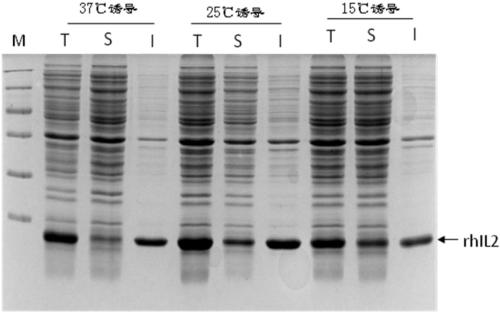
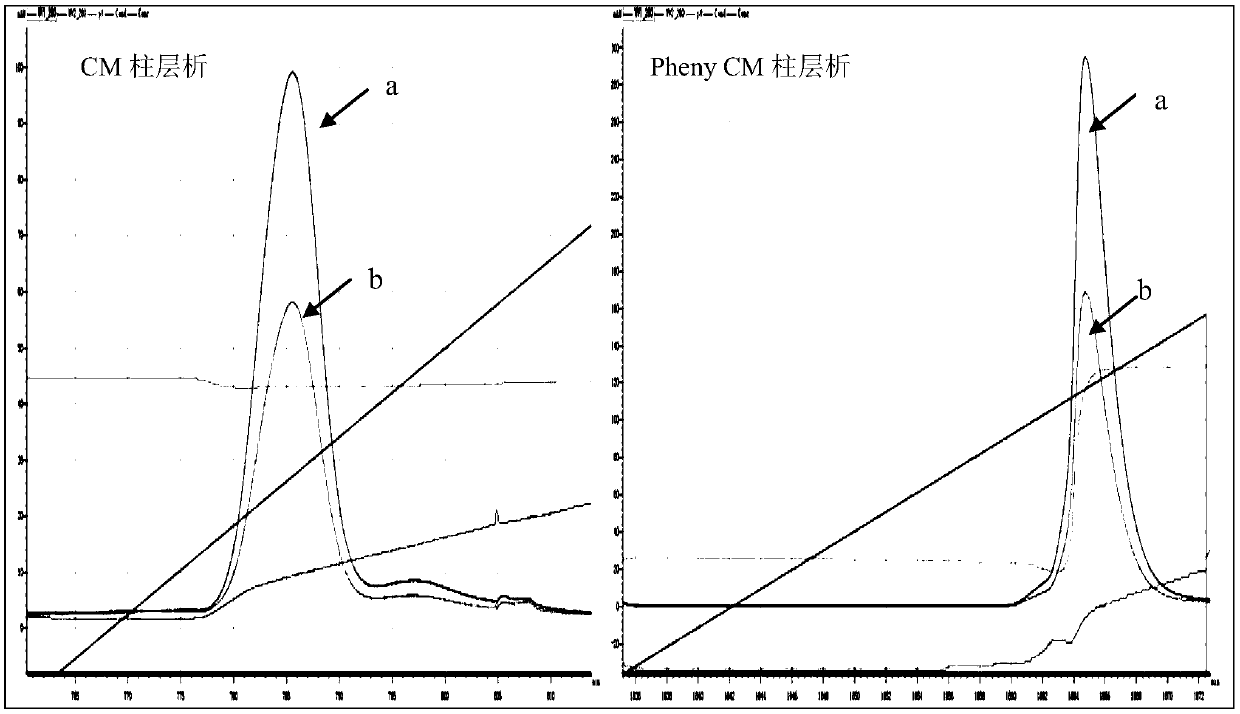
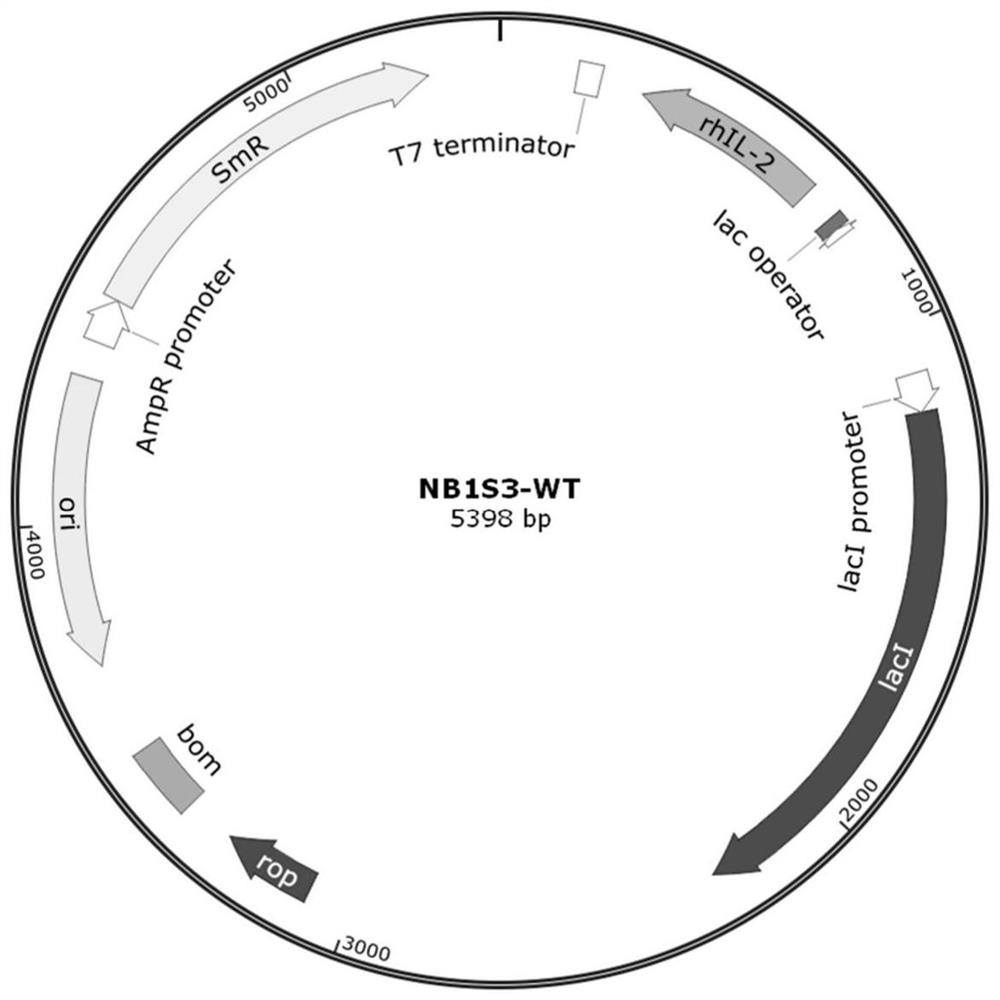
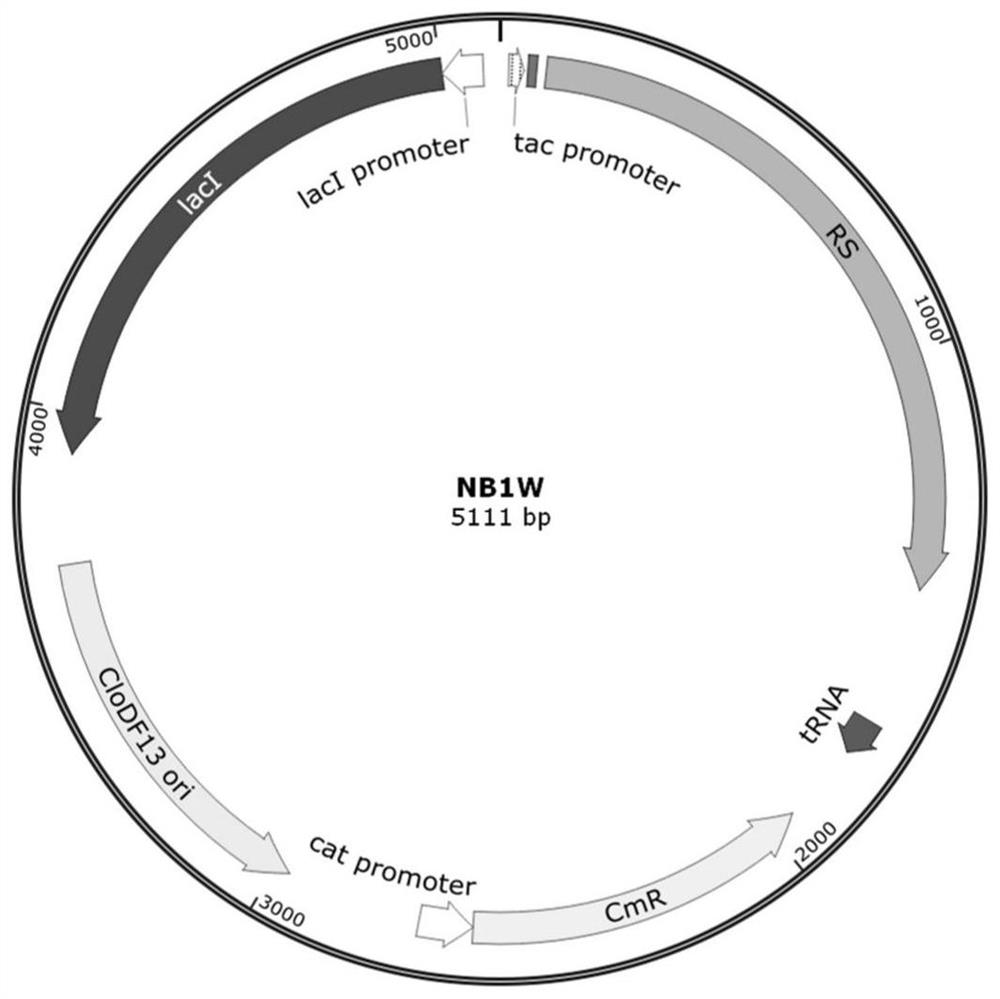
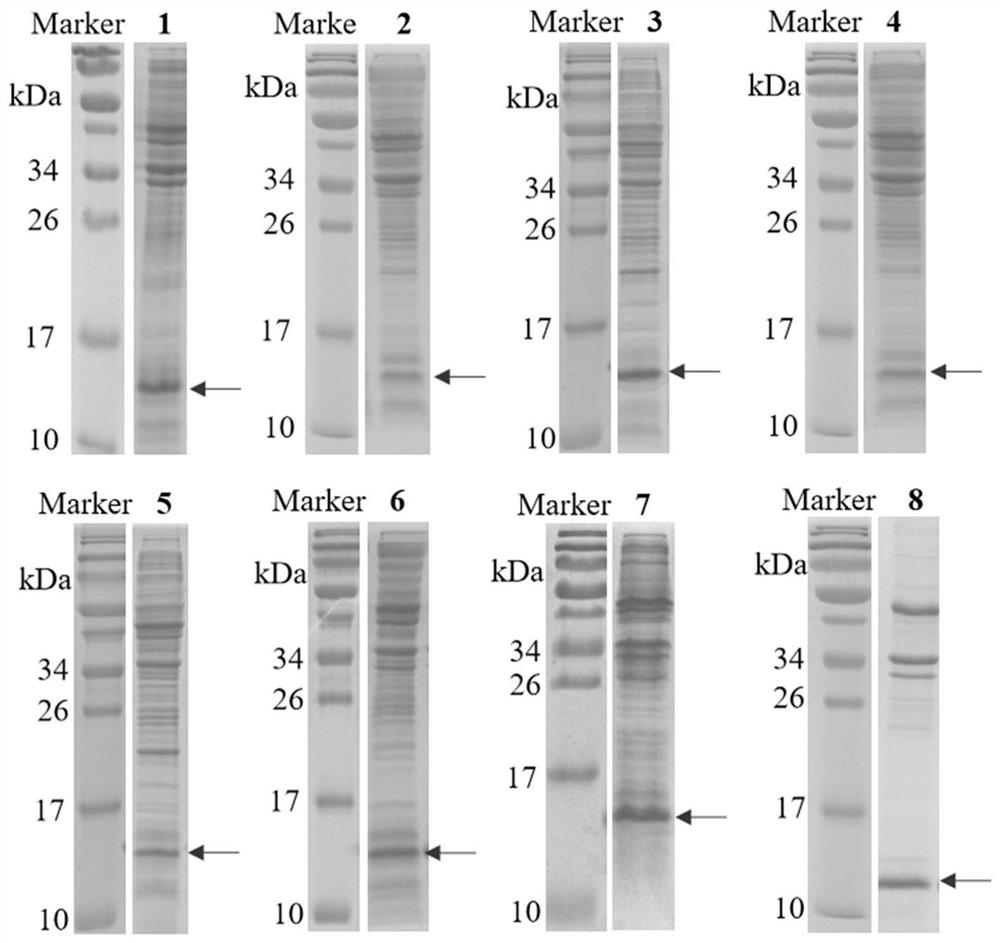
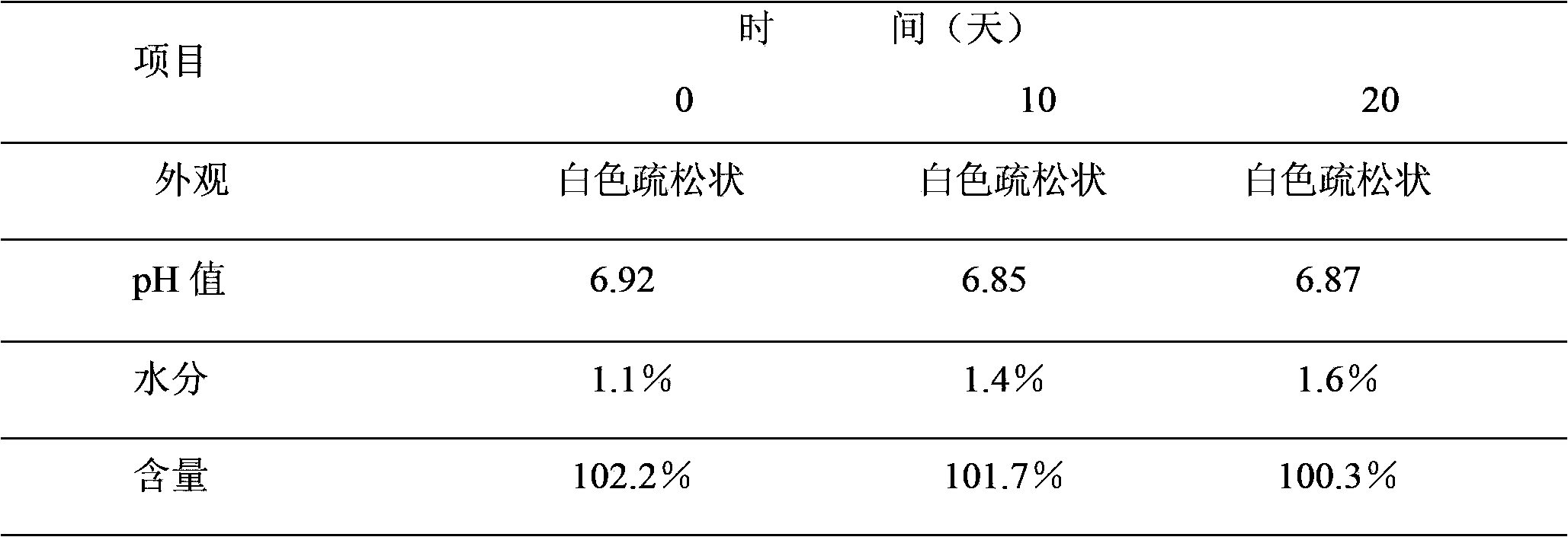
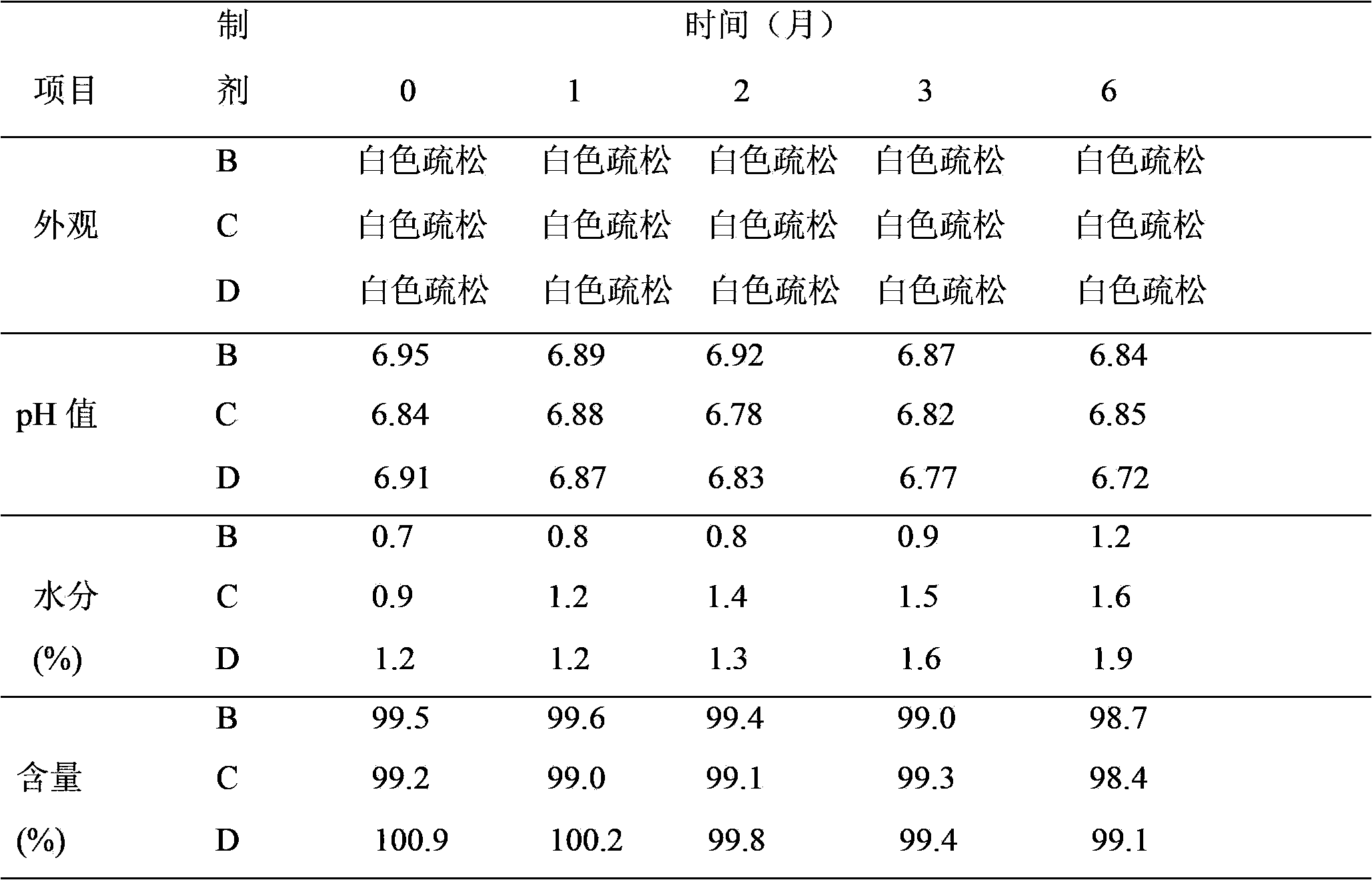
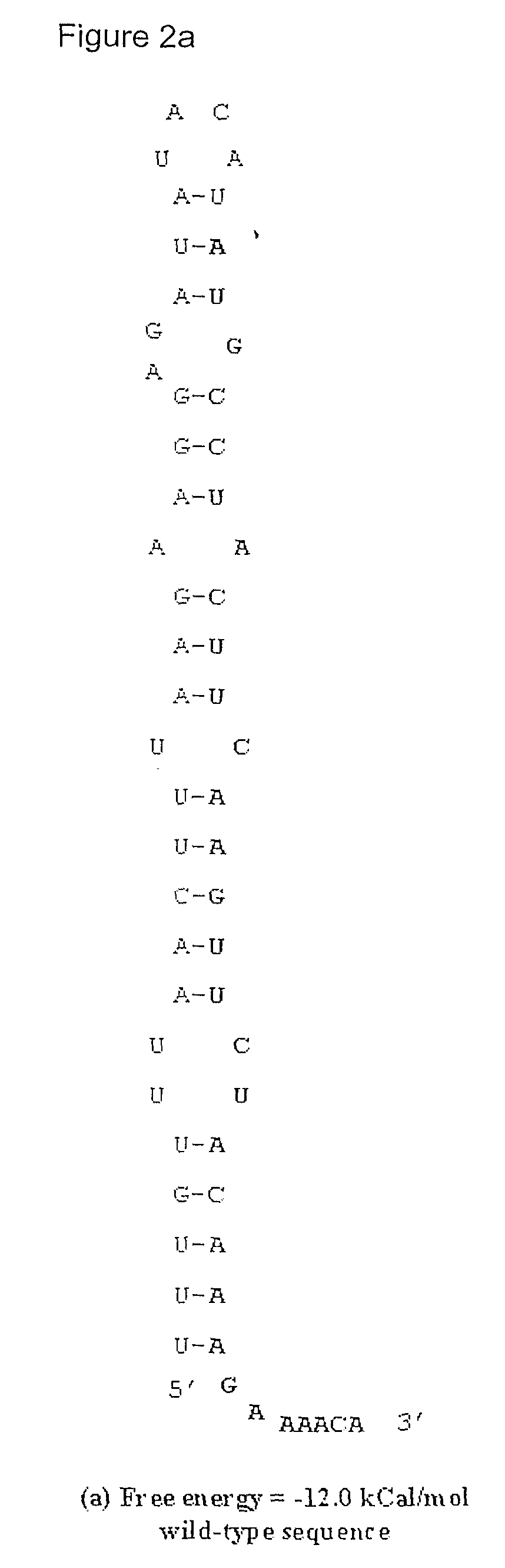Patents
Literature
35 results about "Recombinant Human Interleukin-2" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immune cell cryopreservation solution and application thereof
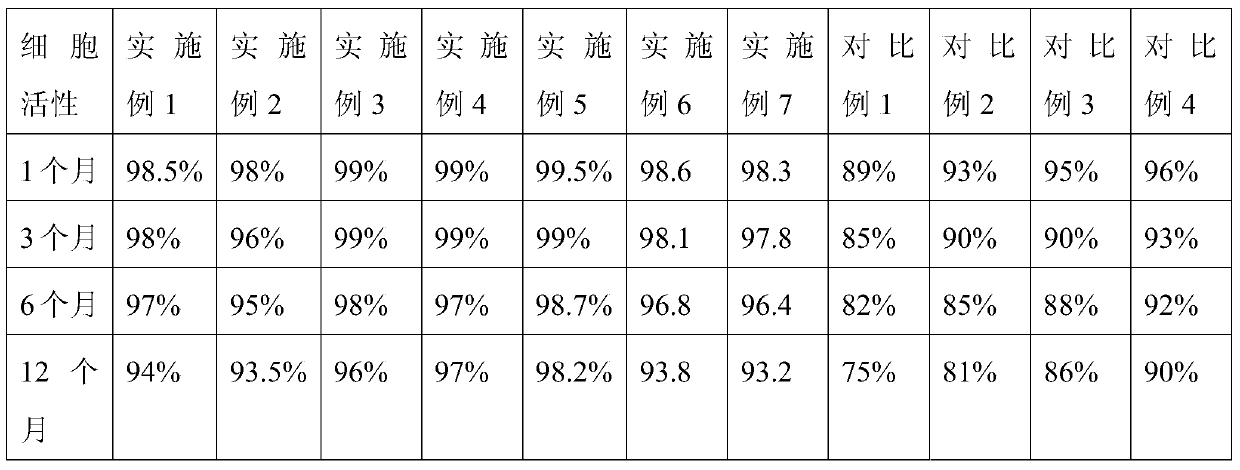
ActiveCN105638642ABiological Property GuaranteeReduced Possibility of ContaminationDead animal preservationPolyethylene glycolDrug biological activity
The invention relates to an immune cell cryopreservation solution and application thereof. The immune cell cryopreservation solution is prepared from 250-200 U / mL of recombinant human interleukin-2, 0.1-0.4 g / mL of polyethylene glycol, 0.1-0.4 g / mL 1,2-propylene glycol and 90-99% basal culture medium or sodium chloride for injection by volume. By using the immune cell cryopreservation solution for immune cell cryopreservation, the survival rate of recovery cells can reach 93% or above, the biological property of the cells is not changed, and the biological activity of the immune cells is ensured; in addition, due to the fact that no animal serum is contained in the immune cell cryopreservation solution, foreign protein cannot be introduced, the possibility of animal pathogeny contamination is reduced, and the problems that immune cells cannot be stored and transported for a long time are effectively solved.
Owner:居李生物科技(北京)有限公司
Culturing method of NK (natural killer) cell
InactiveCN104928242AGuaranteed amplification factorGuaranteed cytotoxicityBlood/immune system cellsHuman bodyLymphocyte culture
The invention discloses a culturing method of an NK (natural killer) cell. The culturing method comprises the following steps of (1) separating a mononuclear cell from peripheral blood or umbilical cord blood of a human body; (2) inoculating the mononuclear cell into a culture medium suitable for culturing lymphocyte, adding a CD3 monoclonal antibody, recombinant human interleukin 2 and autologous plasma, and culturing for 3 to 5 days; (3) adding the recombinant human interleukin 2 and the autologous plasma, and culturing; (4) harvesting the NK cell. According to the culturing method of the NK cell, the culturing cost is reduced, the amplification multiple and cell toxicity of the NK cell are guaranteed, the operation time is saved, meanwhile the probability of error operation is reduced, the obtaining efficiency of the NK cell is higher, and the safety of the NK cell is better.
Owner:WUHAN HAMILTON BIOTECH
Human interleukin 2-polyethylene glycol conjugate as well as preparation method and application thereof
ActiveCN112279906AReduced binding activityReduced stabilityPeptide/protein ingredientsDepsipeptidesWhite blood cellPolyethylene glycol
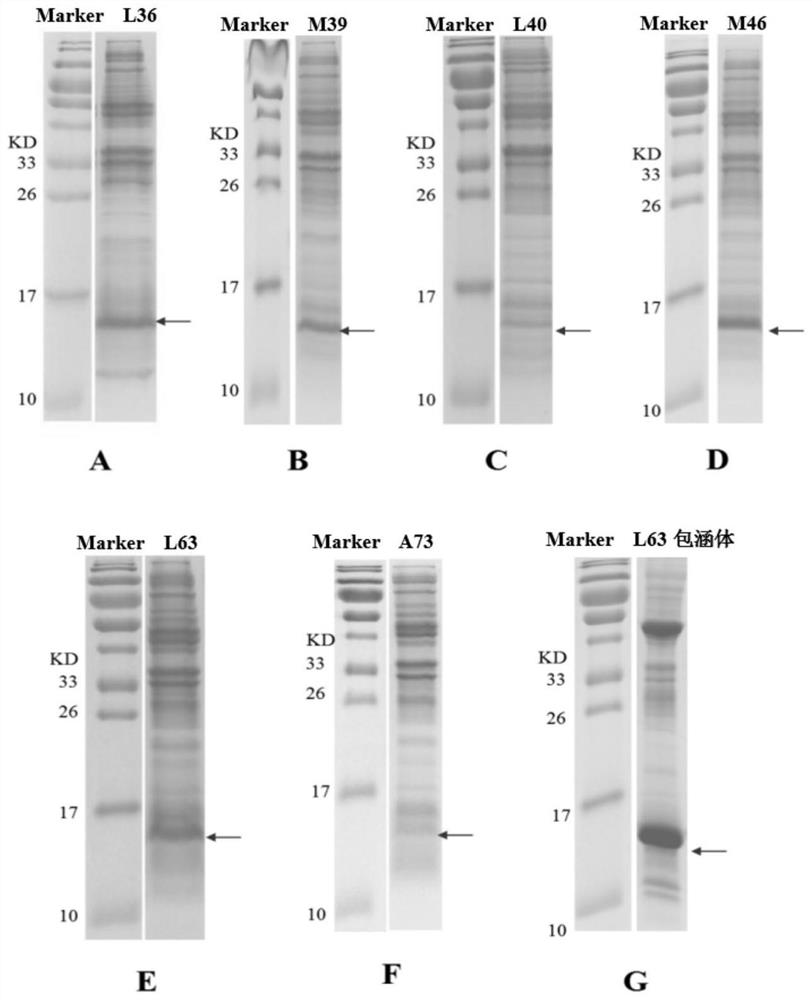
The invention discloses a human interleukin 2-polyethylene glycol conjugate as well as a preparation method and an application thereof. The human interleukin 2-polyethylene glycol conjugate comprisesrecombinant human interleukin 2 of at least one non-natural amino acid and PEG coupled to the at least one non-natural amino acid, the position of the at least one non-natural amino acid is selected from one or more sites of L36, M39, L40, M46, P47, L63, L66, E67, L70 and A73 corresponding to SEQ ID NO: 2, wherein the recombinant human interleukin 2 is a protein as shown in SEQ ID NO: 3 or a functional active fragment thereof. The human interleukin 2-polyethylene glycol conjugate provided by the invention can be used as a single drug or combined with an anti-tumor drug, and is used for treating solid tumors or blood tumors.
Owner:NOVOCODEX BIOPHARMACEUTICALS CO LTD
Adult regulatory T cell in-vitro amplification culture medium and application method thereof
ActiveCN104278012AHigh amplification rateReduce oxidative damageBlood/immune system cellsDiseaseImmunologic disorders
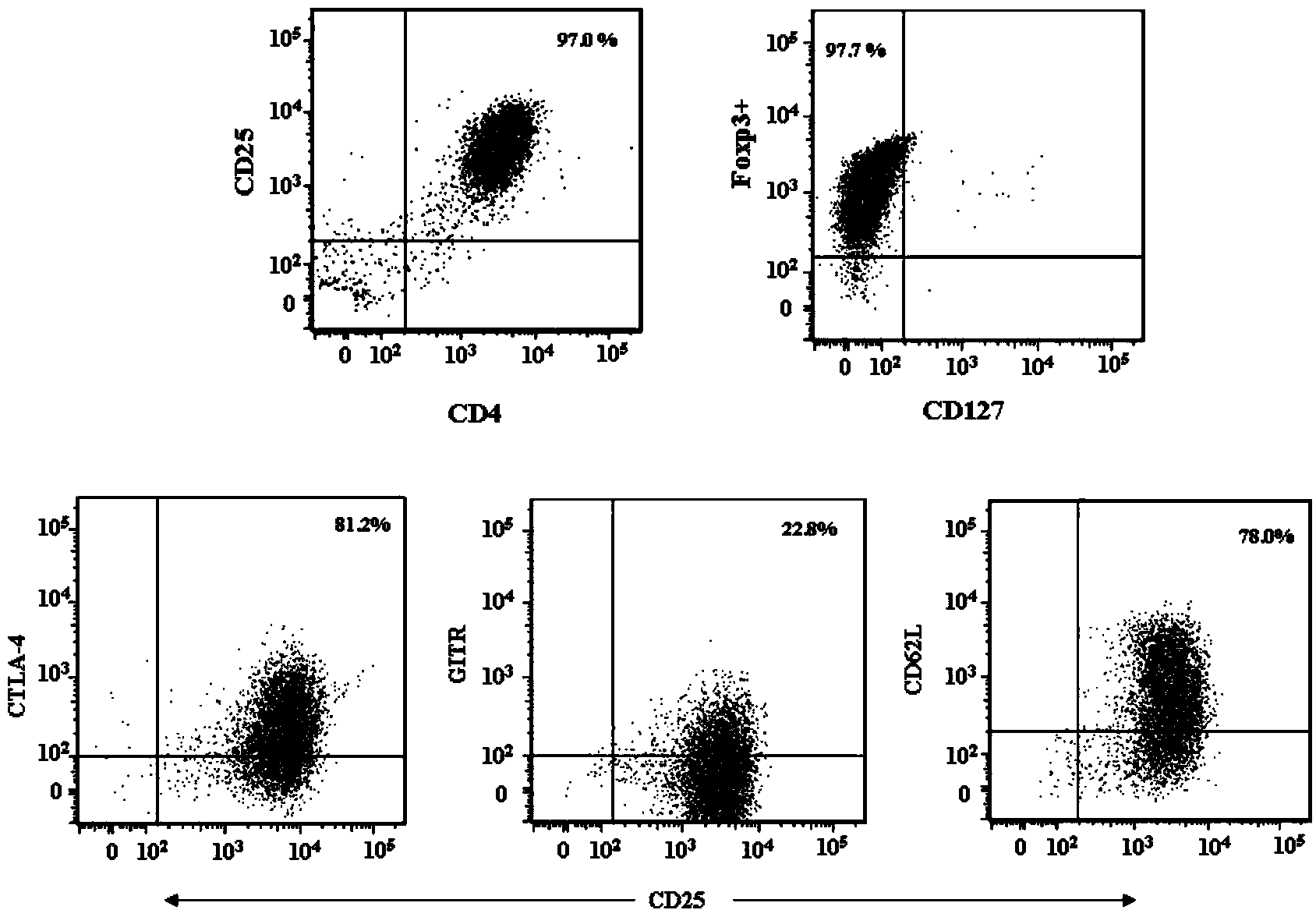
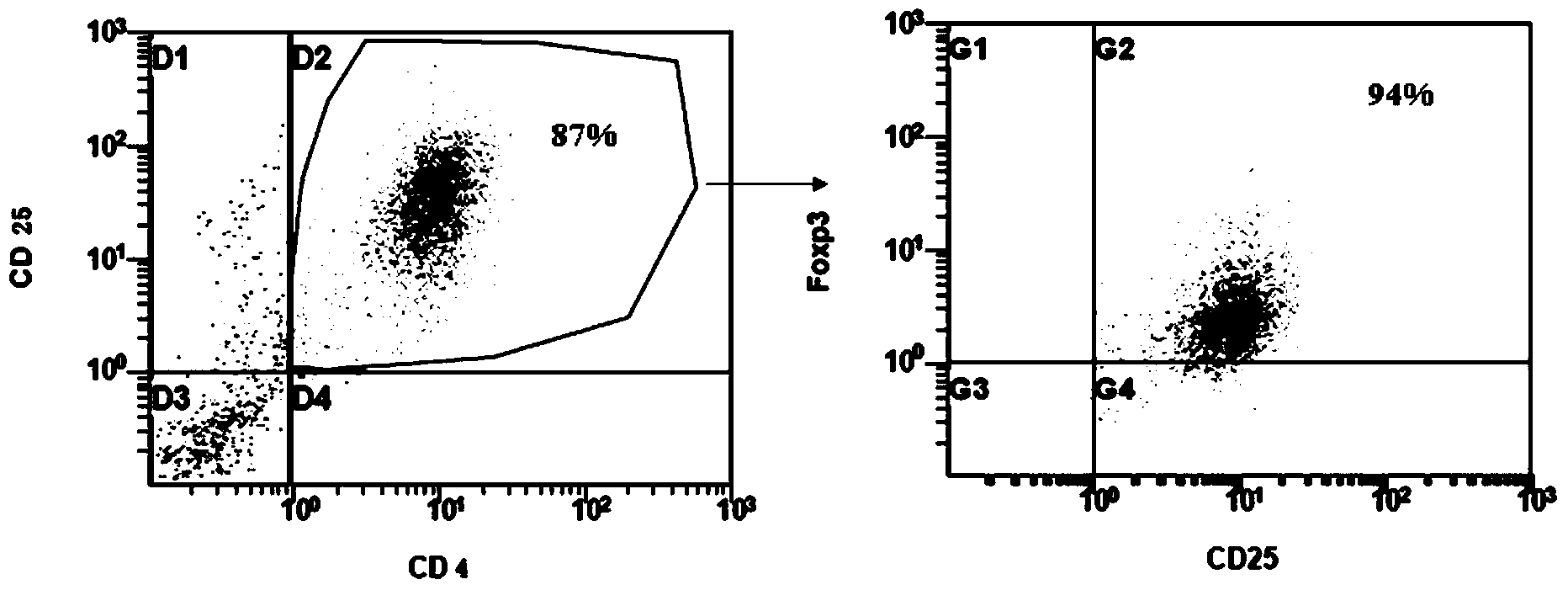
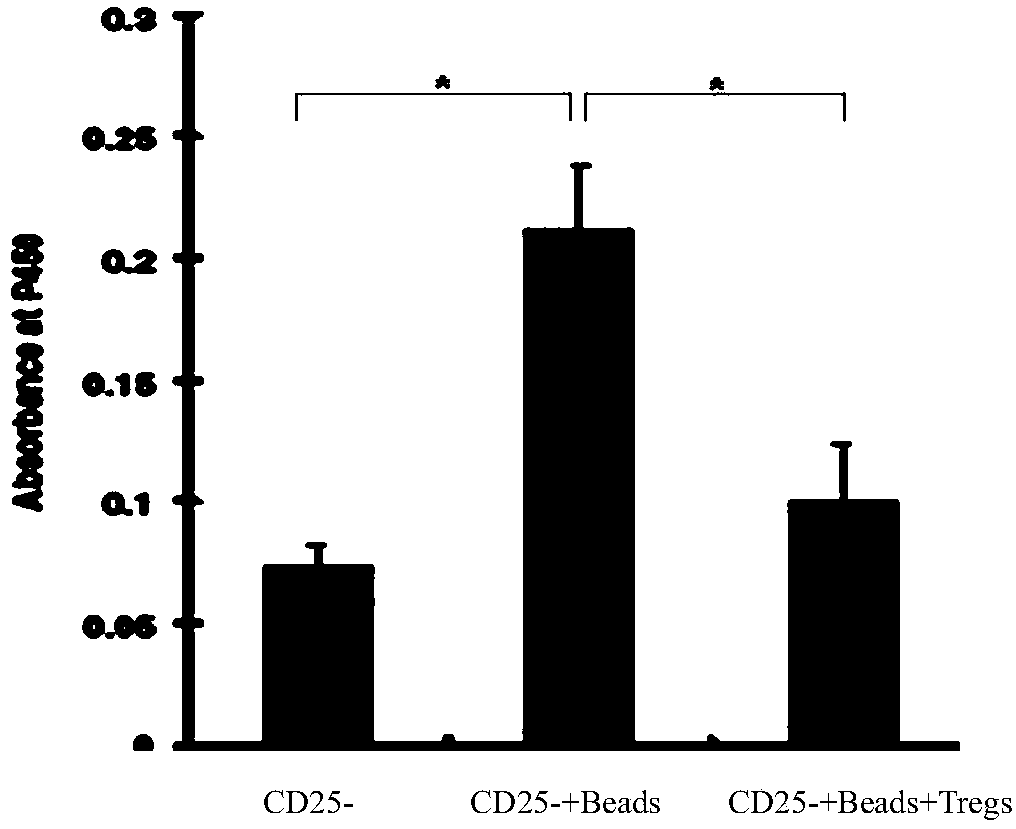
The invention provides an adult regulatory T cell in-vitro amplification culture medium and an application method thereof. The adult regulatory T cell in-vitro amplification culture medium comprises transform growth factor-beta (1-4 ng / ml), bone morphogenesis protein 4 (8-12 ng / ml), recombinant human interleukin-2 (300-500U / ml), rapamycin (80-100nM / ml), al-trans vitamin A acid (1-4 uM / mL), 4-hydroxyethylpiperazinoethylsulfonic acid (20-30mM / ml), L-glutamine (2mM / ml), 2-mercaptoethanol (40-50uM / ml), 5% human AB type serum, CD3CD28 magnetic bead, penicillin (50U / ml) and streptomycin (50ug / ml). When being used for performing amplification culture and induced differentiation on regulatory T cells, the culture medium can shorten the amplification and differentiation time of the regulatory T cells, and can obtain the high-purity Foxp3CD4+CD25+CD127-regulatory T cells. Therefore, the regulatory T cells can be used in the aspect of clinical treatment of anti-transplantation immunity rejection, autoimmune disease, allergic disease and the like to perform a novel clinical cell therapy.
Owner:HUNAN XENO LIFE SCI
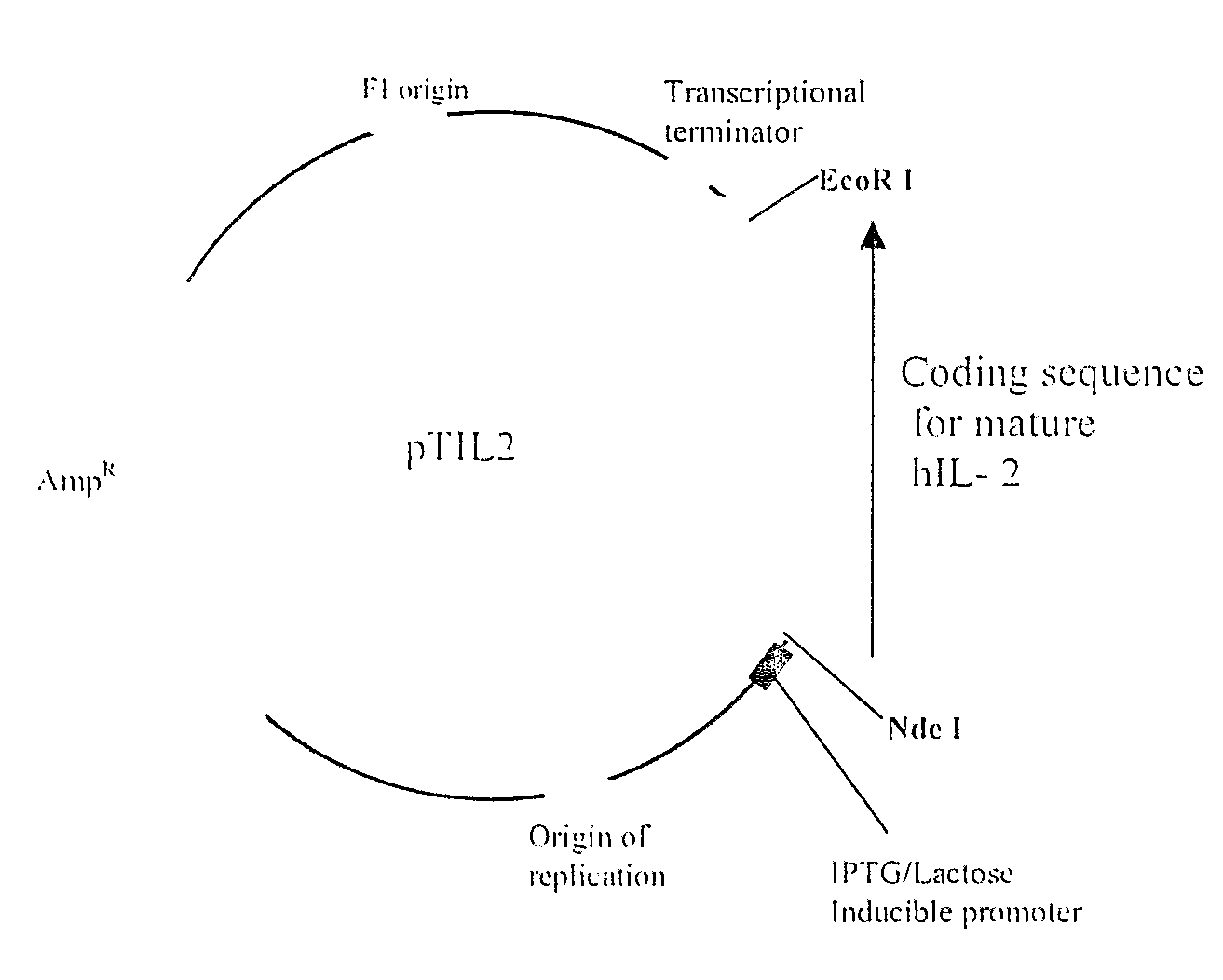
Method for Achieving High-Level Expression of Recombinant Human Interleukin-2 Upon Destabilization of the Rna Secondary Structure
InactiveUS20080268503A1Increase free energyMass productionFermentationVector-based foreign material introductionHigh level expressionFactor ii
The present invention provides a method for achieving high-level expression of the therapeutically important lymphokine (human IL-2). The method comprises of identifying the secondary structure in the 5′ region of human IL-2 mRNA, modifying the 5′ region of the human IL-2 DNA sequence to produce a new DNA sequence wherein the mRNA transcribed from the modified human IL-2 DNA sequence has the predicted 5′ secondary structure destabilized with increased free energy compared to that of the secondary structure of the mRNA transcribed from the native DNA sequence without altering the sequence of the encoded amino acids; and using this modified DNA sequence of human IL-2 for high level recombinant expression in a microbial host for large scale production. This method is also applicable to other expression host like yeasts and mammalian cells.
Owner:ZENOTECH LAB LTD
Serum-free cryopreservation medium and method for peripheral blood monouclear cells
InactiveCN108651439ABiological Property GuaranteeReduced Possibility of ContaminationDead animal preservationPeripheral blood mononuclear cellCytotoxicity
The invention relates to a serum-free cryopreservation medium and method for peripheral blood monouclear cells. The serum-free cryopreservation medium comprises recombinant human interleukin-2, humanserum albumin, polyethylene glycol, trehalose and a basic culture medium or sodium chloride for injection. The serum-free cryopreservation medium has the advantages that the recombinant human interleukin-2 (IL-2) is added into the cryopreservation medium, so that the activity stability of immune cells cultured by thawed cells can be increased greatly, the high activity of original cells can be kept, the immune cells can well keep the physiological function and biological feature of the thawed cells, and the problem of direct large-scale amplification of the thawed cells can be solved effectively; the polyethylene glycol and the trehalose are added into the cryopreservation medium, the cryopreservation medium can replace an existing dimethyl sulfoxide-based (DMSO-based) cryopreservation medium, the toxicity of the cryopreservation medium to the cells is lowered effectively, cell stability is maintained, the direct application safety of the thawed cells is increased, and the thawed cellscan be directly used.
Owner:上海韵飞生物科技有限公司
Reagent kit and method for cultivating immune cells and application thereof
InactiveCN105219708ARaise the ratioImprove systemic immunosuppressionMammal material medical ingredientsBlood/immune system cellsAbnormal tissue growthT lymphocyte
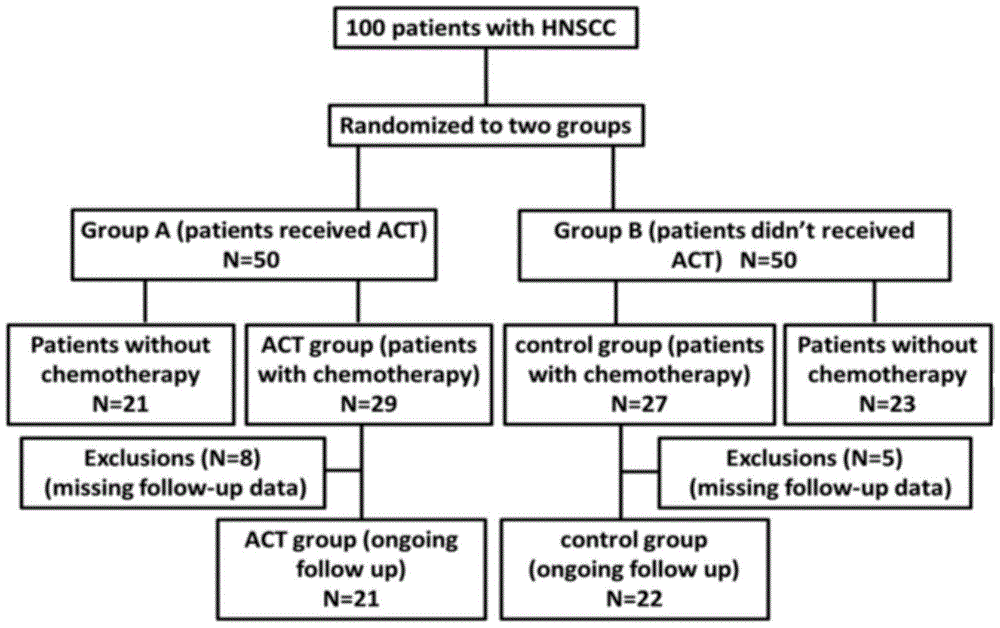
The invention provides a reagent kit for cultivating immune cells, a method for cultivating the immune cells by the aid of the reagent kit and application of the immune cells cultivated by the aid of the method to preparing preparations for preventing or treating tumors. The reagent kit comprises cultivation liquid A. RhIFN-gamma (recombinant human interferon-gamma), rhIL-2 (recombinant human interleukin-2), rhIL-15 and human transferrin are added into serum-free lymphocyte cultivation liquid to obtain the cultivation liquid A. The reagent kit, the method and the application have the advantages that the quantities of the immune cells cultivated by the aid of the method can reach approximately 10 billions, and the immune cells include large quantities of NK (natural killer) cell and NKT (natural killer T) cell masses besides CTL (cytotoxic T lymphocyte) masses; the immune cells cultivated by the aid of the method can be applied to HNSCC (head and neck squamous cell carcinoma) treatment, so that immunosuppression due to chemotherapy can be improved, and the lifetime of patients can be prolonged.
Owner:SUN YAT SEN UNIV +1
Recombinant human interleukin-2 and polyethylene coupling compound
InactiveCN101104077APeptide/protein ingredientsPharmaceutical non-active ingredientsPolyethylene glycolOligopeptide
The invention relates to a coupling compound formed by the recombinant human interleukin-2 connecting with the monomethyl polyethylene glycol through the amino acid or the oligopeptide; the coupling compound has rather good plasma stability, and can release and recompose the recombinant human interleukin slowly to actively play the pharmacological action.
Owner:北京紫辰医药生物技术研究所 +1
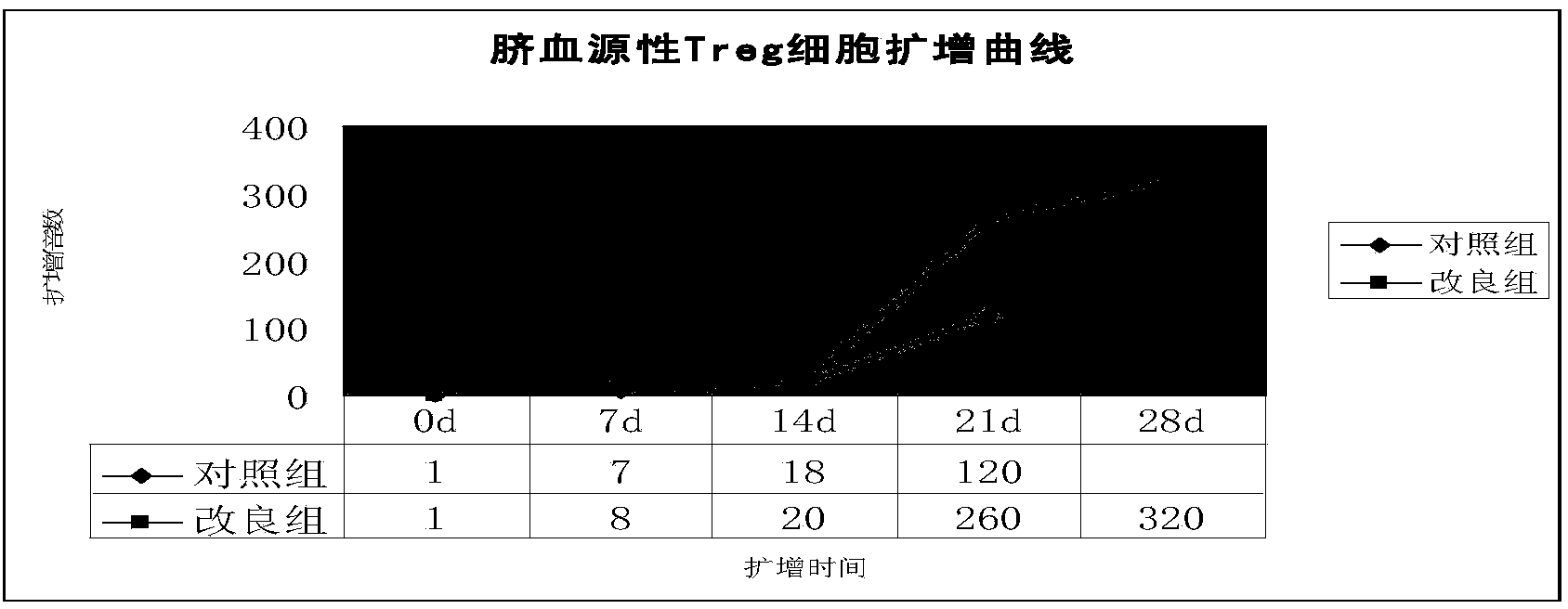
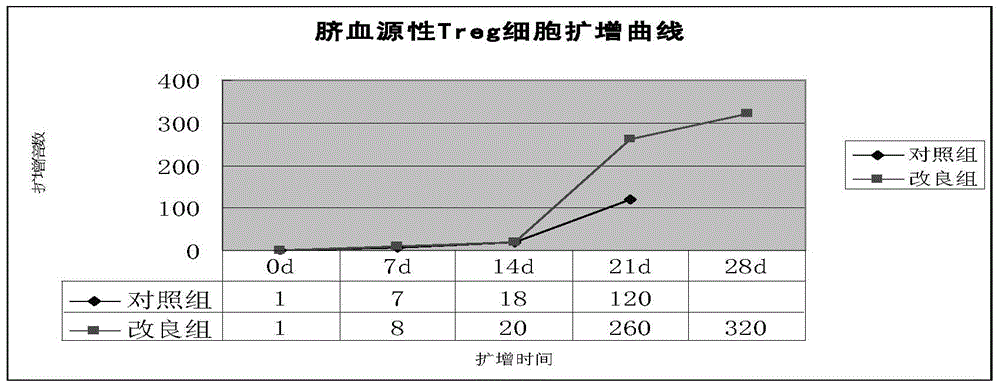
Improved expansion culture medium for regulatory T cells of human cord blood origin and application method of expansion culture medium
ActiveCN104357389AReduce riskPromote growth rateBlood/immune system cellsCulture mediumsClinical disease
The invention relates to an improved expansion culture medium for regulatory T cells of human cord blood origin and an application method of the expansion culture medium. According to the expansion culture medium, heparin anticoagulated autologous cord blood plasma accounting for 10%-12% of the volume of a culture medium, CD3-CD28 antibody co-expressed immunomagnetic beads, recombinant human interleukin 2, 2-mercaptoethanol, rapamycin, HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) and gentamicin are added into the RPMI (Roswell Park Memorial Institute)1640 culture medium; and then separated cell suspension is inoculated in a 96-well plates with a U-shaped bottom, hole-division expansion can be performed every 1-2 days, and the expansion period is 3-4 weeks. All reagents in the culture system reach the GMP (good manufacturing practice) level or are originated from autologous cord blood, so that risks caused by ingredients of animal origin are avoided, and the regulatory T cells can be used for a third-party unrelated donor and directly applied to clinical disease treatment; and compared with a traditional culture system, Treg cells (the regulatory T cells) expanded by the improved culture medium is excellent in aspects of growth speed, purity, activity, lymphocyte inhibition function and the like, and the Treg cells are expected to be used as the regulatory T cells of the third-party unrelated donor and applied to the clinical disease treatment.
Owner:HUNAN XENO LIFE SCI
Preparation method for poly(ethylene glycol) modified recombinant human interleukin-2
InactiveCN103193879AGood molecular weight uniformityStrong antiviral activityDepsipeptidesPeptide preparation methodsPolyethylene glycolP-Toluenesulfonic acid
The invention provides a preparation method for poly(ethylene glycol) modified recombinant human interleukin-2, belonging to the field of biological medicine. The method comprises the following steps: activating mPEG with an activator so as to obtain an activated mPEG molecule; and reacting the activated mPEG molecule with recombinant human interleukin-2, adding a glycine solution to terminate a reaction and separating and purifying an obtained product; wherein the activator is one selected from the group consisting of succinic anhydride and N-hydroxysuccinimide, p-toluenesulfonic acid-chlorine, N,N'-carbonyl diimidazole, N,N'-disuccinimidocarbonate, p-nitrophenyl carbonate, benzotriazole carbonate, phenylsuccinimide carbonate and N-acetoxysuccinimide. The preparation method provided by the invention is simple and convenient to operate and is easy for quality control and enlarged production.
Owner:SHENZHEN YATAIXING IND
Frozen stock solution of immune cells
InactiveCN105994253AEffective protectionLow toxicityDead animal preservationPolyethylene glycolTrehalose
The invention discloses a frozen stock solution of immune cells. The frozen stock solution of the immune cells comprises the following components: 25 to 100 U / mL of recombinant human interleukin-2, 0.5 to 2.5 mg / mL of alpha-1,4-glucan, 0.05 to 0.2 mg / mL of trehalose, 0.1 to 0.5 mL / mL of polyethylene glycol, 0.05 to 0.15 mL / mL of propylene glycol and 0.7 to 0.9 mL / mL of a basic medium. The frozen stock solution of the immune cells disclosed by the invention has the beneficial effects of being high in safety, small in damage to the cells and capable of guaranteeing normal biological functions after the cells are recovered.
Owner:GUANGZHOU ZISHENG BIOLOGICAL TECH CO LTD
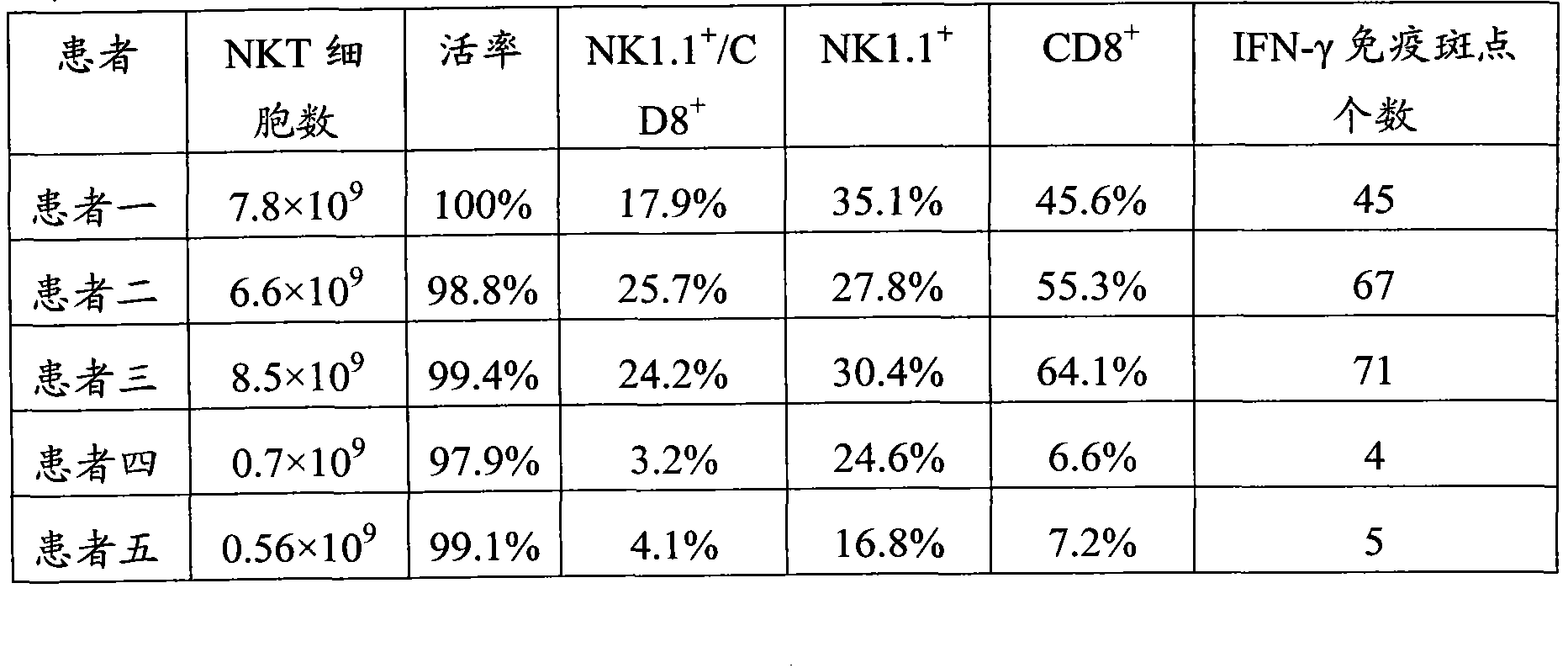
Preparation method for monkshood polysaccharide-induced nature killer T (NKT) cell proliferation and application thereof
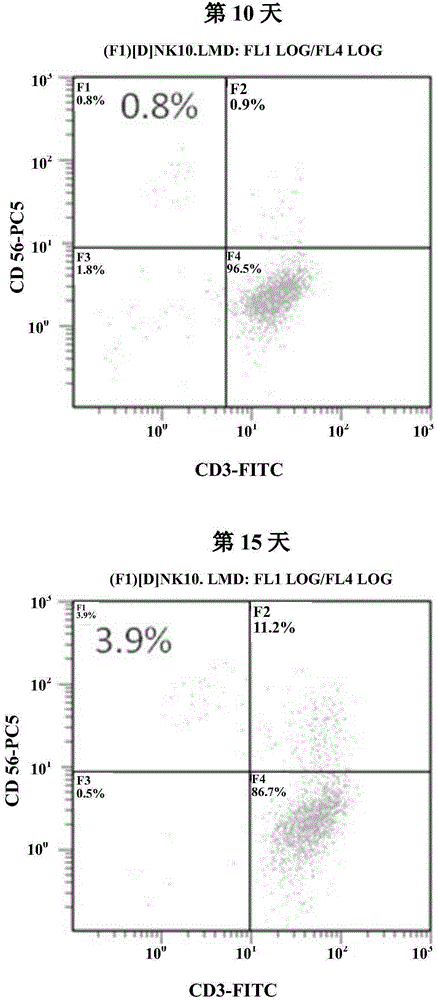
The invention provides a culture method for monkshood polysaccharide-induced nature killer T cell (Nature Killer T cell, NKT) proliferation. The method comprises the characteristics as follows: human peripheral blood, marrow or a mononuclear cell with an umbilical cord blood source are continuously cultivated by traditional Chinese medicine monkshood polysaccharide and recombinant human interleukin 2, a lot of nature kill T cells are obtained, NK<1.1+> and CD<8+> are expressed, interferon gamma is secreted, the ratio in lymphocyte at the 14th day is more than 16.15%, the order of magnitude can be up to over 5*10<9>, and the nature kill T cell have effective anti-tumor and anti-virus functions. A clinical application of the NKT cell obtained by the method is also provided by the invention.
Owner:李金珍
Immune cell preserving fluid
InactiveCN105935051AImprove cell activityConvenient experimentDead animal preservationHigh cellHuman albumin
The invention discloses immune cell preserving fluid. The immune cell preserving fluid comprises 0-50 U / mL of recombinant human interleukin-2, 0-10 mg / mL of human albumin, 0-1.5 mg / mL of alpha-1,4-glucan, 0.5-2.0 mg / mL of glucose, 3.5-5.0 mg / mL of sodium citrate, 2.0-3.5 mg / mL of sodium gluconate, 0.2-0.5 mg / mL of potassium chloride and 0.2-0.5 mg / mL of magnesium chloride. The immune cell preserving fluid has the advantages of being suitable for cell preservation within a wide temperature range, and capable of guaranteeing high cell viability of immune cells and facilitating follow-up cell experiment or clinical treatment.
Owner:GUANGZHOU ZISHENG BIOLOGICAL TECH CO LTD
Improved cryopreservation solution for peripheral blood mononuclear cells
InactiveCN110140716AReduce usageAvoid death phenomenonDead animal preservationPeripheral blood mononuclear cellPolyethylene glycol
Owner:方艳秋 +1
Immune cell cryopreservation solution and application thereof
ActiveCN105638642BBiological Property GuaranteeReduced Possibility of ContaminationDead animal preservationPolyethylene glycolDrug biological activity
The invention relates to an immune cell cryopreservation solution and application thereof. The immune cell cryopreservation solution is prepared from 250-200 U / mL of recombinant human interleukin-2, 0.1-0.4 g / mL of polyethylene glycol, 0.1-0.4 g / mL 1,2-propylene glycol and 90-99% basal culture medium or sodium chloride for injection by volume. By using the immune cell cryopreservation solution for immune cell cryopreservation, the survival rate of recovery cells can reach 93% or above, the biological property of the cells is not changed, and the biological activity of the immune cells is ensured; in addition, due to the fact that no animal serum is contained in the immune cell cryopreservation solution, foreign protein cannot be introduced, the possibility of animal pathogeny contamination is reduced, and the problems that immune cells cannot be stored and transported for a long time are effectively solved.
Owner:居李生物科技(北京)有限公司
Extraction method of recombinant human interleukin-2 fermentation inclusion body
ActiveCN104004082AGuaranteed mass productionSimple componentsPeptide preparation methodsInterleukinsEscherichia coliInclusion bodies
The invention discloses an extraction method of a recombinant human interleukin-2 fermentation inclusion body. The extraction method comprises the following steps: (1) culturing an engineering strain of an escherichia coli expression recombinant interleukin-2; (2) breaking a thallus obtained by fermentation and culturing to collect an inclusion body; (3) extracting and washing the inclusion body to obtain a refined inclusion body, wherein the engineering strain is E.Coli K802(ply-4), the culturing method comprises multiplication culture and fermentation tank culture, which both adopt a yeast and peptone-containing antibiotic-free culture medium, and the thallus is broken by adopting a high-pressure homogenizer, and is degenerated to obtain a high-purity ril-2 stock solution. The extraction method disclosed by the invention is short in production period, high in production efficiency, large in production scale, and especially suitable for industrialized production; the production cost is lowered.
Owner:BEIJING FOUR RINGS BIOPHARM
Application of recombinant human calcineurin B subunit
InactiveCN105435215AGood control effectEnhanced inhibitory effectPeptide/protein ingredientsHydrolasesPositive controlHigh doses
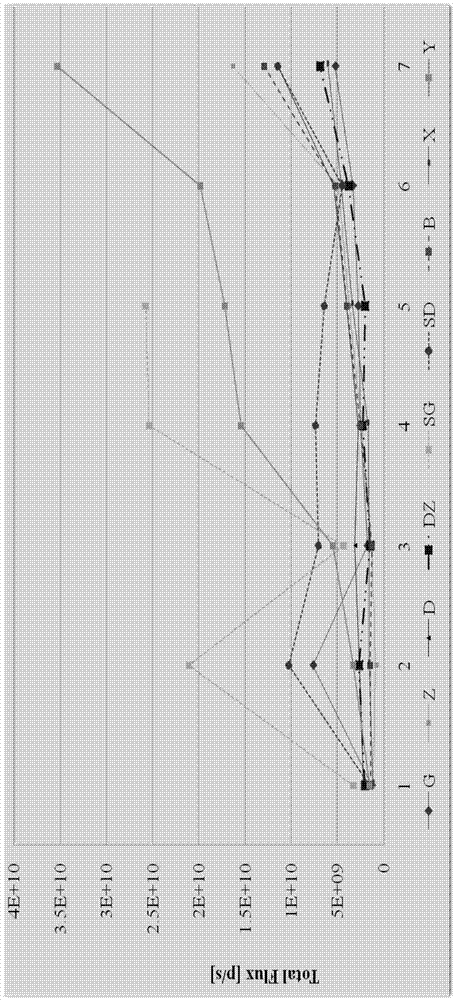
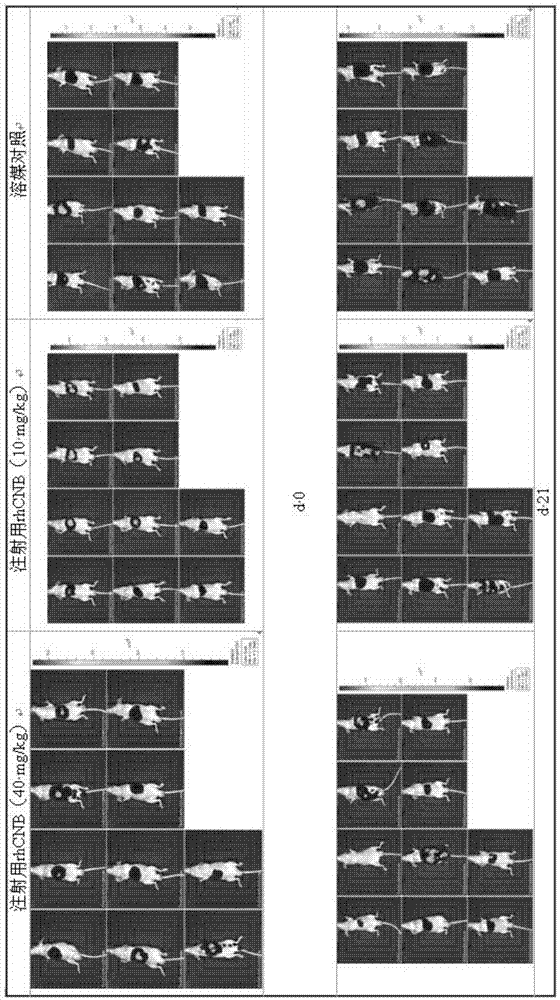
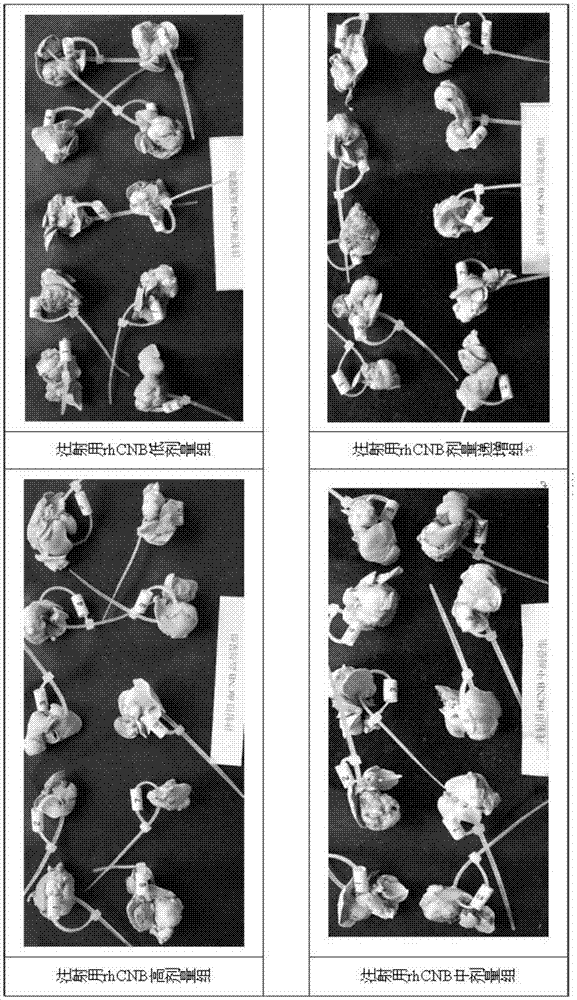
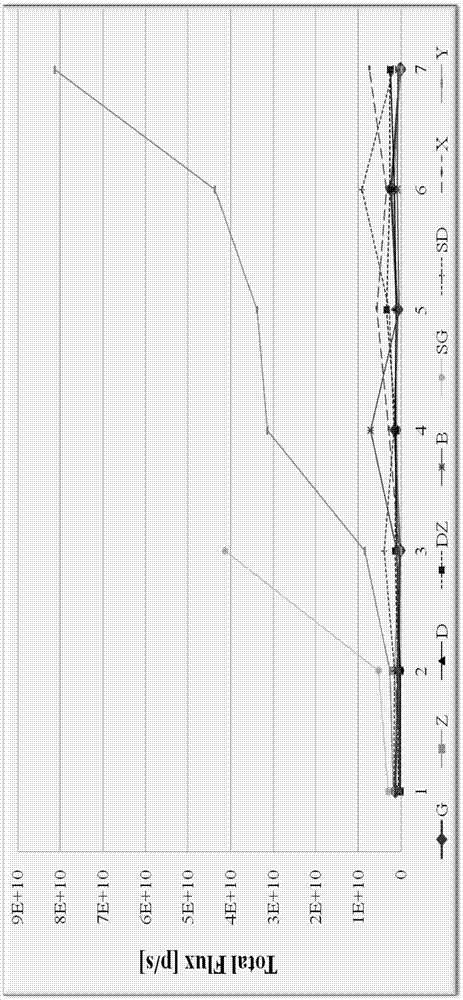
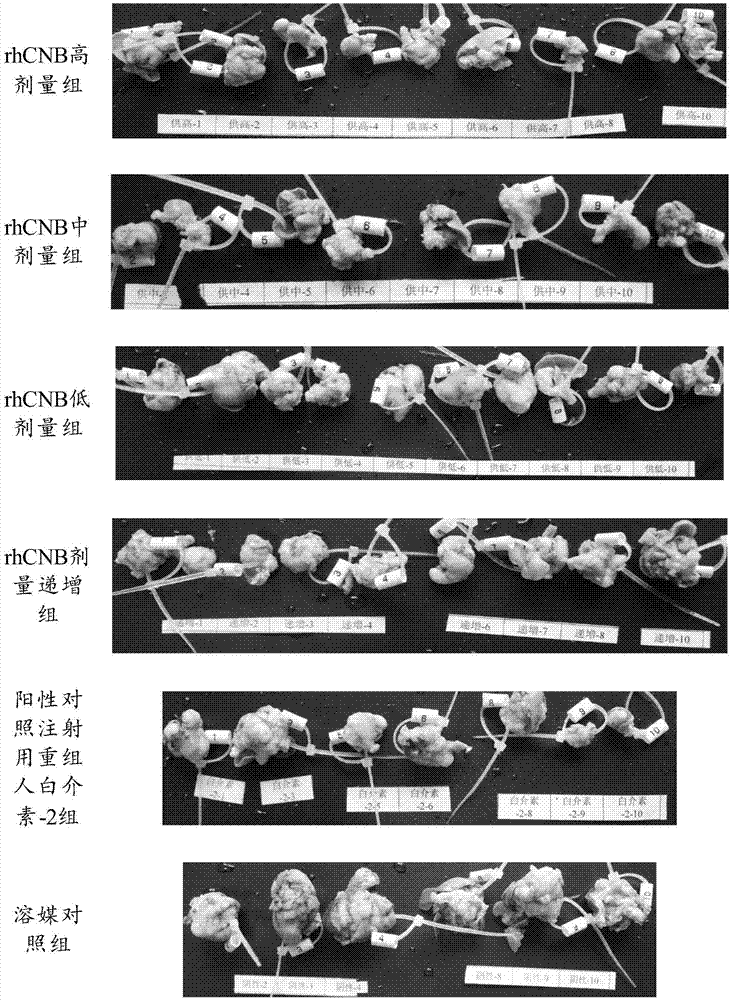
The invention relates to the field of proteins, in particular to application of recombinant human calcineurin B subunit, and mainly provides application of rhCNB to preparation of a medicine for killing and / or inhibiting a liver cancer cell Bel-7402. After continuous medication for 6 times, medication groups of rhCNB for injection with doses of 10 mg / kg, 20 mg / kg and 40 mg / kg all have a relatively good inhibiting effect on the growth of human liver cancer cell Bel-7402 solid tumors. Subsequent testing results show that the effects of high-dose, middle-dose and increasing-dose medication groups are superior to that of a low-dose medication group, and the treating effect of rhCNB for injection is equivalent to those of recombinant human interleukin-2 for injection and hydroxycamptothecine for injection in a solvent control group. The results of d7 testing after final medication show that the high-dose group has the optimum controlling effect (p is smaller than 0.01) on tumor cell proliferation, which is superior to those of other dose groups and a positive control group, and equivalent to those of a hydroxycamptothecine medication group and the increasing-dose group (p is smaller than 0.05).
Owner:HAIKOU QILI PHARMA
Long-acting recombinant human interleukin 2 fusion protein and preparation method and application thereof
InactiveCN113789346AProlonged plasma half-lifeHigh expressionPeptide/protein ingredientsAntipyreticDiseaseWhite blood cell
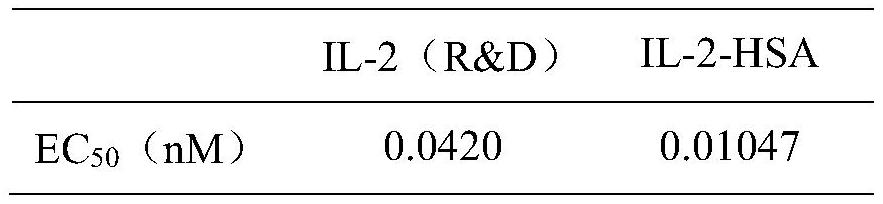
The invention discloses a fusion protein of interleukin 2 and human serum albumin. Plasmids with a fusion gene of interleukin 2 and human serum albumin are electrically transferred into CHO cells to obtain a CHO monoclonal cell strain capable of stably and efficiently expressing human recombinant protein. The monoclonal cell strain of the invention can secrete and express the fusion protein of the interleukin 2 and the human serum albumin, and the fusion protein can prolong the plasma half-life period of the human interleukin 2 and can be used for preparing human interleukin 2 drugs or drugs for treating various diseases, such as tumors and immunodeficiency diseases.
Owner:BEIJING VDJBIO
Method for improving yield of recombinant human interleukin-2 (rhIL-2)
InactiveCN109721652ABiologically activeHigh yieldDepsipeptidesPeptide preparation methods2-MercaptoethanolDrug biological activity
The invention discloses a method for improving the yield of recombinant human interleukin-2 (rhIL-2). The method comprises the steps of purification, eluent recovery after the purification, reductionof rhIL-2 dimer and wrong ligand by using 2-mercaptoethanol, reoxidation renaturation, and purification. The method performs reduction of rhIL-2 dimer and wrong ligand by using the 2-mercaptoethanol,opens disulfide bonds, and performs reoxidation renaturation and purification, so that correctly-paired disulfide bonds with biological activity can be formed so as to obtain the rhIL-2 with the biological activity, and the yield is improved by about 20 percent.
Owner:江苏金丝利药业股份有限公司
Application of recombinant human calcineurin B subunit
The invention relates to the field of proteins, in particular to application of recombinant human calcineurin B subunit, and mainly provides application of rhCNB to preparation of a medicine for killing and / or inhibiting a gastric cancer cell MGC-803. When rhCNB for injection plays a role in treatment of human gastric cancer cell MGC-803 carcinoma in situ transplantation tumors in BALB / c nude mice, the effective tumor treatment (compared with that of a solvent control group, p is smaller than 0.05) time starts after continuous medication for 6 times; in the whole medication process, the tumors can be effectively controlled; one week after medicine withdrawal, rhCNB for injection still has a continuous and effective treating effect (compared with that of the solvent control group, p is smaller than 0.001) on the tumors; the performance of rhCNB for injection is superior to those of cisplatin for positive control injection and hydroxycamptothecine for injection, and equivalent to recombinant human interleukin-2 for injection.
Owner:HAIKOU QILI PHARMA
Application of interleukin 2 and its derivative in preparing medicine for treating rhinitis
InactiveCN101125199ARelief of symptoms and signsImprove lesionPeptide/protein ingredientsAerosol deliveryMedicineCurative effect
The present invention discloses an application of the interleukin-2 (IL-2) and the derivatives in the preparation of the drugs for treating rhinitis and pertains to the field of biomedicine. The present invention has the advantages that: the present invention firstly uses the recombinant human interleukin-2 in the treatment of rhinitis, the results show that the interleukin-2 can obviously alleviate the symptoms and physical signs of the rhinitis, significantly alleviate the nasal lesions and have the accurate efficacy.
Owner:BEIJING FOUR RINGS BIOPHARM +1
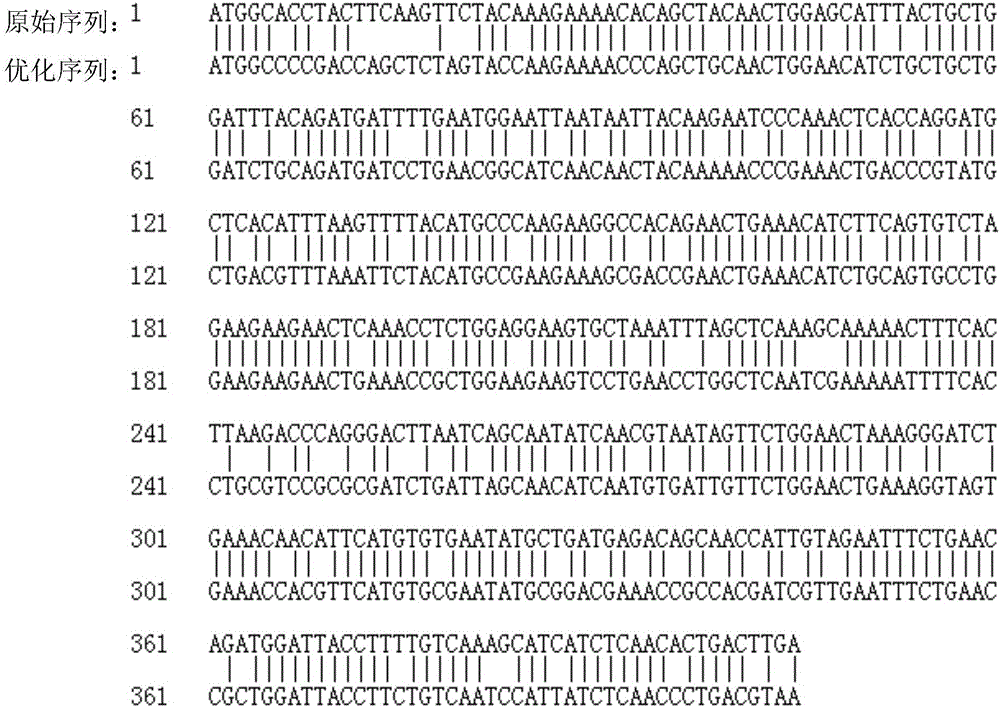
Optimized nucleotide sequence of recombinant human interleukin-2 and high-efficiency soluble expression method
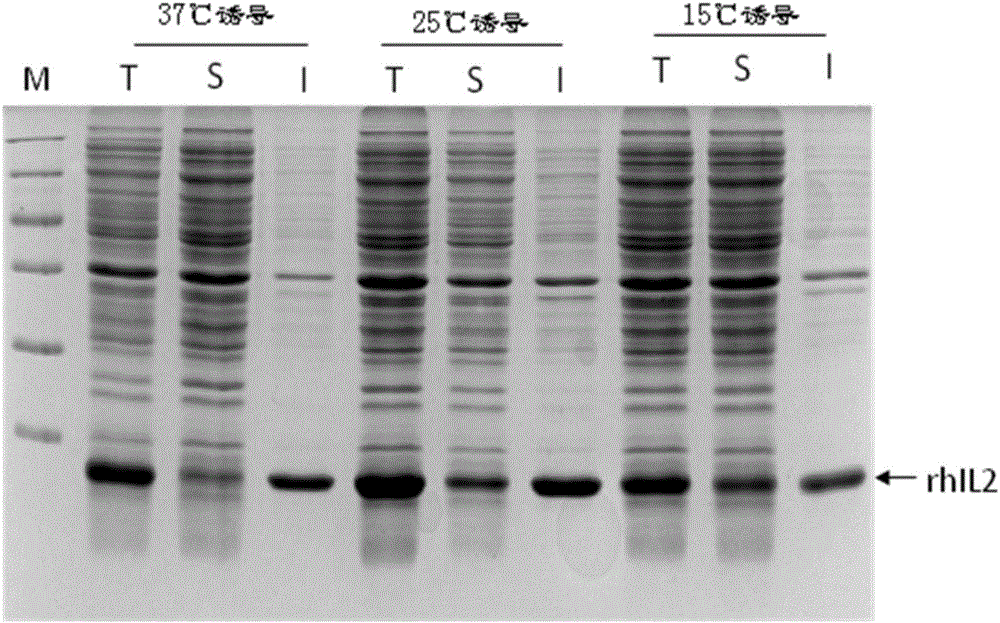
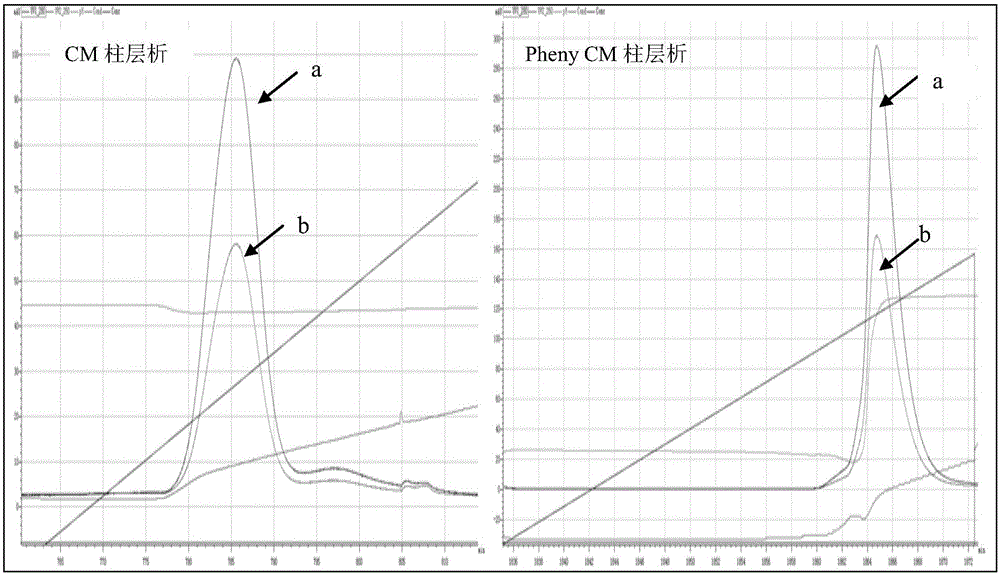
ActiveCN107435045AHigh expressionEfficient expressionBacteriaMicroorganism based processesEscherichia coliInclusion bodies
The invention discloses an optimized nucleotide sequence of recombinant human interleukin-2. The nucleotide sequence is optimized according to preferred codons of Escherichia coli under the circumstance of not changing amino acid coding sequence. The nucleotide sequence is as shown in SEQ ID NO:1, or is complementary nucleotide sequence. Furthermore, expression vector and engineering bacteria of the nucleotide sequence are constructed, and recombinant human interleukin-2 is expressed in the engineering bacteria. The expressed recombinant human interleukin-2 accounts for 10-30% of total mycoprotein, and soluble expression amount accounts for 3-15% of total expression amount. The amount of recombinant human interleukin-2 inclusion body is reduced, expression amount of soluble protein is raised, and conditions are created for mass production of soluble recombinant human interleukin-2.
Owner:SHANGHAI HUAXIN HIGH BIOTECH
A method for optimizing the nucleotide sequence and efficient soluble expression of recombinant human interleukin-2
ActiveCN107435045BImprove solubilityReduce formationBacteriaMicroorganism based processesEscherichia coliMycoprotein
The invention discloses an optimized nucleotide sequence of recombinant human interleukin-2. The nucleotide sequence is optimized according to preferred codons of Escherichia coli under the circumstance of not changing amino acid coding sequence. The nucleotide sequence is as shown in SEQ ID NO:1, or is complementary nucleotide sequence. Furthermore, expression vector and engineering bacteria of the nucleotide sequence are constructed, and recombinant human interleukin-2 is expressed in the engineering bacteria. The expressed recombinant human interleukin-2 accounts for 10-30% of total mycoprotein, and soluble expression amount accounts for 3-15% of total expression amount. The amount of recombinant human interleukin-2 inclusion body is reduced, expression amount of soluble protein is raised, and conditions are created for mass production of soluble recombinant human interleukin-2.
Owner:SHANGHAI HUAXIN HIGH BIOTECH
Human interleukin 2-polyethylene glycol conjugate and application thereof
ActiveCN113698468AAchieve precise point couplingOvercome the disadvantage of inaccurate couplingPeptide/protein ingredientsDepsipeptidesDiseaseHydroxylamine
The invention provides a human interleukin 2-polyethylene glycol conjugate and application thereof. The human interleukin 2-polyethylene glycol conjugate provided by the invention comprises recombinant human interleukin 2 containing at least one non-natural amino acid and PEG coupled to the at least one non-natural amino acid, the non-natural amino acid is a carbonyl terminal group-containing compound with a structure as shown in a formula (I) or an enantiomer thereof, and PEG is coupled to the at least one non-natural amino acid by forming an oxime bond between the carbonyl end group and PEG containing a hydroxylamine end group. The human interleukin 2-polyethylene glycol conjugate provided by the invention can be singly used or combined with other anti-tumor drugs, and is used for treating diseases such as solid tumors and hematologic tumors.
Owner:NOVOCODEX BIOPHARMACEUTICALS CO LTD
Recombinant human interleukin-2 freeze-dried preparation for injection and preparation method thereof
InactiveCN103565757AImprove performanceEasy to transportPowder deliveryPeptide/protein ingredientsSucroseFreeze-drying
The invention provides a recombinant human interleukin-2 freeze-dried preparation for injection and a preparation method thereof. The preparation is a freeze-dried solution comprising the following components: recombinant human interleukin-2 and a pharmaceutically acceptable solubilizer, wherein the pH (potential of Hydrogen) of the solution is 6.0-8.0; the solubilizer is a mixture of one or more than one of mannitol, sorbitol, sodium chloride, SDS (Sodium Dodecyl Sulfate), glucose, sucrose, lactose or dextran. The preparation method comprises the steps of (1) preparing a solution containing the recombinant human interleukin-2 and the pharmaceutically acceptable solubilizer, wherein the pH of the solution is 6.0-8.0; (2) carrying out pyrogen removal and filtration sterilization on the solution obtained in the step (1) by using a conventional method; and (3) carrying out freeze drying on the solution obtained in the step (3). The recombinant human interleukin-2 freeze-dried powder-needle preparation provided by the invention is stable in performance, easy to transport and store and convenient in clinic use; the preparation method provided by the invention is simple, practicable and low in cost and is beneficial for mass production.
Owner:江苏金丝利药业股份有限公司
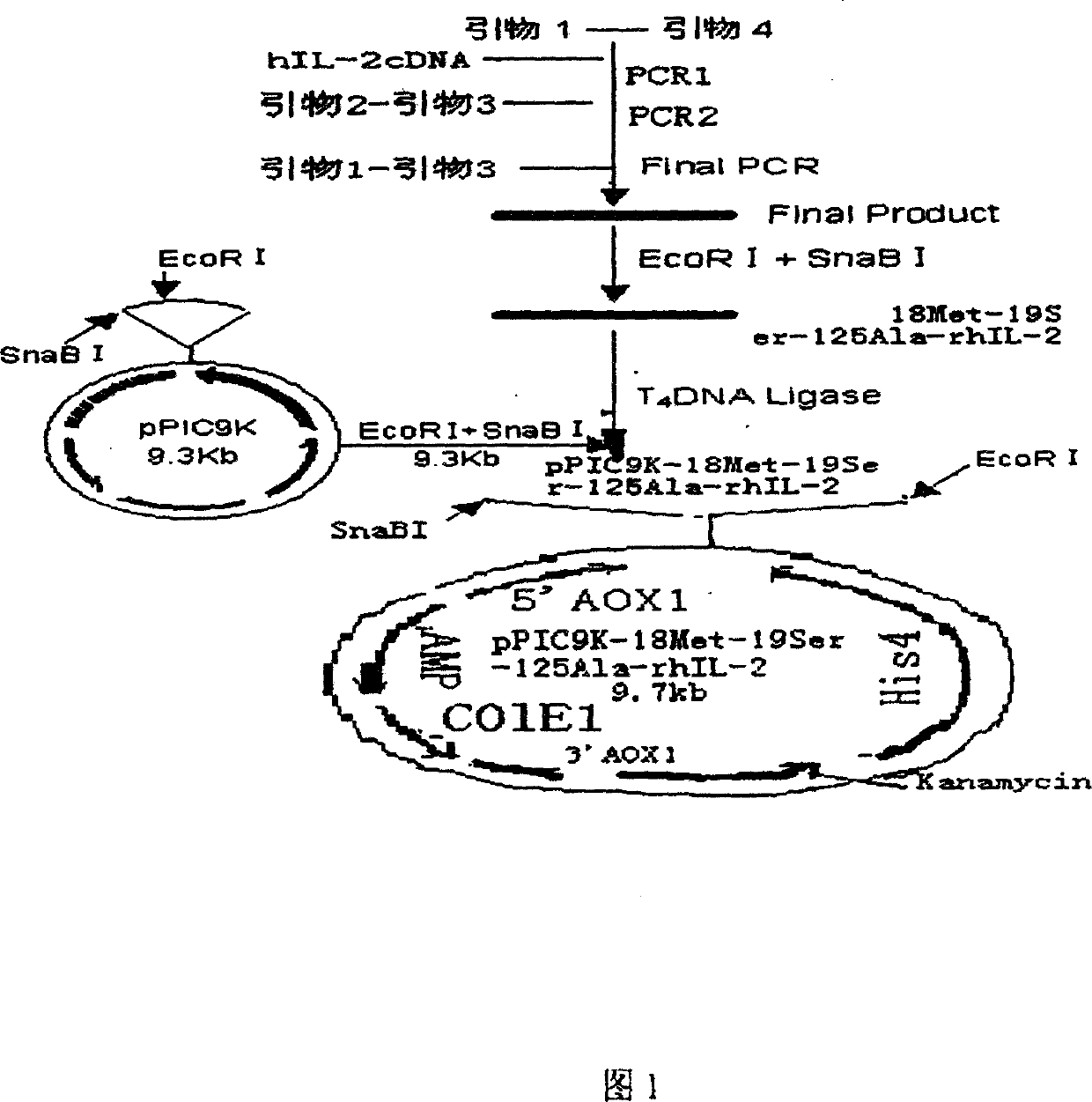
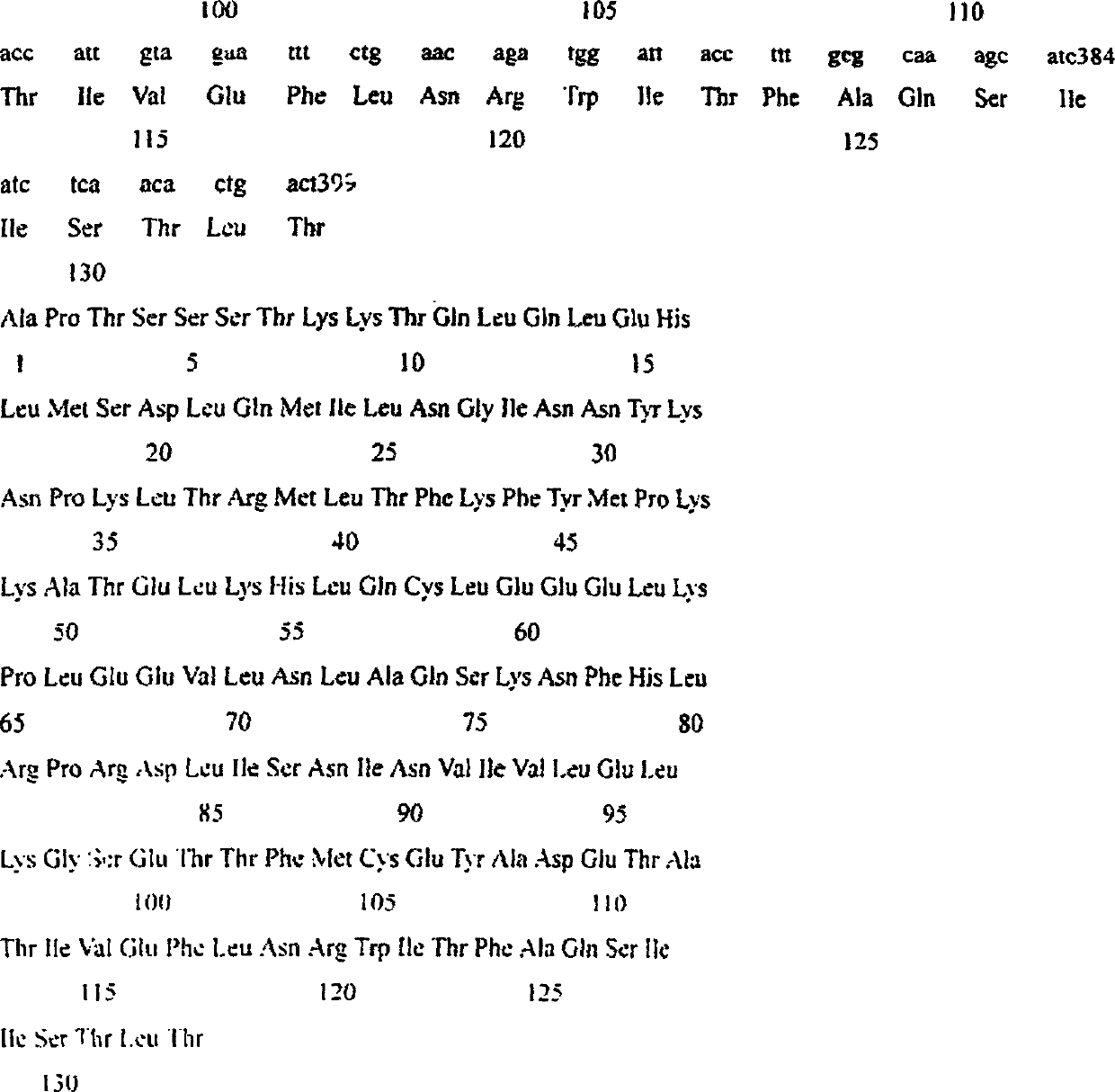
Trimutant of recombinant human interleukin-2 and its preparation method
InactiveCN1208345CHigh activityImprove stabilityDepsipeptidesFermentationPichia pastorisWhite blood cell
The present invention relates to a triple mutant of recombinant human interleukin-2 and a preparation method thereof. The purpose is to transform human interleukin-2 (hIL-2) by means of genetic engineering, and prepare three-site mutant interleukin-2 Proved to be 4.3 times more active than natural human interleukin. By obtaining the three-site mutant interleukin-2 cDNA, constructing the pPIC9K-IL-2-3m plasmid, and then transforming it into KM71 Pichia pastoris, and obtaining the three-site mutation from the pPIC9K-IL-2-3m KM71 yeast fermentation supernatant Site mutations in the interleukin-2 protein. That is, the 18th, 19th and 125th amino acids of natural interleukin-2 are replaced by methionine, serine and alanine respectively.
Owner:CHONGQING UNIV +1
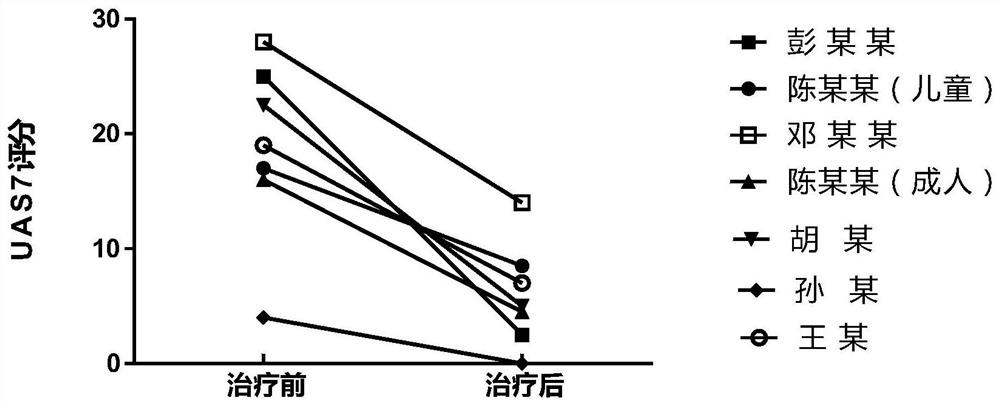
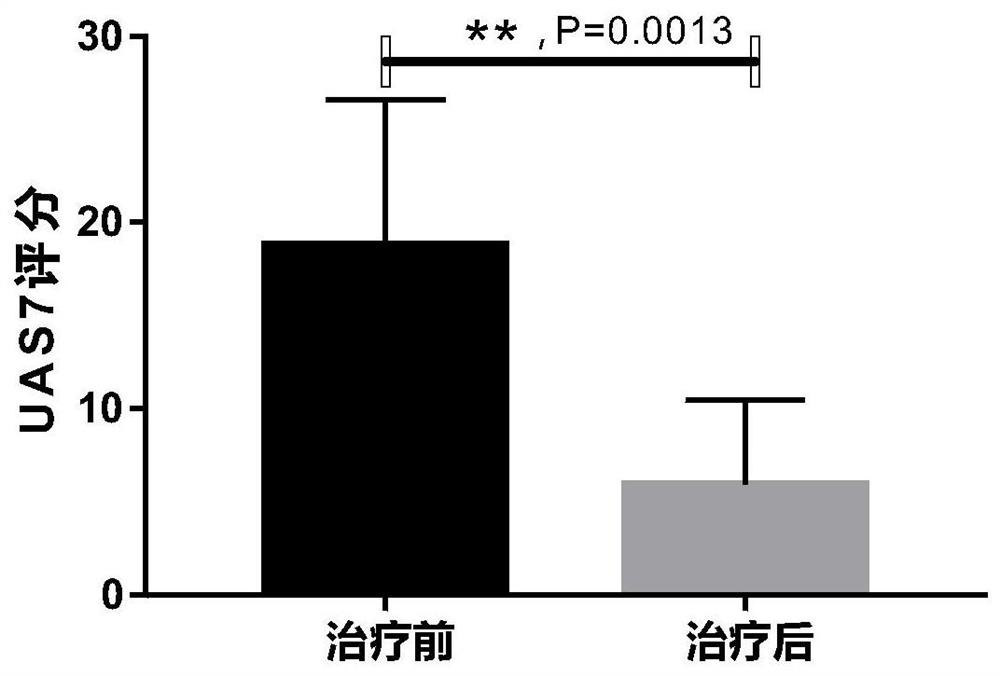
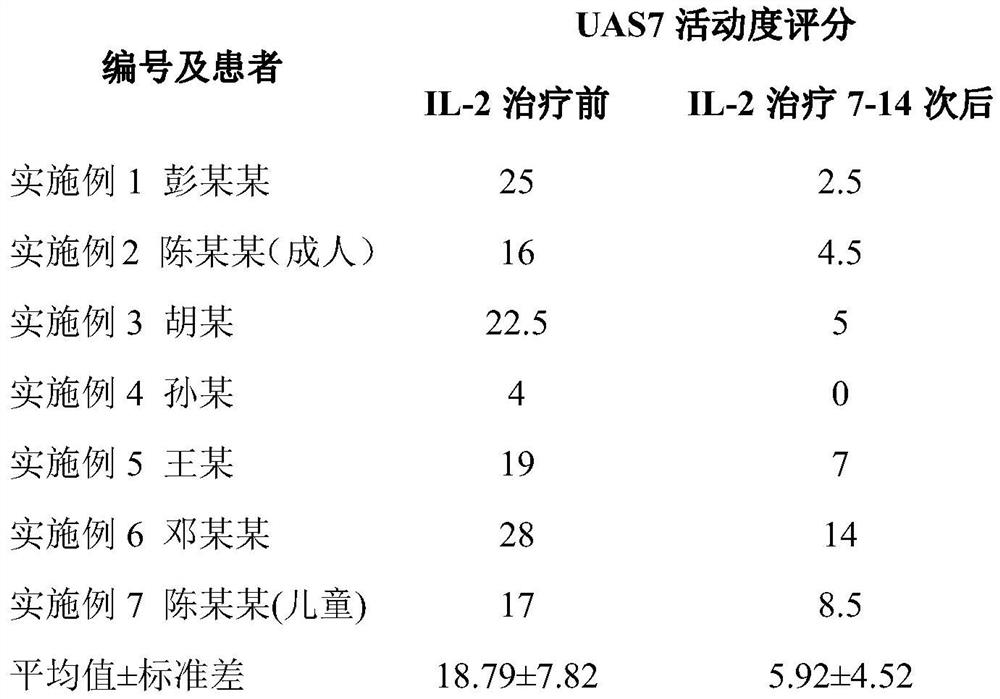
Application of interleukin 2 for treatment of chronic spontaneous urticaria
ActiveCN111821423ALSolve bottlenecksPeptide/protein ingredientsDermatological disorderIntramuscular injectionPharmaceutical drug
The invention belongs to the field of medicine preparation, and particularly discloses application of low-dose interleukin 2 in medicine for treating chronic spontaneous urticaria. The low-dose recombinant human interleukin 2 is innovatively injected to the subcutaneous or intramuscular part of a patient suffering from chronic spontaneous urticaria, the severity and frequency of erythema and whealattack of the patient suffering from chronic spontaneous urticaria with poor treatment control of conventional antihistamine can be reduced, the pruritus degree can be reduced, or the medicine takingdose and medicine taking frequency of the antihistamine can be reduced, and the antihistamine can even be completely stopped from being taken for part of patients.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
Application of interleukin 2 in preparing medicine for treating rhinitis
InactiveCN101125199BRelief of symptoms and signsImprove lesionPeptide/protein ingredientsAerosol deliveryMedicineCurative effect
The present invention discloses an application of the interleukin-2 (IL-2) and the derivatives in the preparation of the drugs for treating rhinitis and pertains to the field of biomedicine. The present invention has the advantages that: the present invention firstly uses the recombinant human interleukin-2 in the treatment of rhinitis, the results show that the interleukin-2 can obviously alleviate the symptoms and physical signs of the rhinitis, significantly alleviate the nasal lesions and have the accurate efficacy.
Owner:BEIJING FOUR RINGS BIOPHARM +1
A human umbilical cord blood-derived regulatory T cell expansion medium and its application method
ActiveCN104357389BReduce riskPromote growth rateBlood/immune system cellsRegulatory T cellCord blood stem cell
The invention relates to an improved expansion culture medium for regulatory T cells of human cord blood origin and an application method of the expansion culture medium. According to the expansion culture medium, heparin anticoagulated autologous cord blood plasma accounting for 10%-12% of the volume of a culture medium, CD3-CD28 antibody co-expressed immunomagnetic beads, recombinant human interleukin 2, 2-mercaptoethanol, rapamycin, HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) and gentamicin are added into the RPMI (Roswell Park Memorial Institute)1640 culture medium; and then separated cell suspension is inoculated in a 96-well plates with a U-shaped bottom, hole-division expansion can be performed every 1-2 days, and the expansion period is 3-4 weeks. All reagents in the culture system reach the GMP (good manufacturing practice) level or are originated from autologous cord blood, so that risks caused by ingredients of animal origin are avoided, and the regulatory T cells can be used for a third-party unrelated donor and directly applied to clinical disease treatment; and compared with a traditional culture system, Treg cells (the regulatory T cells) expanded by the improved culture medium is excellent in aspects of growth speed, purity, activity, lymphocyte inhibition function and the like, and the Treg cells are expected to be used as the regulatory T cells of the third-party unrelated donor and applied to the clinical disease treatment.
Owner:HUNAN XENO LIFE SCI
Application of a kind of interleukin 2 in the preparation of a medicine for treating pemphigus vulgaris oral erosion
ActiveCN109939223BPromote healingRelieve painPeptide/protein ingredientsDigestive systemMouth mucosaPharmaceutical drug
The invention belongs to the field of medicine preparation, and particularly discloses application of interleukin 2 in preparing medicine for treating pemphigus vulgaris oral erosion. Accordingly, lowdose of recombinant human interleukin 2 is innovatively and externally applied to the oral mucosa erosive surface caused by the pemphigus vulgaris, the oral mucosa erosion caused by the pemphigus vulgaris can be quickly recovered, and pain can be effectively relieved.
Owner:THE SECOND XIANGYA HOSPITAL OF CENT SOUTH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com