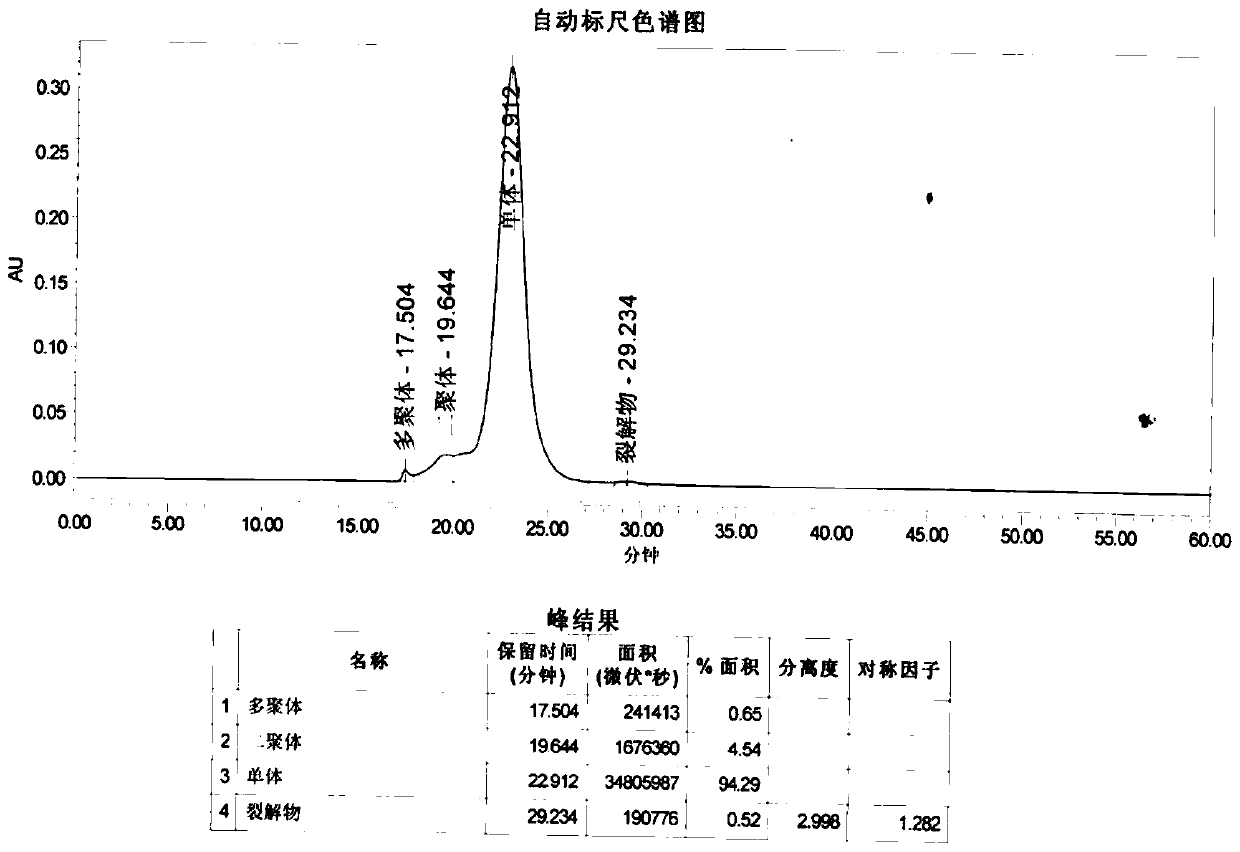
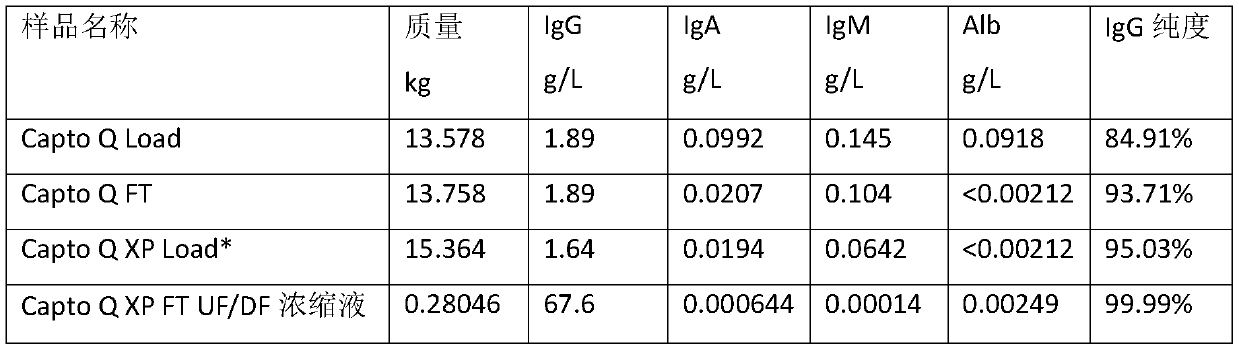
Method for extracting intravenous injection human immunoglobulin from plasma separation component I and plasma separation component III
A technology for separation of human immunoglobulin and plasma, applied in the field of extraction of human immunoglobulin for intravenous injection, which can solve the problems of unstable components and difficult to remove
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1, Sodium Octanoate Precipitation F Ⅰ+Ⅲ Removal of Miscellaneous Protein Technology Research
[0031] The purpose of this step is to optimize the octanoic acid precipitation conditions: not only meet the requirement of removing the impurity proteins in F Ⅰ+III as much as possible, but also meet the requirement of high IgG content when loading on the column.
[0032] Experimental reagents and equipment:
[0033] 1. Reagent: 100ml of 1M sodium octanoate mother liquor; half-diluted hydrochloric acid; F Ⅰ + Ⅲ precipitation.
[0034] 2. Equipment: 6 200ml and 500ml beakers each; 36 50ml molded bottles; 2 glass rods; 1 PH meter; 1 balance; 6 magnetic stirrers, 1 electric bacteria collector; tower.
[0035] Experimental program:
[0036] 1. Dissolving amount of F Ⅰ+Ⅲ precipitate:
[0037] (1) Dissolving amount of normal sodium caprylate precipitate 5g F Ⅰ+III precipitate is dissolved in 200ml water for injection;
[0038] (2) Dissolve 20g of F Ⅰ+III precipitate in...
Embodiment 2
[0053] Embodiment 2, ion exchange chromatography
[0054] Equipment used: AKTA Purifier 100
[0055] Software: Unicorn 5.3
[0056] Reagents and solutions:
[0057] Buffer A: 200mM NaAC-HAC buffer, pH5.4;
[0058] Buffer B: 10mM NaAC-HAC buffer, pH5.4;
[0059] Buffer D: 200mM NaAC-HAC buffer, pH6.0;
[0060] Buffer E: 10mM NaAC-HAC buffer, pH6.0;
[0061] Buffer C: 1M NaOH;
[0062] 2M acetic acid;
[0063] 0.5M NaOH;
[0064] 1, pretreatment (carry out according to embodiment 1 optimization result)
[0065] (1) Dissolution of F Ⅰ+III precipitate: F Ⅰ+III precipitate was dissolved in water for injection at 20% (wt).
[0066] (2) Dosage of sodium octanoate: 50mM.
[0067] (3) Adjust pH: 4.9.
[0068] 2. Precipitation process:
[0069](1) Completely stir and dissolve the F Ⅰ+III precipitate with water for injection at room temperature according to the set amount, then slowly add 1M octanoic acid to the set concentration while stirring;
[0070] (2) After stirring f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com