Method of preparing albumin from a solution comprising albumin and a method for inactivating viruses using caprylate in solutions containing albumin
A technology of albumin and octanoate, which is applied in the field of preparing albumin from albumin-containing solution and using octanoate in albumin-containing solution to inactivate viruses, which can solve the problems of cost, explosion and fire
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] human albumin
[0051] Suspend Cohn Fraction V in cold water for injection. Alternatively, effluent IV-1 can be used instead of fraction V. Add cold denatured ethanol (SDA-3A) to obtain an approximately 10% alcohol solution while cooling the solution to a temperature of approximately 0 °C. The solution was mixed for about 1 hour at about 0°C, then clarified by depth filtration.
[0052] The albumin fraction was then precipitated from the flow-through IV-1 or fraction V by adjusting the pH, adding cold denatured ethanol (SDA-3A), and incubating the solution at low temperature. After incubation at low temperature, the albumin fraction was isolated by centrifugation.
[0053] Acetone inactivates virus
[0054] The albumin fraction was then suspended in cold acetone and kept at about 0°C for several minutes. The protein was separated from the slurry and rinsed with cold acetone. Dry the wet albumin powder by passing dry nitrogen and / or air through the powder.
[0055...
Embodiment 2
[0061] Virus inactivation experiment
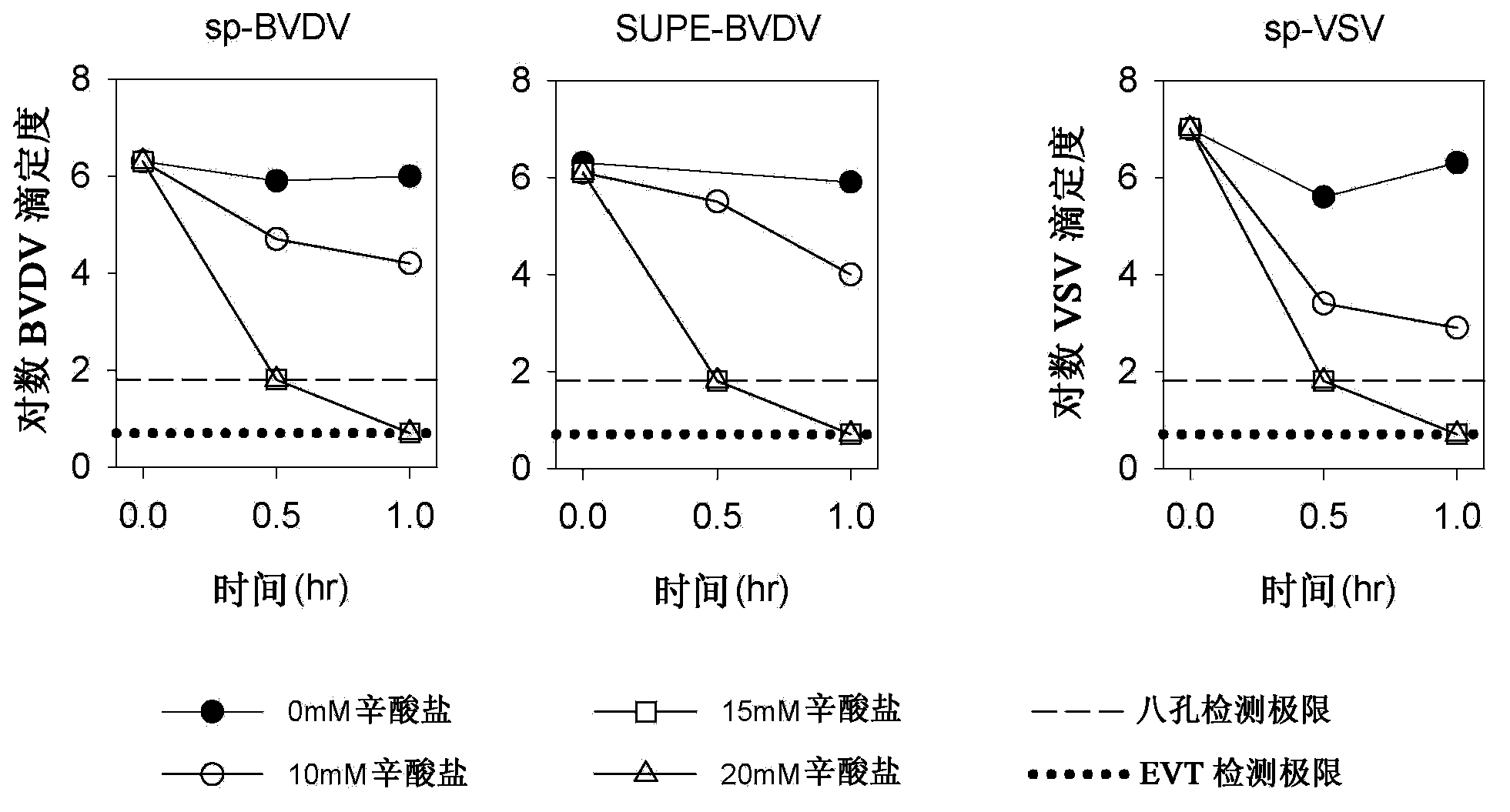
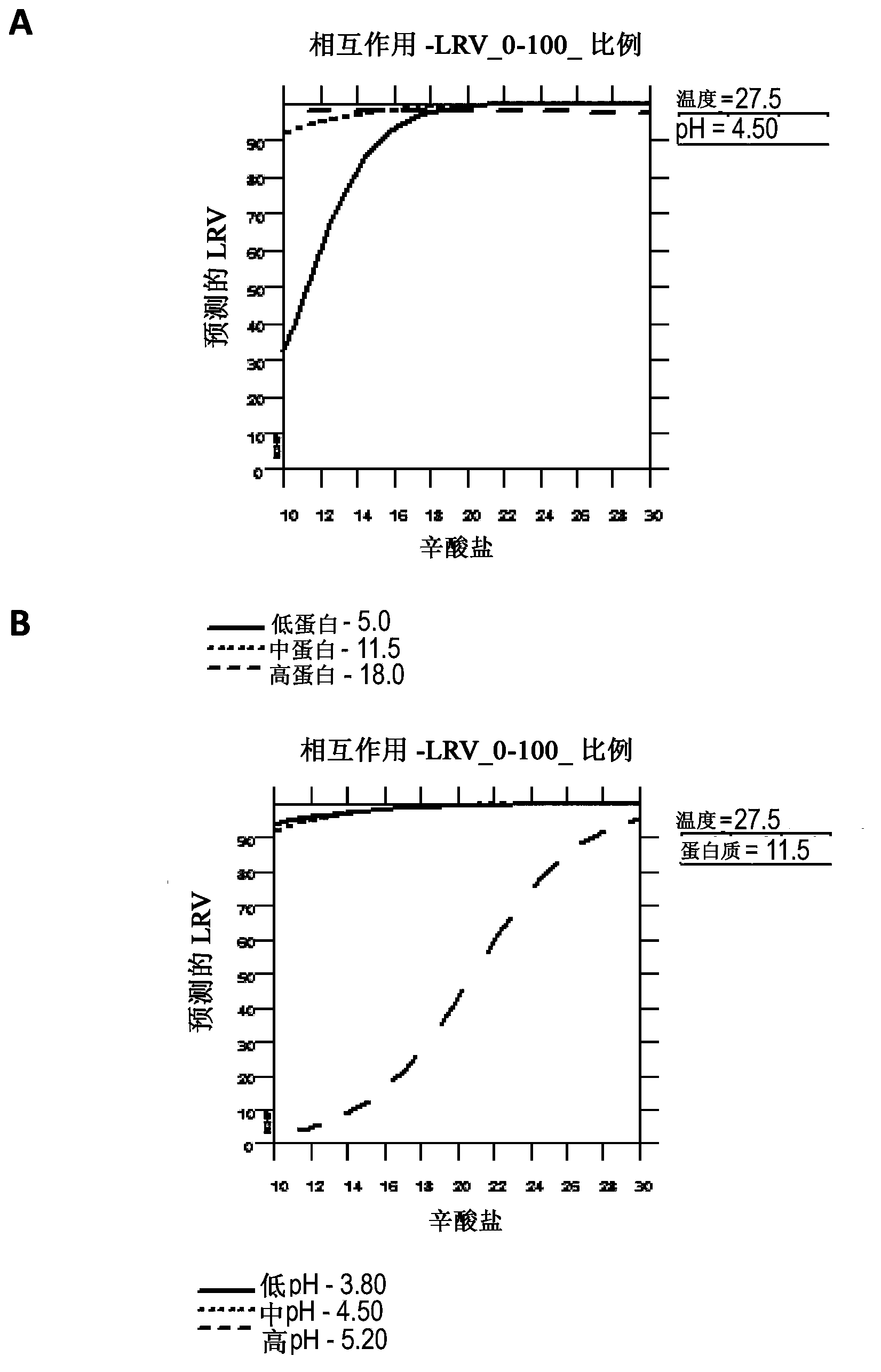
[0062] Acetone treatment of albumin slurries is a very effective enveloped virus inactivation step, but this step is a bottleneck in the process and is also associated with numerous cleaning and safety issues (fires). Therefore, caprylate treatment may replace acetone suspension and drying. Experiments were performed to evaluate the inactivation of enveloped viruses by treatment of albumin serum suspensions with octanoate. For this study, albumin slurry suspensions with a protein concentration of 25 AU were spiked with bovine viral diarrhea virus (BVDV) or vesicular stomatitis virus (VSV) and incubated at 27°C, pH 5.1, with 0 mM, 10 mM, 15 mM, or 20 mM octanoate was incubated for 1 hour. The data showed that the virus was inactivated to the limit of detection following treatment with 15 mM octanoate. See Table 1 and figure 1 .
[0063]
[0064]
Embodiment 3
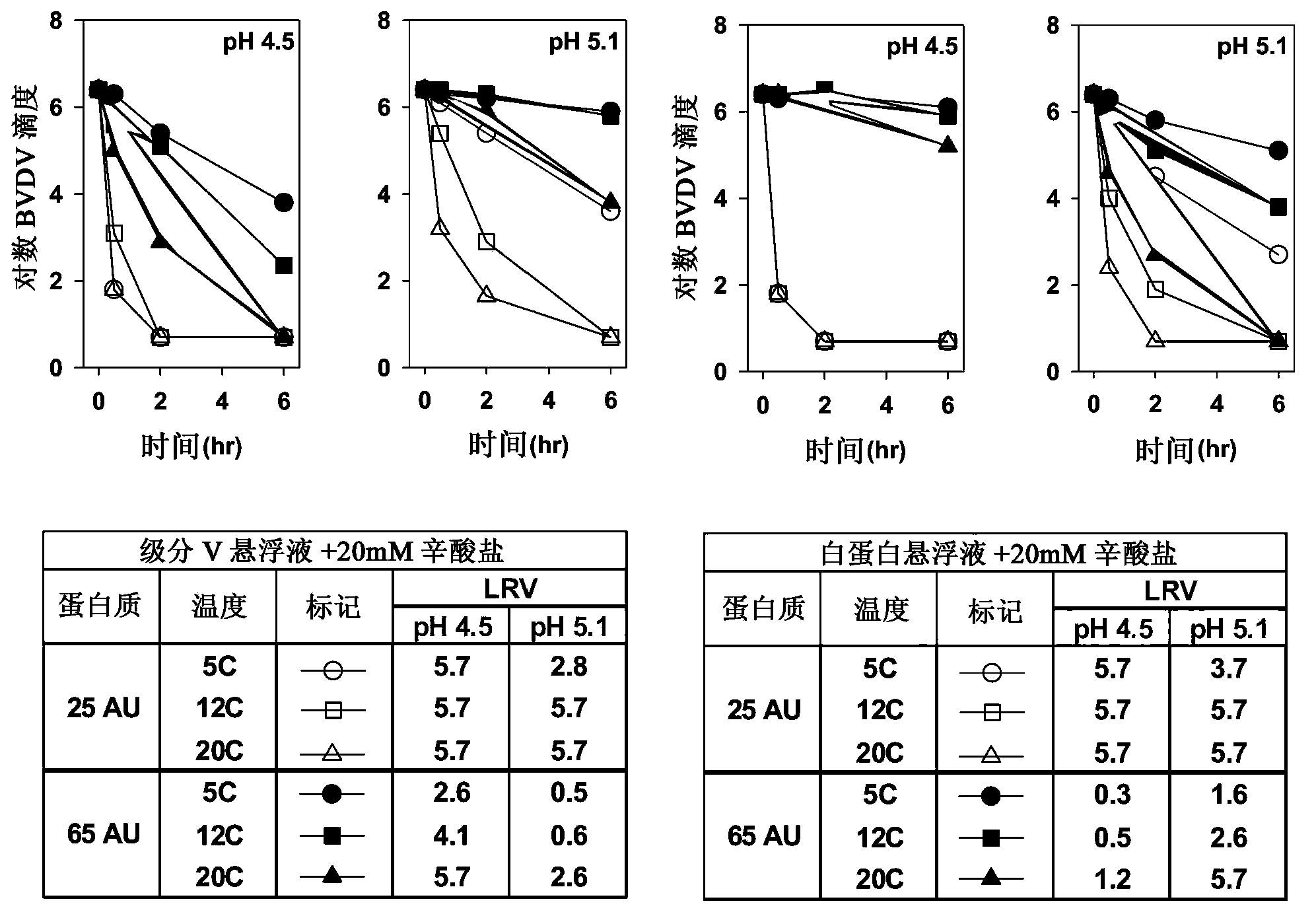
[0066] Experiments were performed at low temperature to evaluate the virucidal ability of caprylate. Albumin and Fraction V serum suspensions were diluted to absorbance units (AU) of 25 and 65, and sodium caprylate was added to a final concentration of 20 mM. The solution was adjusted to pH 4.5 or 5.1 and the pH adjusted BVDV was spiked prior to incubation at 5°C, 12°C, or 20°C. Pipette for titration, pH, and / or protein concentration (i.e., A 280 )sample.
[0067] Virus inactivation by caprylate was greater at higher temperatures and lower protein concentrations for both albumin and Fraction V serum suspensions. see figure 2 . Virus inactivation was higher at pH 4.5 than at pH 5.1 for Fraction V suspensions at 25 AU and 65 AU. There was also a higher virus inactivation at pH 4.5 for the albumin suspension at 25 AU. However, virus inactivation was higher at pH 5.1 than at pH 4.5 for the albumin suspension at 65 AU. Since the mechanism of virus inactivation by caprylate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com