A human umbilical cord blood-derived regulatory T cell expansion medium and its application method
A technology of umbilical cord blood source and culture medium, applied in the direction of blood/immune system cells, animal cells, vertebrate cells, etc., to achieve superior activity and lymphocyte suppression function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1. Separation and purification of umbilical cord blood regulatory T cells
[0038] The obstetrics department of our hospital collected umbilical vein blood from 30 cases of normal delivery full-term fetuses. Immediately after ligation and cutting of the umbilical cord, blood was collected by umbilical vein puncture and anticoagulated with heparin. The general blood collection volume was 50-100ml.
[0039] Collected into a 50ml centrifuge tube, centrifuged at 2500rpm for 10 minutes, took the upper plasma layer (the umbilical cord blood autologous plasma as the medium component of the present invention) for subsequent use, and diluted the lower blood cell layer with 3 times the volume of PBS. After the blood cells are resuspended, place 10ml of 1.077-1.080g / ml density lymphatic separation solution on the lower layer of a 50ml centrifuge tube, and gently add 40ml of blood cell suspension to the upper layer, with a clear boundary between the two layers, centrifuge at...
Embodiment 2
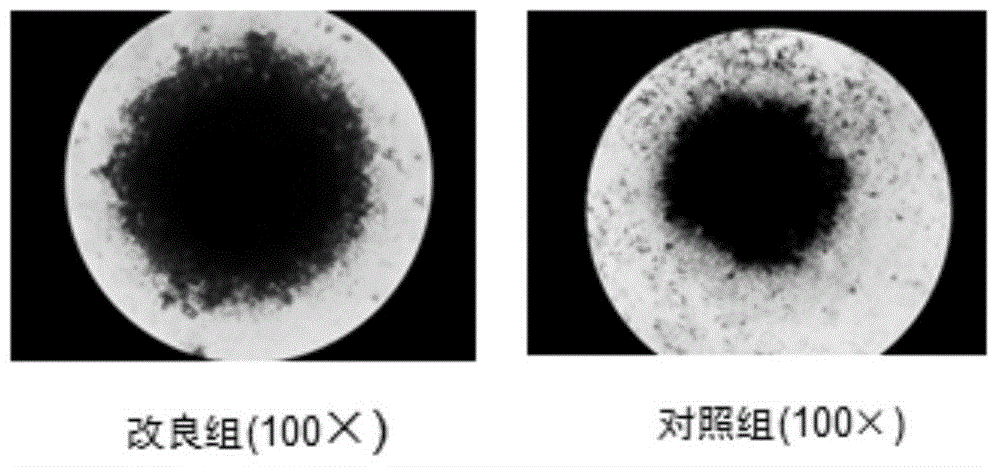
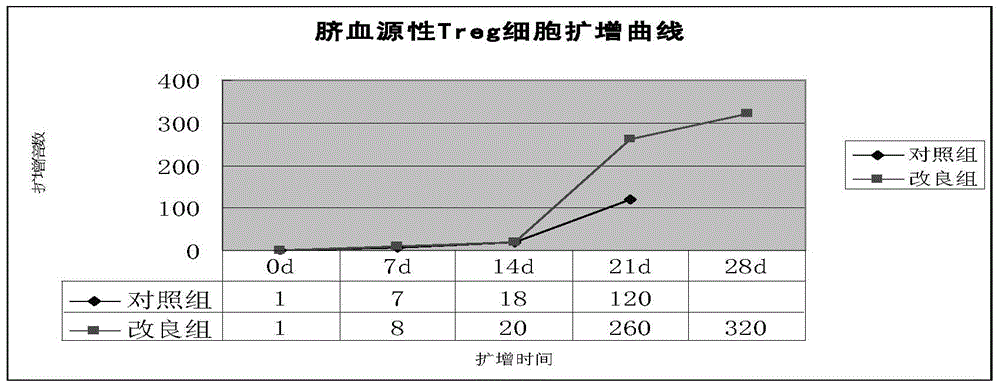
[0046] Example 2. Conditional expansion of umbilical cord blood regulatory T cells
[0047] 1. Improved group: collect CD4+CD25+ regulatory T cells every 5×10 5 Each cell was resuspended in 250μl RPMI1640 conditioned medium containing 10-12% autologous plasma, 800IU / ml IL-2, 50uM 2-ME, 100nM Rapamycin, and CD3CD28 Microbeads were added at a ratio of 1:3-1:4 to the number of cells.
[0048] After culturing for 3-4 days to form cell colonies, break up the cells and subculture with 250μl RPMI1640 conditioned medium containing 10-12% autologous plasma, 800IU / ml IL-2, 50uM 2-ME, 100nM Rapamycin., and CD3CD28 Microbeads For well expansion, the ratio of CD3CD28 Microbeads to cells is 1:2, and the number of cells at the beginning of the split well is about 2×10 5 pcs / hole.
[0049] After that, divide the wells once every 1-2 days (the conditions for dividing the wells are the same as before), the total expansion time is 3-4 weeks, and the cell expansion times of 7, 14, 21, and 28 da...
Embodiment 3
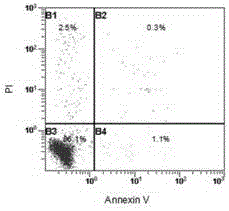
[0054] Example 3. Detection of the phenotype of regulatory T cells in umbilical cord blood by flow cytometry.
[0055] 1.2.1 Collect the CD4+CD25+ regulatory T cells expanded in vitro for 14 days using the medium of the control group and the modified group, and resuspend the cells to 2×10 with PBS 5 cells / 100 μl.
[0056] 1.2.2 Immunofluorescence staining and intracellular factor staining: add CD4-FITC, CD4-PE, CD45-PE-Cy5, CD19-PE-Cy5, CD25-PE-Cy5, HLA-DR-PE, CD62L-PE, Put 5-20 μl each of GITR-PE, CD127-PE, CD45-RO-PE, CD45-RA-PE-Cy5, and CTLA-PE into each well of the present invention, mix well, incubate at 4°C in the dark for 30 minutes, add Wash once with cold PBS, centrifuge at 1600r / min for 5min, discard the supernatant, add 0.5mL freshly prepared fixation / permeabilization solution if intracellular staining is required, protect from light at 4°C for 60min, wash with 1mL fixation / permeabilization buffer Resuspend the cells in 100 μl once, then directly add 5 μl each of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com