Optimized nucleotide sequence of recombinant human interleukin-2 and high-efficiency soluble expression method
A technology of interleukin and nucleotide sequence, which is applied in the field of optimizing the nucleotide sequence and highly efficient soluble expression of recombinant human interleukin-2, which can solve complex steps, contribution of interleukin-2 solubility, error To reduce the growth temperature, reduce the formation of inclusion bodies, and reduce the formation of inclusion bodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Codon Optimization of Recombinant Human Interleukin-2 Nucleotide Sequence
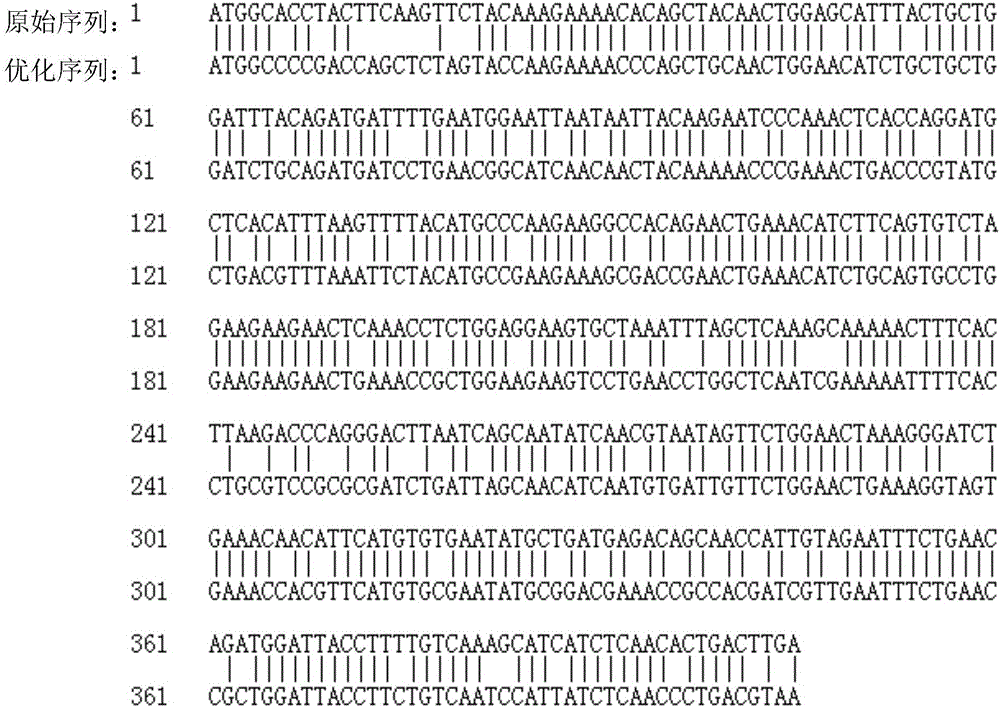
[0047] The nucleotide sequence of recombinant human interleukin-2 is based on NCBI (LOCUS: NM_000586), the signal peptide sequence is removed, and the free energy and stability of the secondary structure of the mRNA are considered while the amino acid sequence is kept unchanged. sequence, etc., replace and optimize the gene sequence using the preferred codons in E.coli, and then carry out the whole gene synthesis.
[0048] The recombinant human interleukin-2 nucleotide sequence was synthesized by Nanjing GenScript Biotechnology Co., Ltd., and the specific sequence is shown in SEQ ID NO:1; the synthesized nucleotide sequence is 405bp in length, and it was constructed in a cloning vector for sequencing verification correct. Compared with the natural human interleukin-2 gene (specific sequence such as SEQ ID NO: 2), the recombinant human interleukin-2 nucleotide sequence synthesized by t...
Embodiment 2
[0049] The construction of embodiment two recombinant expression vectors
[0050] Carriers and reagents: The vector pET21a and strain DH5α were donated by Professor Tan Xiaoli of Jiangsu University; restriction enzymes and DNA markers were purchased from Dalian Bao Biological Engineering Co., Ltd.
[0051] pET21a includes ampicillin selection marker, inducible promoter Ptac, multiple cloning site. The cloning vector with 405bp optimized rhIL-2 sequence and the pET21a plasmid were double digested with NdeI and XhoI respectively, and the two fragments were ligated with T4 ligase to obtain the pET21a / rhIL-2 plasmid, and rhIL-2 was inserted into pET21a Among the multiple cloning sites of , the upstream is Ptac. The ligation product was transformed into Escherichia coli DH5α competent cells by heat shock, clones were selected on LB plates containing ampicillin, plasmids were prepared in small quantities, and positive clones were screened by double enzyme digestion and sequencing i...
Embodiment 3
[0053] Example 3: Screening, acquisition and induction culture of highly efficient soluble expression engineered bacteria
[0054] (1) The recombinant plasmid obtained in Example 2 was transformed into a host bacterium, ie, Escherichia coli BL21 (DE3), which is the engineering bacterium of the present invention, and positive transformants were obtained by screening on an ampicillin solid medium plate.
[0055] (2) Select a positive transformant, add 50ml of LB culture medium, shake culture overnight at 37°C in a 250ml shake flask to mid-late logarithm, that is, overnight activation, inoculate 50ml 2× at 1:100 (v / v) In YT liquid medium (containing 0.1mg / ml ampicillin), culture on a shaker at 37°C until OD600=0.6;
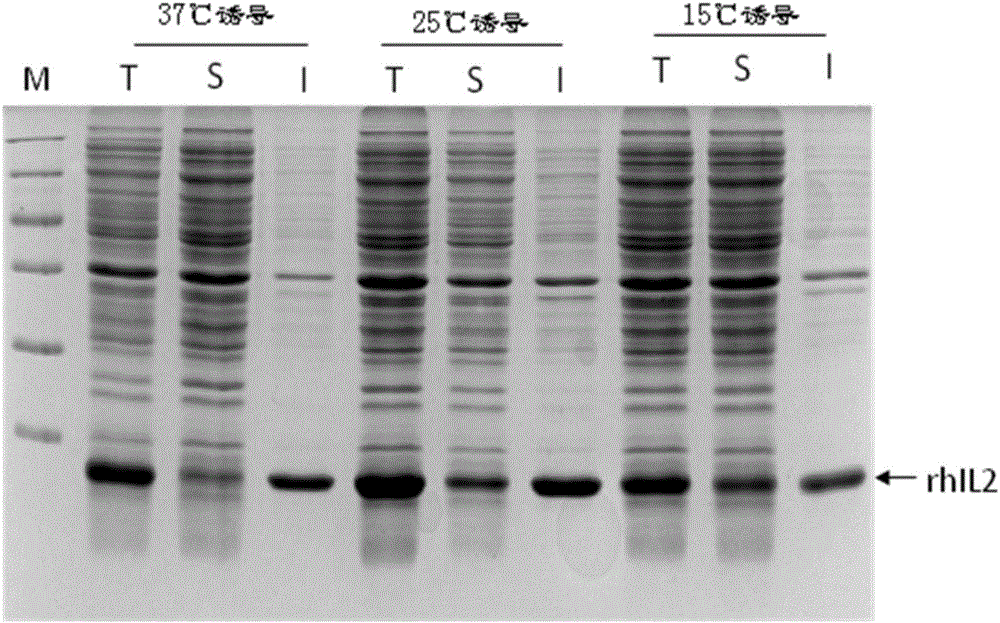
[0056] (3) Add the inducer IPTG to make the final concentration 1mM; culture under different conditions (A, 37°C shaker culture for 4h; B, 25°C shaker culture for 6h; C, 15°C shaker culture for 20h); The cells were collected by centrifugation, the supernatant was di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com