Recombinant human interleukin-2 freeze-dried preparation for injection and preparation method thereof
The technology of interleukin and freeze-dried preparation is applied in the field of recombinant human interleukin-2 freeze-dried preparation for injection and its preparation, which can solve the problem that the performance of recombinant human interleukin-2 for injection is prone to change, the activity is decreased, and the Affect product quality and expiration date and other issues, and achieve the effect of being conducive to large-scale production, stable performance, easy transportation and storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
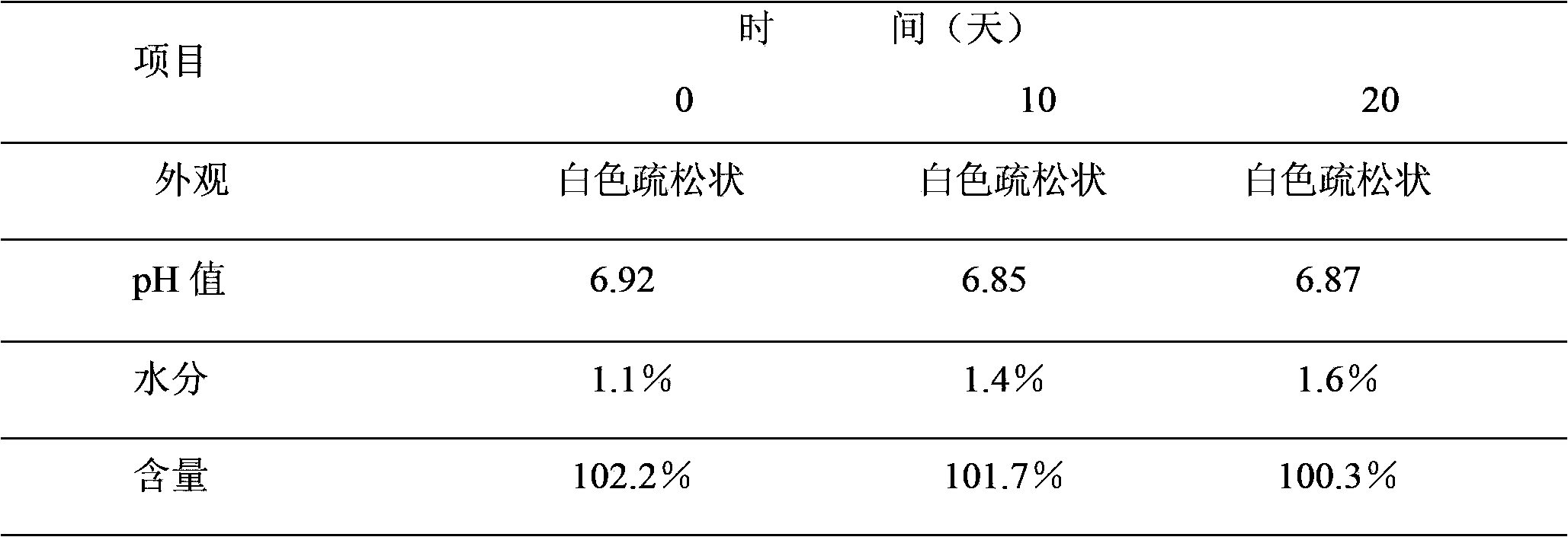
[0029] Take an appropriate amount of recombinant human interleukin-2 stock solution and an appropriate amount of mannitol injection, add sodium citrate solution, add SDS, add human serum albumin, stir well, control the pH value between 6.0 and 8.0, and dilute with water for injection , to obtain a solution containing recombinant human interleukin-2.1 million IU and 0.1 g of mannitol per milliliter. The solution was sterilized by filtration with a 0.22 μm microporous membrane, and under aseptic conditions, the resulting solution was placed in a sterile vial of 1.0 ml per bottle, half-stoppered with a venting rubber stopper, and freeze-dried in a freeze dryer for about 24 hours. Hours later, full vacuum plugging was performed to obtain the recombinant human interleukin-2 freeze-dried powder injection A of the present invention.
Embodiment 2~4
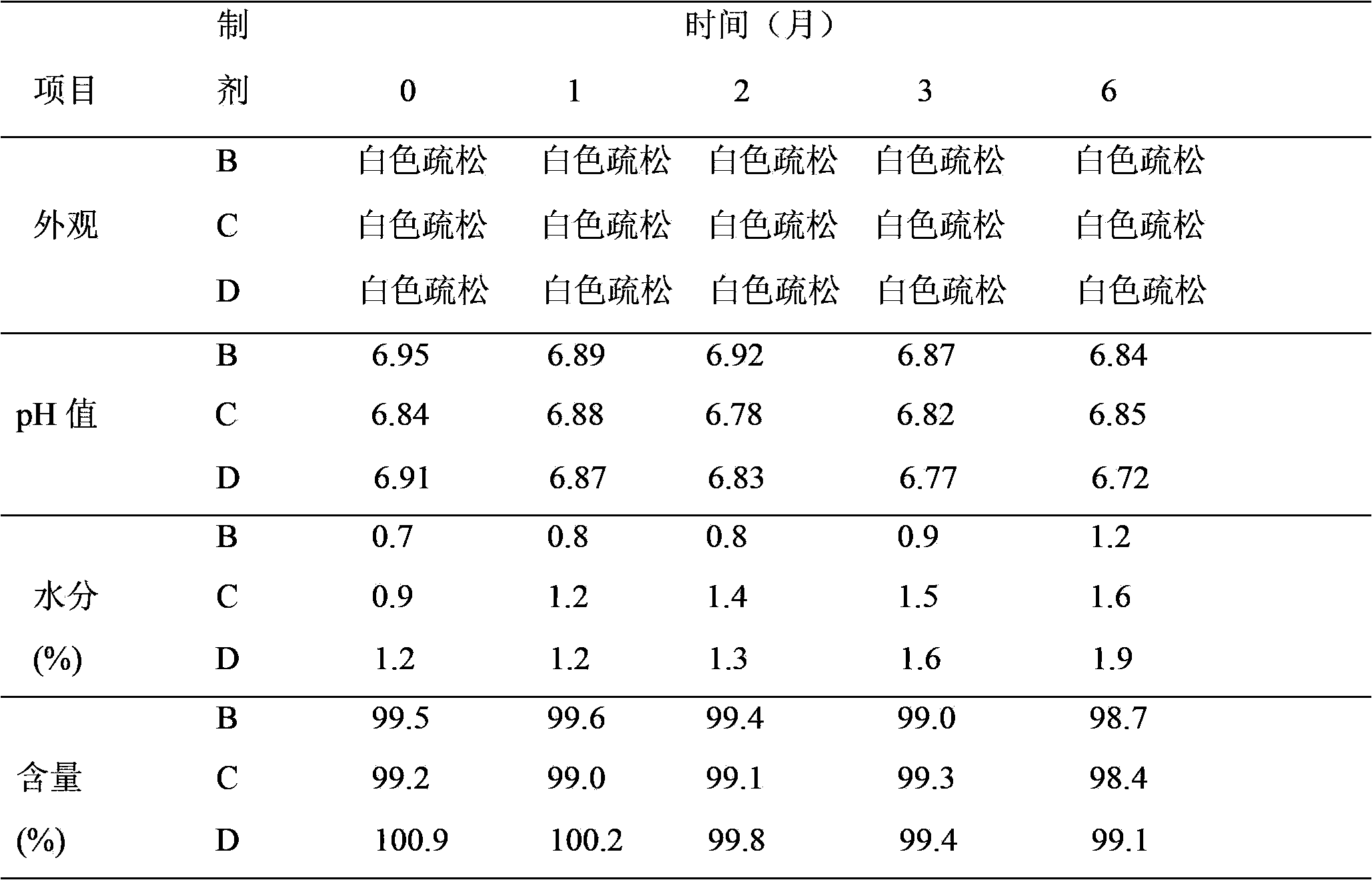
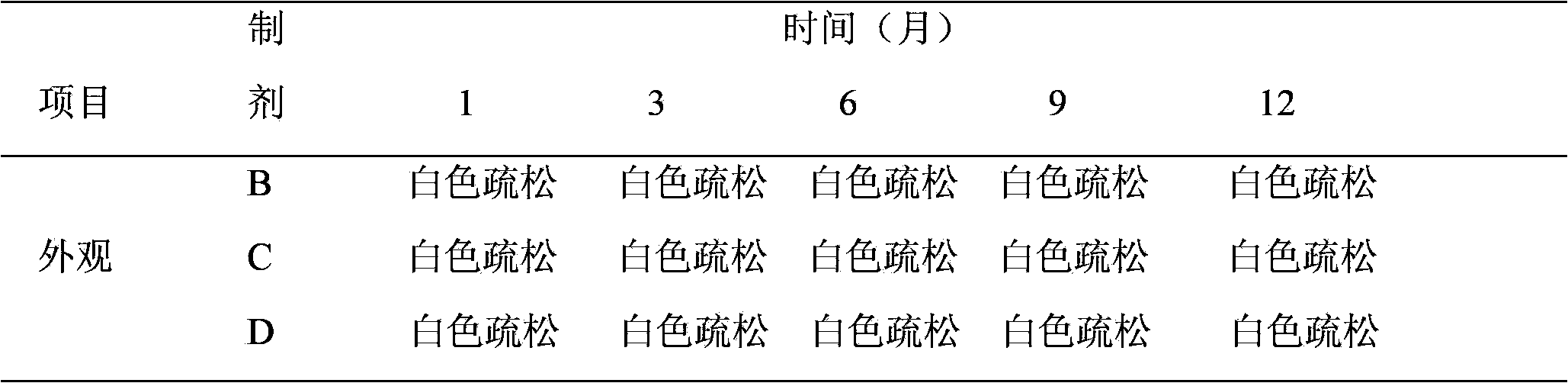
[0031] Take appropriate amount of recombinant human interleukin-2 stock solution and appropriate amount of mannitol injection, add sodium dihydrogen phosphate and disodium hydrogen phosphate buffer, add SDS, add human serum albumin, stir well, and control the pH value at 6.0-8.0 Between them, dilute with water for injection to obtain solutions containing 0.05g, 0.1g, and 0.15g of recombinant human interleukin-2.1 million IU and mannitol per milliliter. The solution is sterilized by filtration with a 0.22 μm microporous membrane, and the resulting solution is placed in a sterile vial at 1.0 ml per bottle under aseptic conditions, half stoppered with a venting rubber stopper, and placed in a freeze dryer according to Example 8 After freeze-drying by the method according to the above method, full vacuum plugging is used to obtain recombinant human interleukin-2 freeze-dried powder injections B, C, and D of the present invention.
Embodiment 5
[0033] Take an appropriate amount of recombinant human interleukin-2 stock solution and an appropriate amount of lactose aqueous solution, add sodium dihydrogen phosphate and disodium hydrogen phosphate buffer, add SDS, add human serum albumin, stir well, and control the pH value between 6.0 and 8.0, Dilute to volume with water for injection to obtain a solution containing recombinant human interleukin-2.1 million IU and lactose 0.1 g per milliliter. The solution is sterilized by filtration with a 0.22 μm microporous membrane, and the resulting solution is placed in a sterile vial at 1.0 ml per bottle under aseptic conditions, half stoppered with a venting rubber stopper, and placed in a freeze dryer according to Example 8 After freeze-drying according to the method described above, full vacuum plugging is used to obtain the recombinant human interleukin-2 freeze-dried powder injection E of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com