Trimutant of recombinant human interleukin-2 and its preparation method
A technology of interleukin and mutants, applied in the fields of botanical equipment and methods, biochemical equipment and methods, chemical instruments and methods, etc., can solve the problems of many background proteins, troublesome purification, low expression level, etc., and it is not easy to achieve Aggregation and precipitation, improved stability, and improved activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
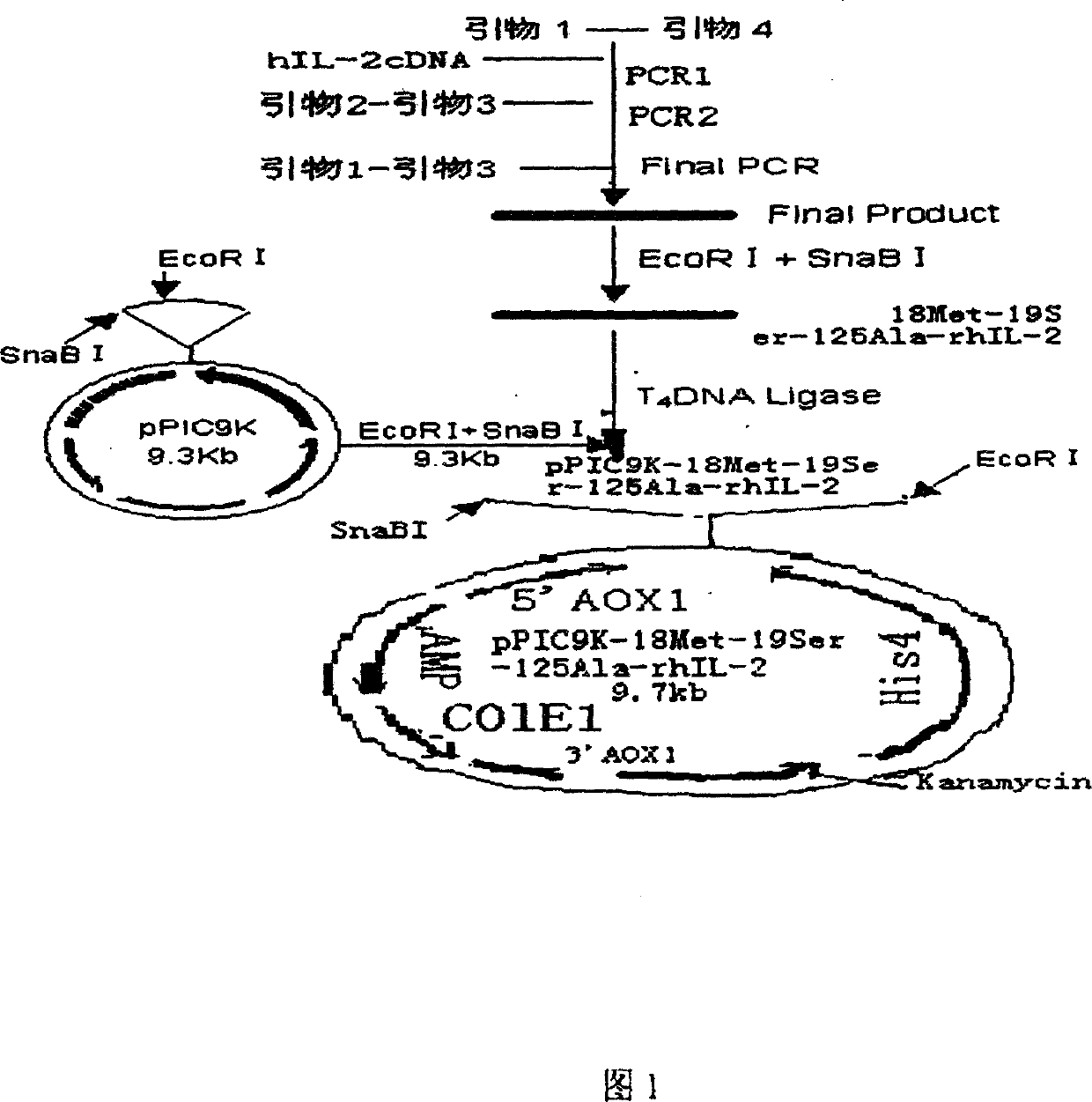
[0027] 1. Cloning and amplification of three-site mutation hIL-2 gene and construction of pPIC9K plasmid
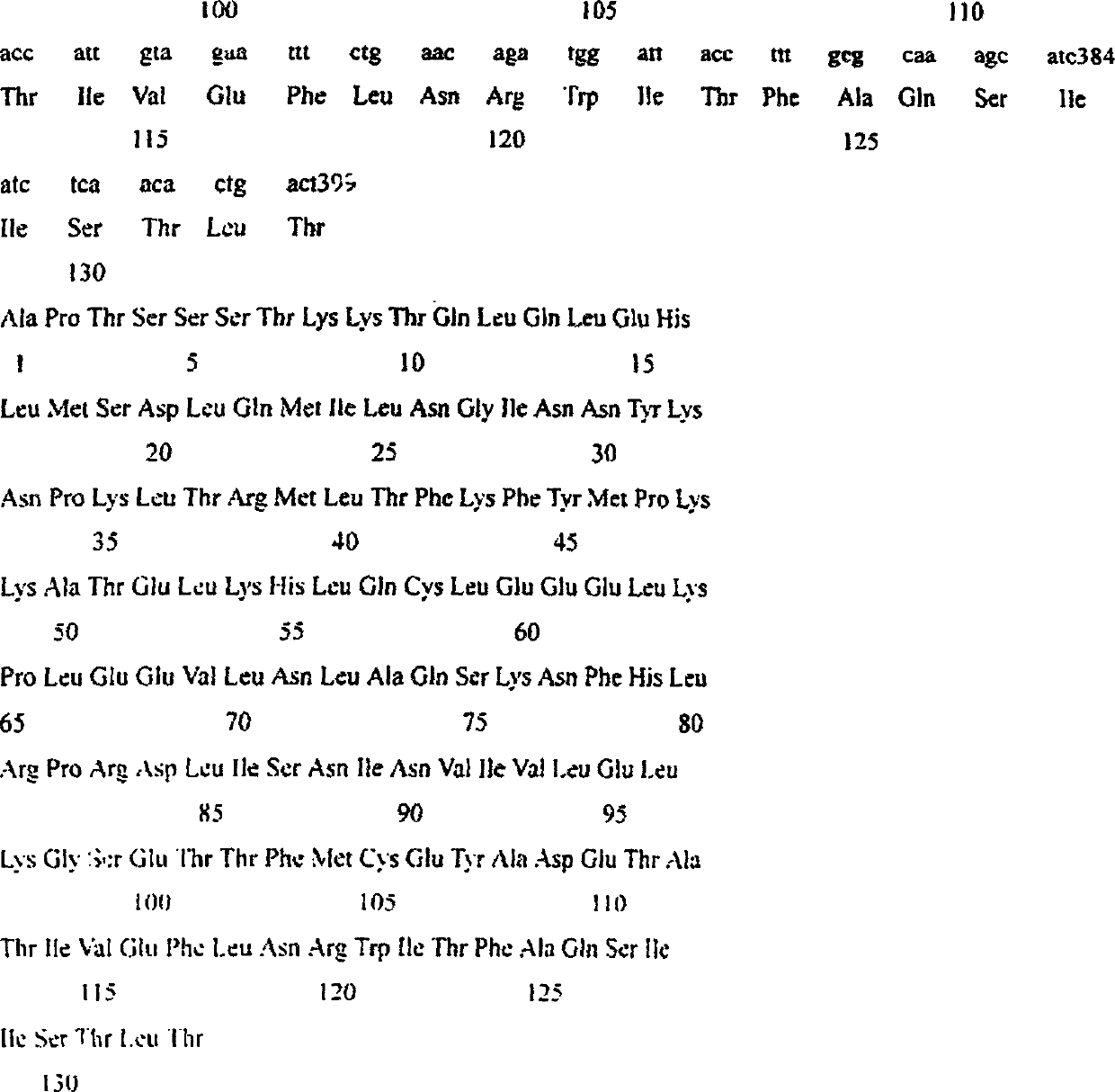
[0028] We designed and automatically synthesized in a DNA synthesizer by the phosphite triester method, purified by 20% acrylamide 7mol / L urea denaturing gel to obtain four primers, primer 1, 5′GCG AAGTCAGTGTTGAGATGATGCTTTG AAAGG3', Primer2, 3'CTGTAAATCAGACAAATTAAATG5', Primer3, 5'GCACGT AGCACCTACTTCAAGTTCTAC 3′, Primer 4, 5′ GCATTTA TCTGATTTACAG 3'. Primer 1 contains EcoR I restriction site, stop codon, 125 Ala and a complementary sequence of the amino acid coding gene at the 3′ end of mature human IL-2, a total of 43 bases, primer 2, a total of 22 bases, primer 3 contains a SnaB I restriction site, start codon and mature hIL -2 The amino acid coding sequence at the 5′ end, with a total of 33 bases, primer 4 is a mutation primer, in which 18 and 19 leucines are mutated into 18 Met, 19 Ser, a total of 22 bases. The full-length cDNA sequence of hIL-2 was amplifi...
Embodiment II
[0087] 1. Purification and activity determination of three-site mutant rhIL-2
[0088] The purification of rhIL-2 is mostly limited to extraction from Escherichia coli. It is the first time to express and purify secreted natural and mutant rhIL-2 with Pichia pastoris. Purifying three-site mutant rhIL-2 from this expression system is a A whole new process of exploration. We explored a set of the simplest and fastest purification steps.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com