Serum-free cryopreservation medium and method for peripheral blood monouclear cells
A technology of peripheral blood mononuclear cells, applied in the field of immune cell cryopreservation, peripheral blood mononuclear cells completely serum-free cryopreservation solution, to maintain cell stability, reduce possibility, and ensure the effect of biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
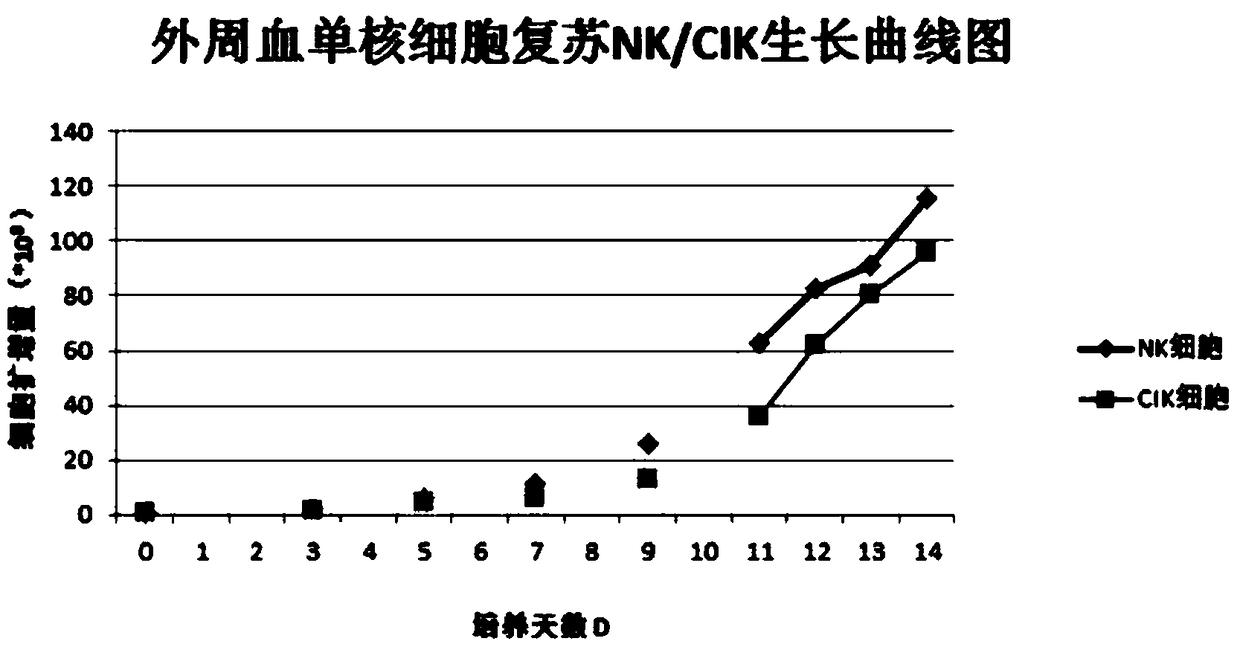
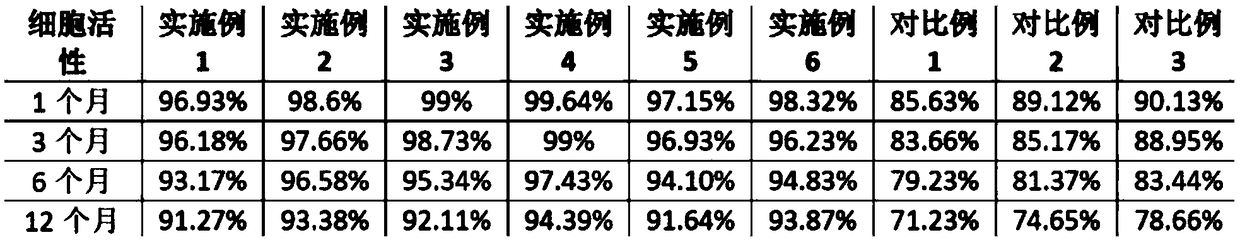
[0036] Example 1: Preparation of peripheral blood mononuclear cell cryopreservation solution (recombinant human interleukin-2+polyethylene glycol+trehalose+DMEM / F12 medium+human albumin): by volume content, 0.05g / ml human Serum albumin, 50U / mL recombinant human interleukin-2, 0.1g / mL polyethylene glycol, 0.1g / mL trehalose and 99% DMEM / F12 medium were mixed to obtain peripheral blood mononuclear cells Freeze the solution and store it at 4°C for later use.
Embodiment 2
[0037] Embodiment 2: Preparation of peripheral blood mononuclear cell cryopreservation solution (recombinant human interleukin-2+polyethylene glycol+trehalose+DMEM / F12 medium+human albumin): by volume content, 0.05g / ml human Serum albumin, 100 U / mL recombinant human interleukin-2, 0.2 g / mL polyethylene glycol, 0.15 g / mL trehalose and 98% DMEM / F12 medium were mixed to obtain peripheral blood mononuclear cells Freeze the solution and store it at 4°C for later use.
Embodiment 3
[0038] Example 3: Preparation of peripheral blood mononuclear cell cryopreservation solution (recombinant human interleukin-2+polyethylene glycol+trehalose+DMEM / F12 medium+human albumin): by volume content, 1.0g / ml human Serum albumin, 200U / mL recombinant human interleukin-2, 0.0.3g / mL polyethylene glycol, 0.2g / mL trehalose and 95% DMEM / F12 medium were mixed to obtain peripheral blood mononuclear The cell freezing solution was stored at 4°C for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com