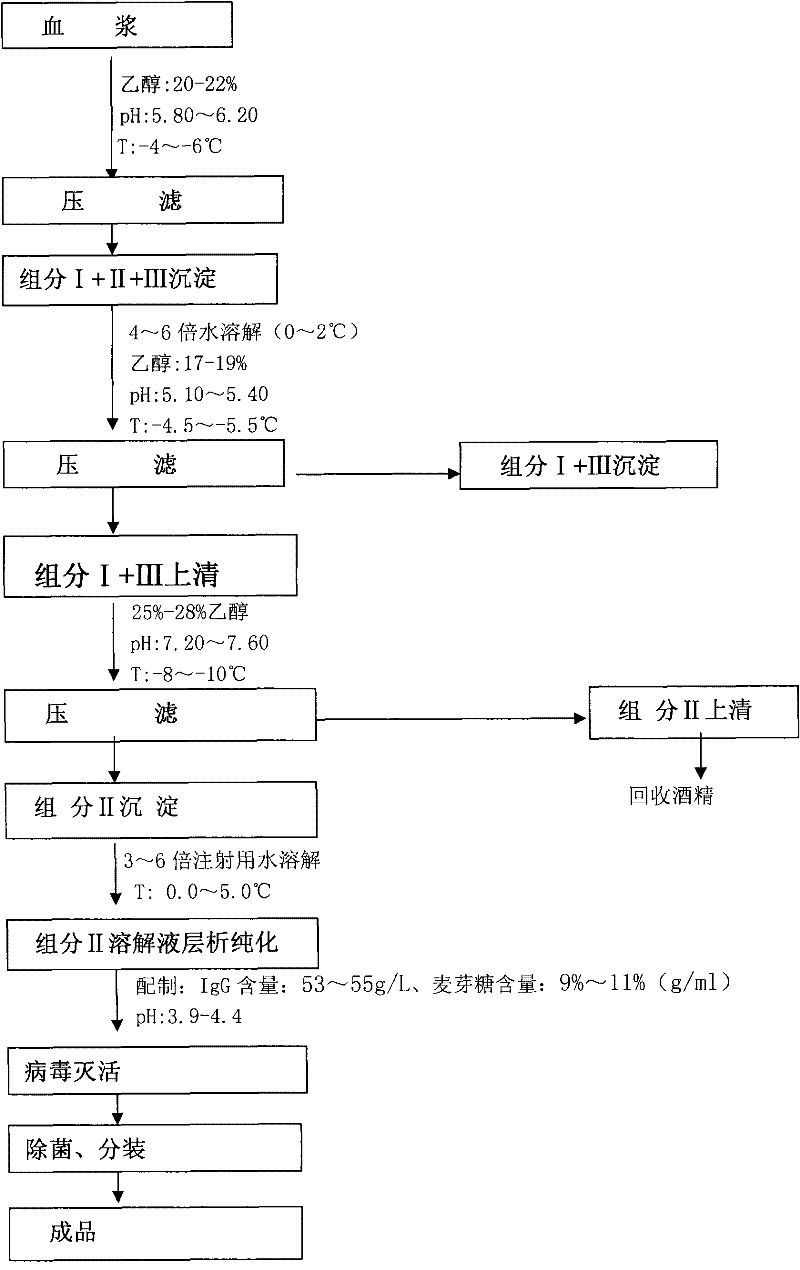
[0013] A. Separation of component I+II+III precipitation from plasma: 1000L of frozen plasma was thawed at 35°C, the plasma temperature was adjusted to 1°C, and the pH value of plasma was adjusted to 5.8 with acetate buffer with pH value of 4.0 , add 95% ethanol to make the final ethanol concentration in the plasma 20%, adjust the temperature of the reaction solution to -4°C, add 1% (g / ml) diatomaceous earth as a filter aid, press The filter is pressurized and filtered, and the filtered clear liquid is put into another reaction tank. When the filtration is completed, the filter press is dried with compressed air, the filter press is opened, and the components I+II+III are collected for precipitation.
[0014] B. Precipitation and separation of component I+III from component I+II+III: Dissolve the precipitation of component I+II+III in 4 times the amount of water for injection pre-cooled at 0°C, stir for more than 3 hours, and cool the reaction solution to a temperature of 0°C ...
Embodiment 2
[0021] A. Separation of fraction I+II+III precipitation from plasma: 1000L of frozen plasma was thawed at 32°C, the plasma temperature was adjusted to 2°C, and the pH value of the plasma was adjusted to 6.0 with acetate buffer with a pH value of 4.0 , add 95% ethanol to make the final ethanol concentration in the plasma 21%, adjust the temperature of the reaction solution to -5°C, add 1.5% (g / ml) diatomaceous earth as a filter aid, press The filter is pressurized and filtered, and the filtered clear liquid is put into another reaction tank. When the filtration is completed, the filter press is dried with compressed air, the filter press is opened, and the components I+II+III are collected for precipitation.
[0022] B. Precipitate and separate component I+III from component I+II+III: dissolve the precipitation of component I+II+III in 5 times the amount of water for injection pre-cooled at 1°C, stir for more than 3 hours, and cool the reaction solution to a temperature of 1°C ...
Embodiment 3
[0029] A. Separation of component I+II+III precipitation from plasma: 1000L of frozen plasma was thawed at 30°C, the plasma temperature was adjusted to 4°C, and the pH value of plasma was adjusted to 6.2 with acetate buffer with pH value of 4.0 , add 95% ethanol to make the final ethanol concentration in the plasma 22%, adjust the temperature of the reaction solution to -6°C, add 2.0% (g / ml) diatomaceous earth as a filter aid, press The filter is pressurized and filtered, and the filtered clear liquid is put into another reaction tank. When the filtration is completed, the filter press is dried with compressed air, the filter press is opened, and the components I+II+III are collected for precipitation.
[0030] B. Precipitate and separate component I+III from component I+II+III: dissolve the precipitation of component I+II+III in 6 times the amount of water for injection pre-cooled at 2°C, stir for more than 3 hours, and cool the reaction solution to a temperature of 2°C and s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com