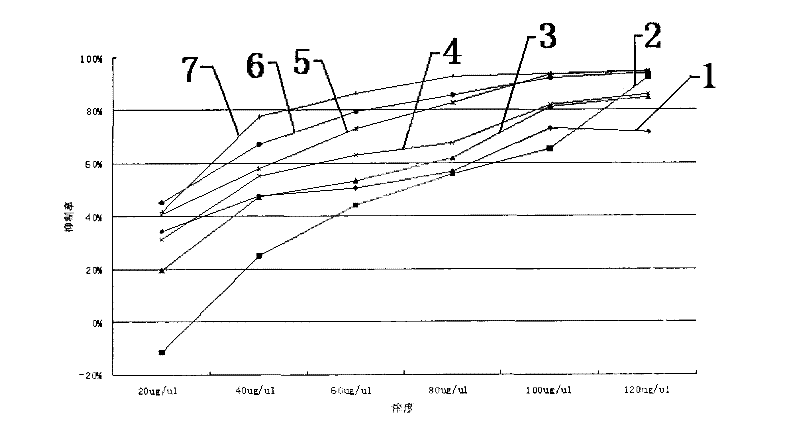
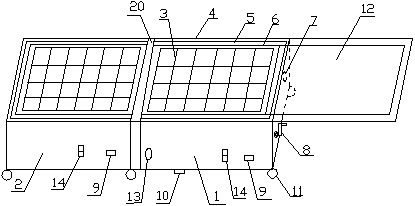
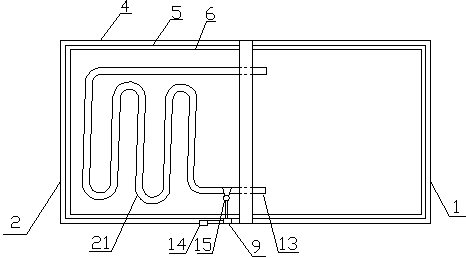
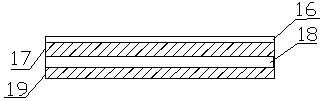
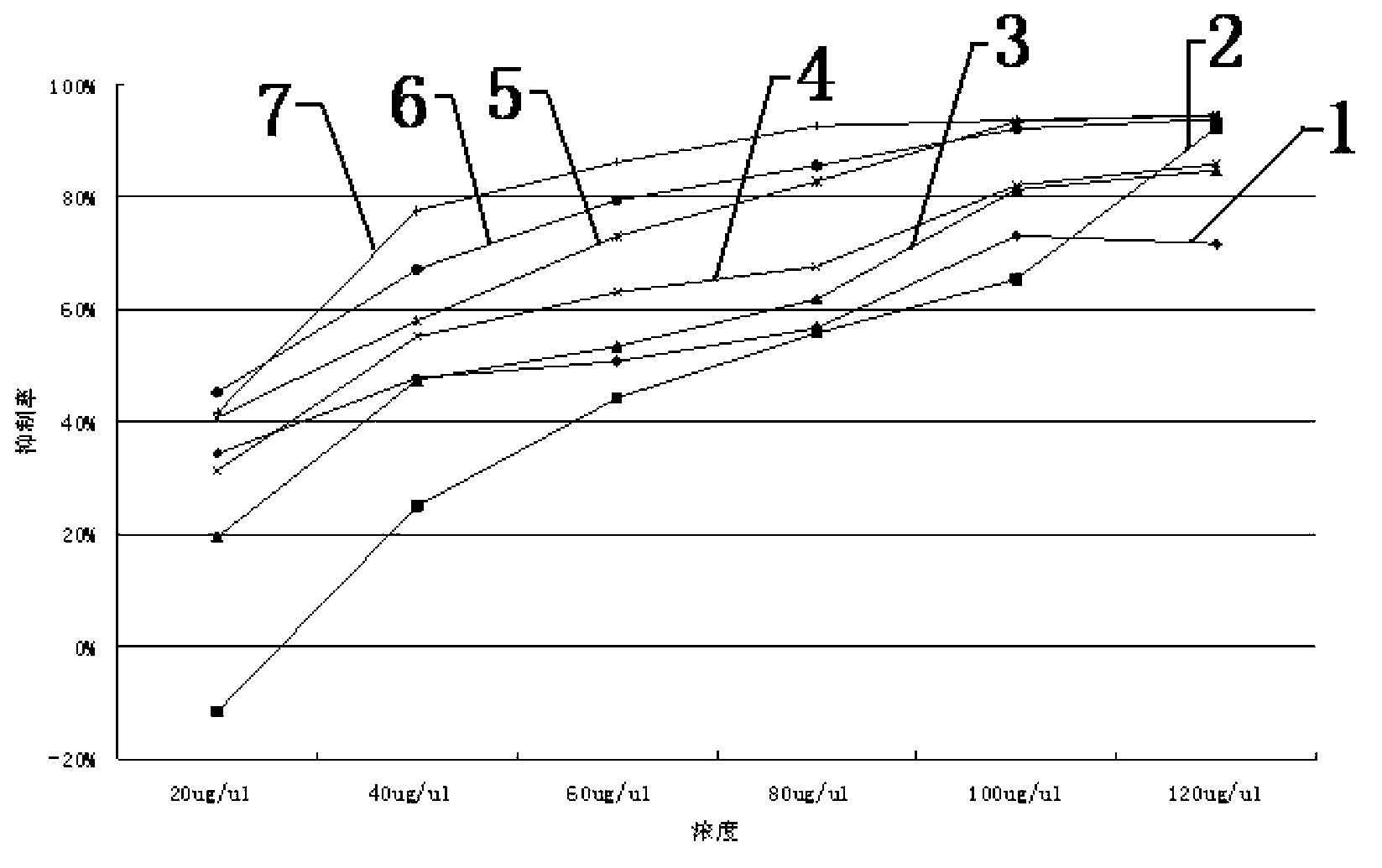
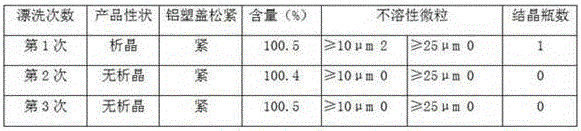
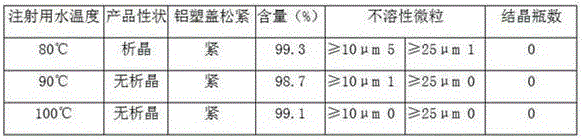
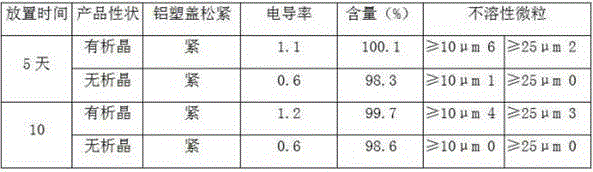
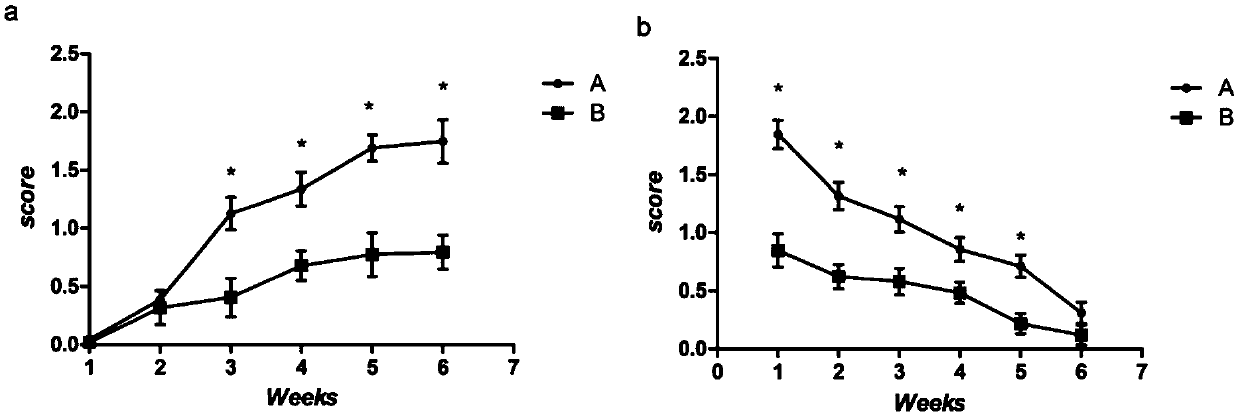
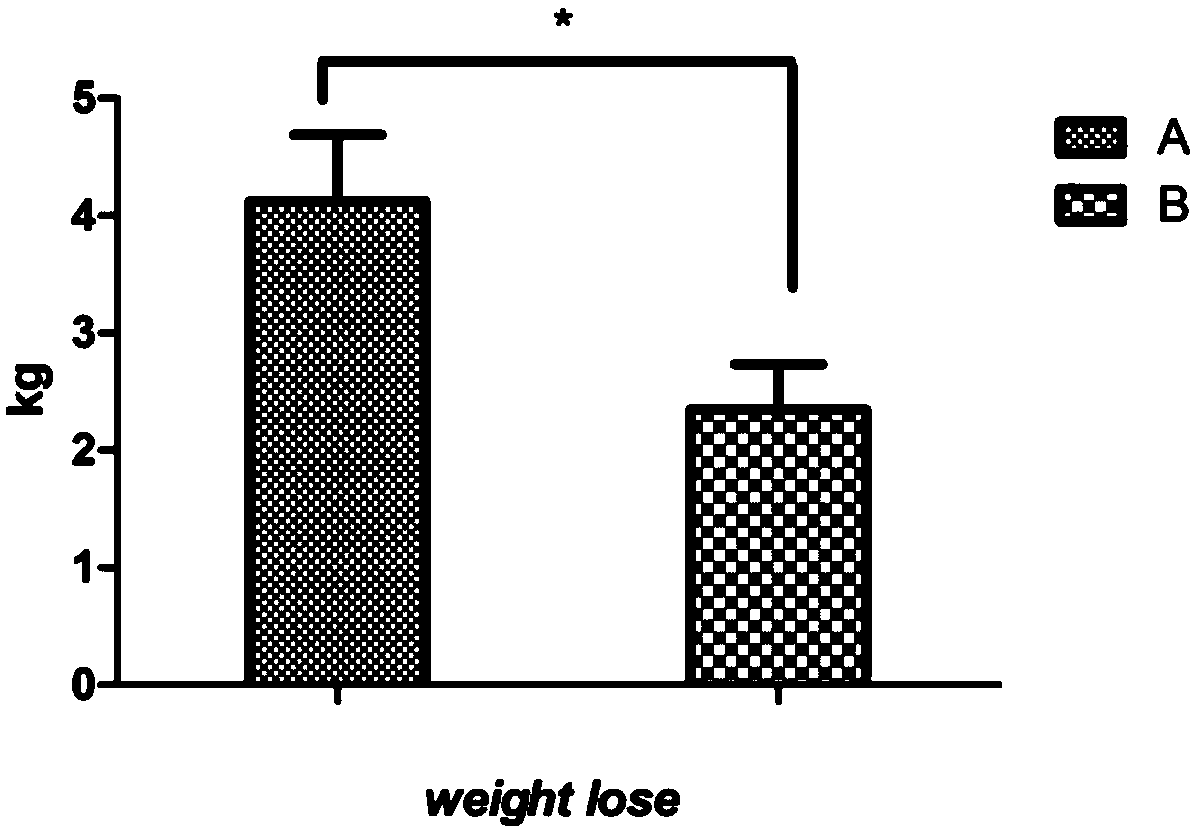
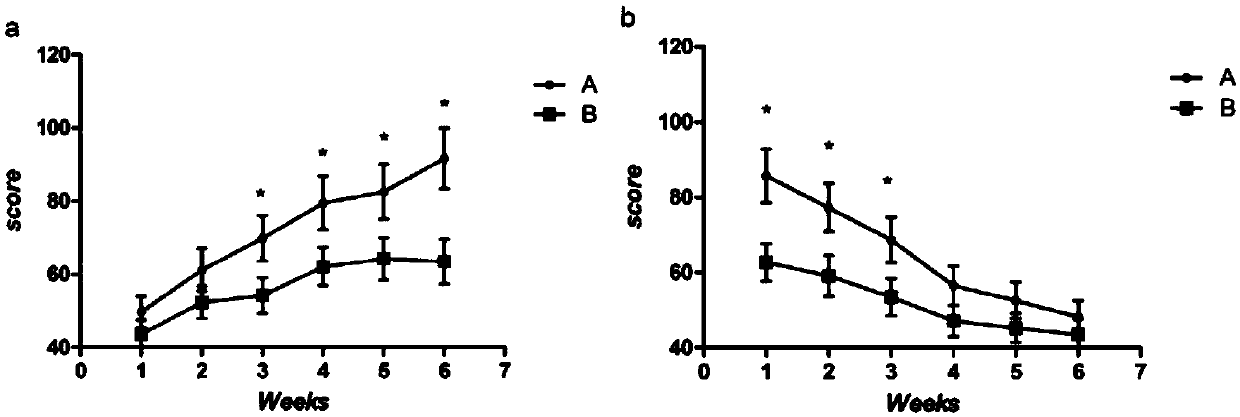
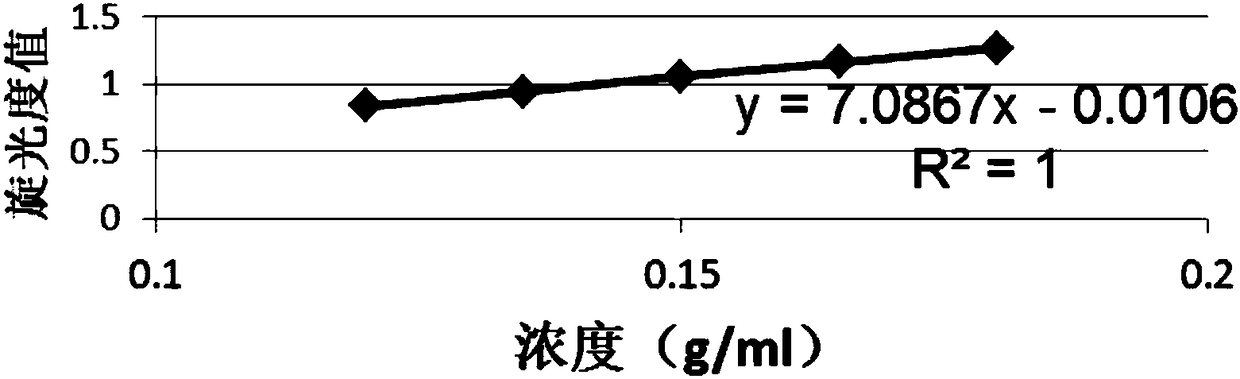
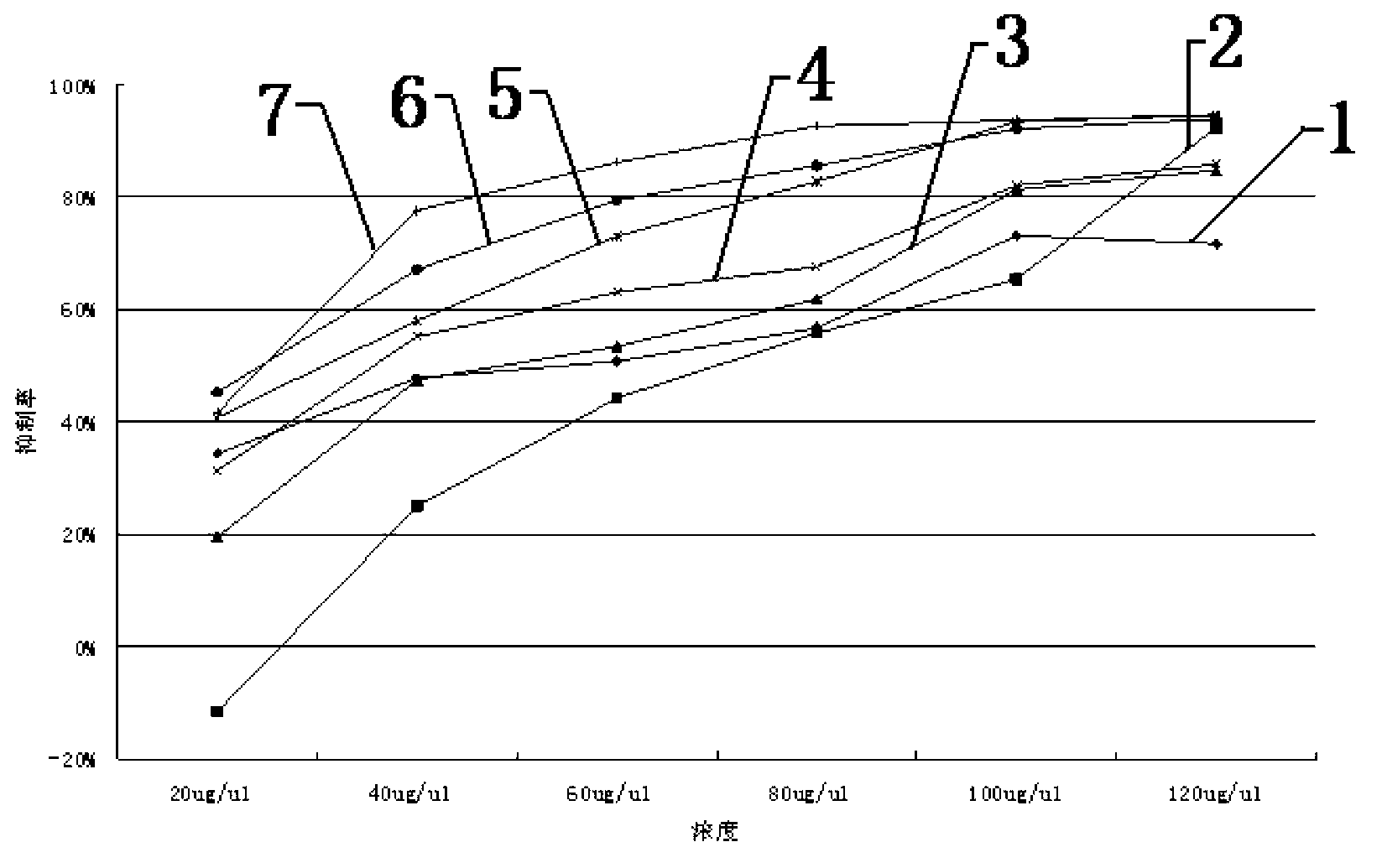
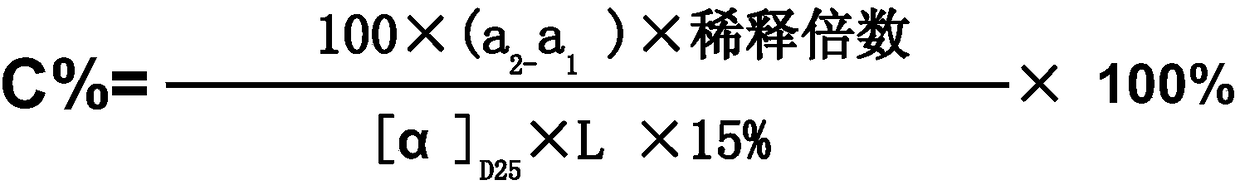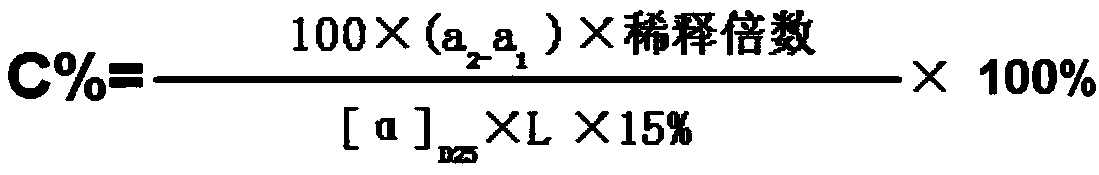Patents
Literature
45 results about "Mannitol Injection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
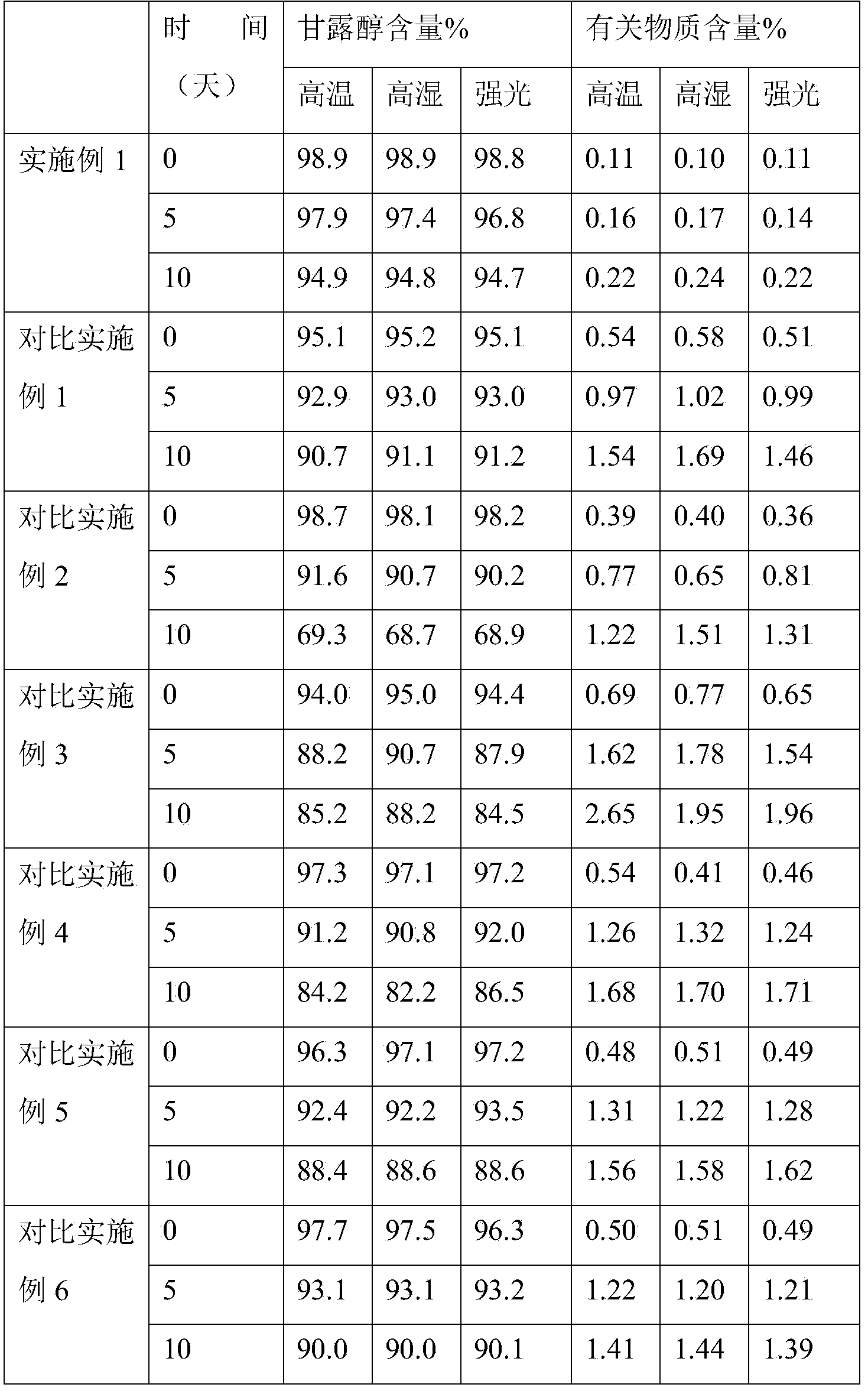
20% Mannitol Injection USP is a sterile, nonpyrogenic solution of Mannitol USP in a single dose container for intravenous administration. It contains no antimicrobial agents. Mannitol is a 6-carbon sugar alcohol prepared commercially by the reduction of dextrose. Although virtually inert ...
Compound mannitol injection and preparation method thereof
InactiveCN103432066AMild process conditionsEasy to operateSenses disorderHydroxy compound active ingredientsPropylene glycolMannitol Injection
The invention relates to a compound mannitol injection which is added with a small amount of propylene glycol based on a conventional compound mannitol injection. The invention also relates to a preparation method of the compound mannitol injection. The compound mannitol injection provided by the invention is substantially improved in stability, and has no crystallization even after long-term storage, and thus the injection is substantially improved in quality and extremely improved in pharmacy safety. The preparation method provided by the invention is mild in technological conditions and simple in operation, and is suitable for large-scale industrialized application.
Owner:蚌埠丰原涂山制药有限公司
Compound formula mannitol injection liquid
InactiveCN1823746AReduce concentrationIncrease concentrationHydroxy compound active ingredientsPharmaceutical delivery mechanismMANNITOL/SORBITOLMannitol Injection
Owner:夏运岳
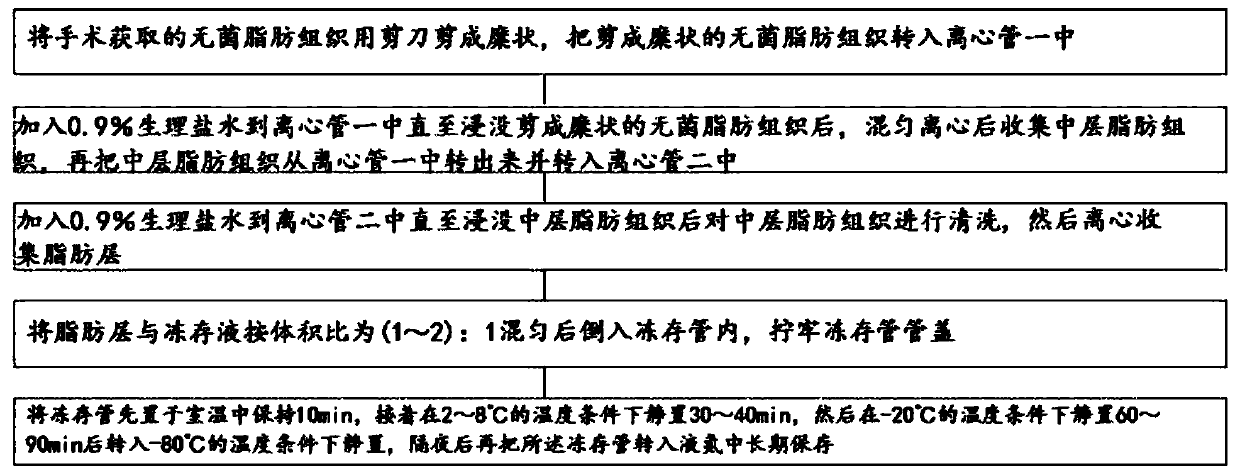
Adipose tissue cryopreservation liquid at clinical application level and cryopreservation method
Adipose tissue cryopreservation liquid at a clinical application level and a cryopreservation method. The adipose tissue cryopreservation liquid at the clinical application level comprises 15-50-mmol / L saccharose, 15-50-mmol / L mannitol injection, 1-5-mmol / L glucose injection, 1-3-mol / L adenosine injection, 0.5-5-mmol / L reduced glutathione, 1-4.5-mol / L trehalose and 0.1-1-mmol / L EDTA-2Na; and the components are mixed to form mixed liquid, and the mixed liquid is the adipose tissue cryopreservation liquid at the clinical application level. By means of the adipose tissue cryopreservation liquid,in combination with the cryopreservation method thereof, at the clinical application level, the defect of adverse reaction due to 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES), phenol red, DMSO, animal serum, blood platelet extracts and the like which are used in the prior art is effectively overcome.
Owner:陕西医赛尔生物科技有限公司
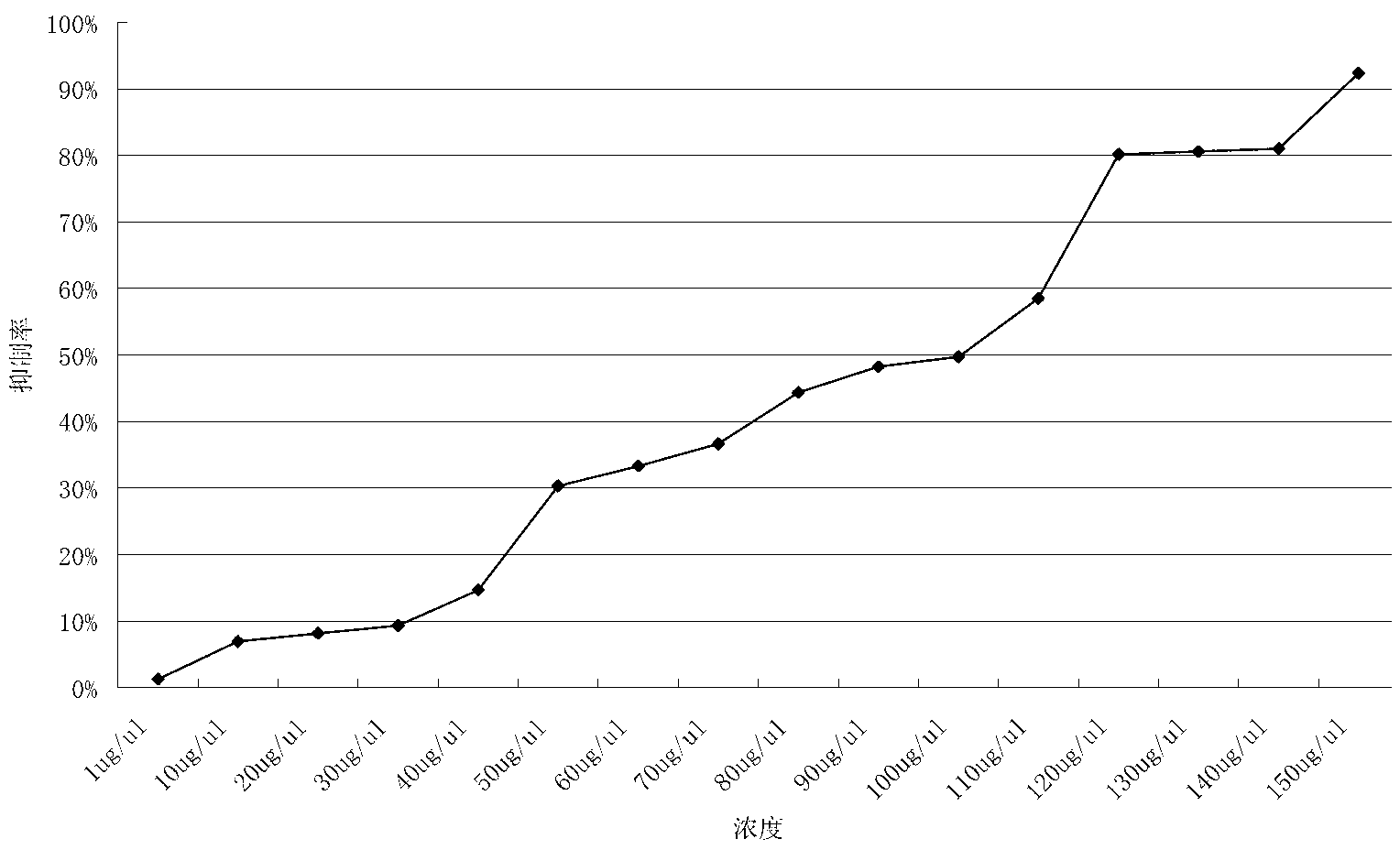
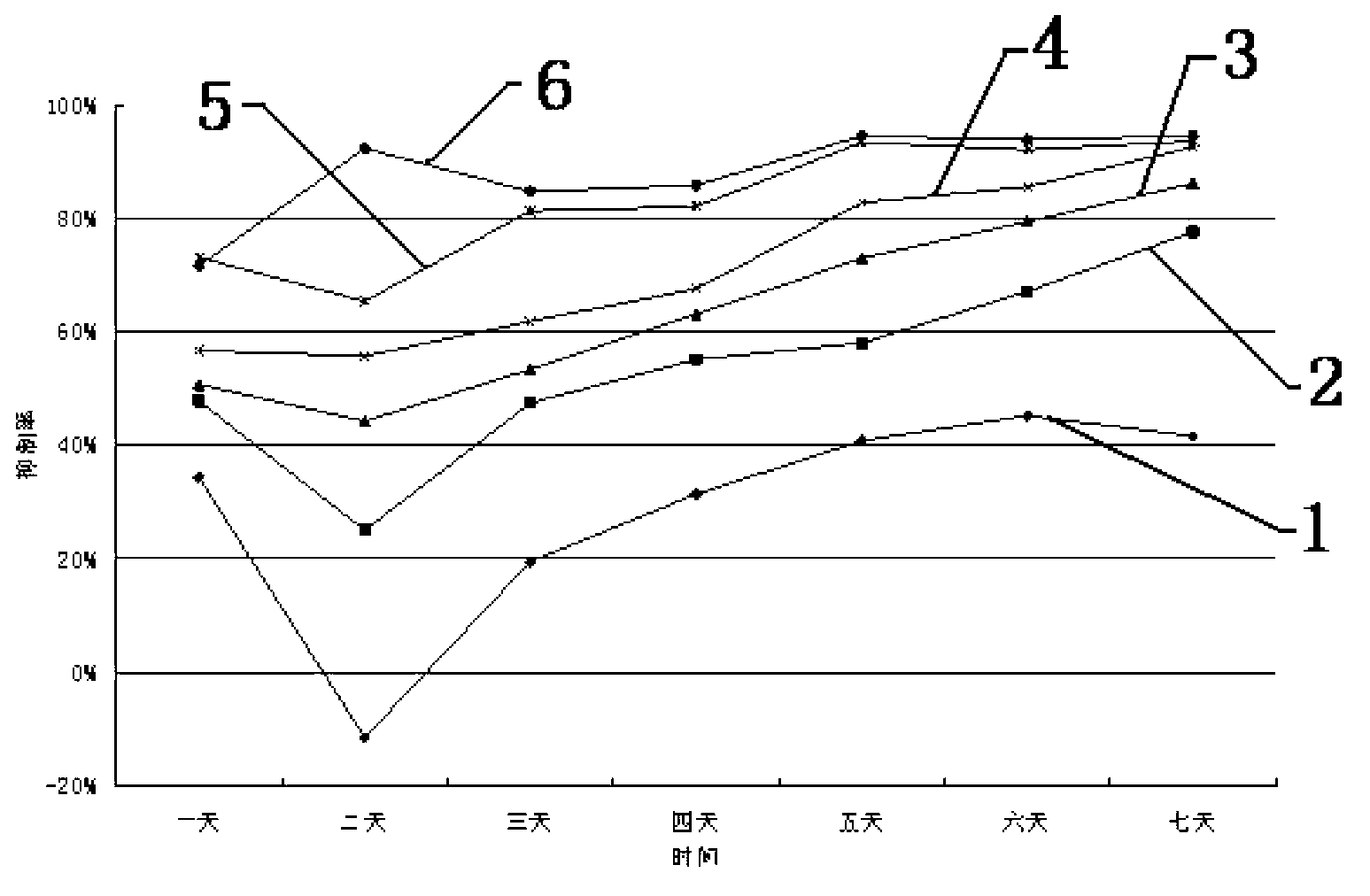
Application of mannitol in preparing antitumor medicines
InactiveCN102225059AHydroxy compound active ingredientsPharmaceutical delivery mechanismApoptosisStomach cancer
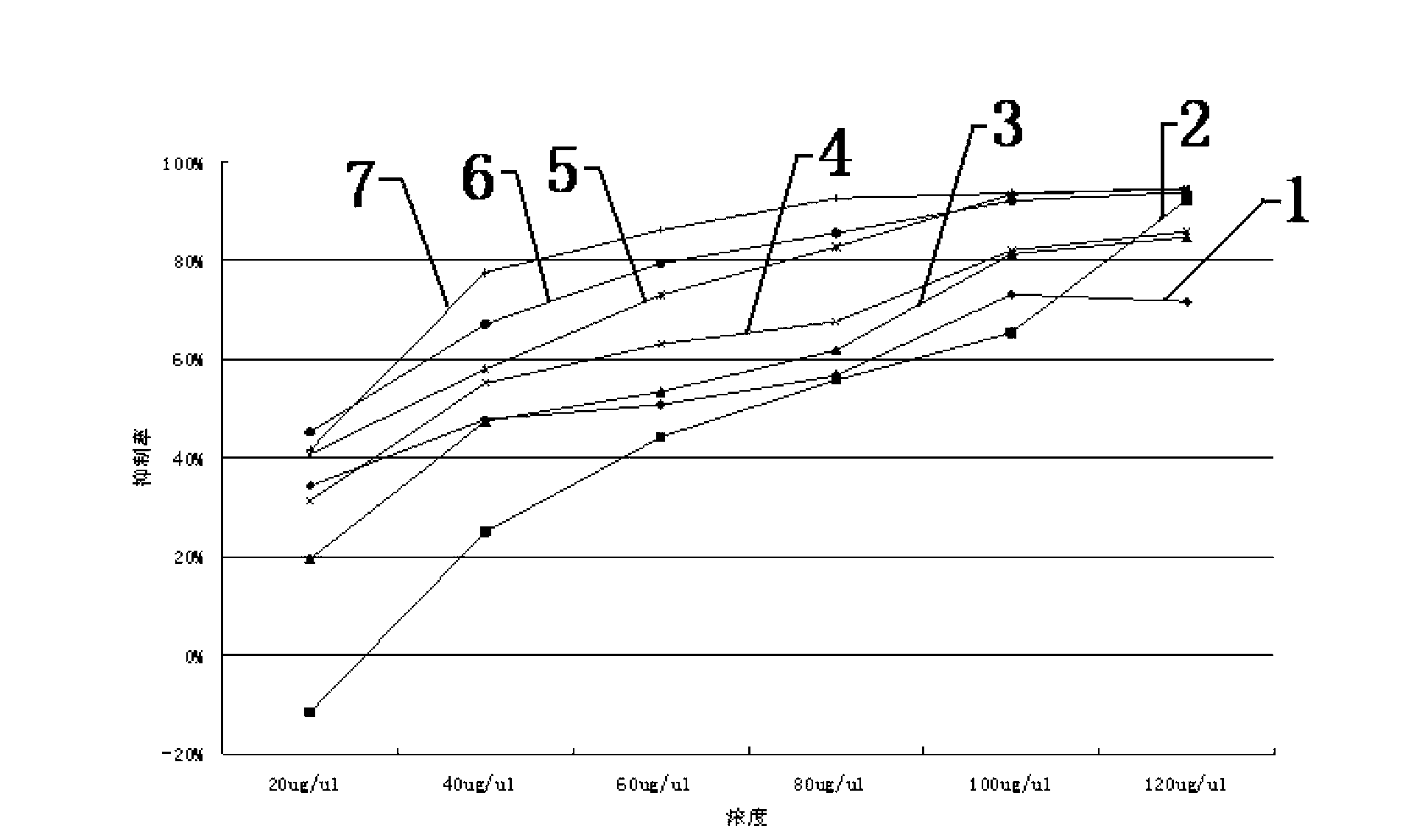
The invention discloses an application of mannitol in preparing antitumor medicines, and particularly relating to an application of a mannitol injection of a specific concentration (10-19%) in treating lung cancer, liver cancer, breast cancer, pancreatic cancer, stomach cancer, adenocarcinoma of stomach, and so on. Traditionally, mannitol is only used as a diuretic, a high-penetration antihypertensive agent, and so on. The inventor finds that mannitol has antitumor activity through scientific experiments, confirms that by using a multiterm apoptosis detection technique and detecting apoptosis-associated genes bc1-2 and bax, mannitol of a specific concentration can induce distinct apoptosis of a great variety of cancer cells, and masters the effect relationship between concentration and time, and preliminarily elucidates the possible mechanism. In addition, the inventor applies the research results in treating tumor patients and obtains distinct curative effects. The results in the invention provide theoretical basis and practical cases for the application of mannitol in preparing antitumor medicines and the clinical applications of mannitol.
Owner:广西壮族自治区肿瘤防治研究所
Thermal insulation device for water-bath heating and dissolving of mannitol
ActiveCN103920401AEvenly heatedSolution is not easy to dissolveMixer accessoriesDissolvingTemperature controlInsulation layer
The invention discloses a thermal insulation device for water-bath heating and dissolving of mannitol. The thermal insulation device comprises a heating box and a thermal insulation box, wherein a thermal-protective coating is arranged between the heating box and the thermal insulation box; the heating box and the thermal insulation box comprise box bodies and box covers; box body walls and the box covers structurally comprise housings, thermal insulation layers and water-resisting layers; a heating device is arranged above the thermal insulation layer inside each box body and is connected with a temperature controller; a filter net is arranged inside each box body; a vertical lead rail is also arranged at the inner side of each water-resisting layer; a bump matched with the corresponding lead rail is arranged on the edge of each filter net; a filter net crank is arranged on the outer side of each box body, and controls the corresponding filter net to slide up and down along the corresponding lead rail through a cam device; a heat-conducting coil is also arranged on the bottom of the thermal insulation box; a water inlet and a water outlet of the heat-conducting coil are formed inside the heating box; a miniature temperature control electric pump is arranged at the water inlet section of the heat-conducting coil inside the thermal insulation box, and connected with the temperature controller. The device can be used for heating and constantly keeping warm, the problems that mannitol injection is not easy to inject at low temperature or high temperature are solved, and the thermal insulation device has an energy-saving effect, and is convenient to use, low in manufacturing and use costs, and convenient to popularize and use.
Owner:滨州医学院附属医院
Intestinal flora reconstruction kit and application thereof
ActiveCN108891725AEasy to useEasy to takeDigestive systemUnknown materialsIntestinal structurePolyethylene glycol electrolyte powder
The invention discloses an intestinal flora reconstruction kit and application thereof. The kit comprises a kit body, which comprises a plurality of chambers opened sequentially and regularly, whereinthe chambers are an n chamber to an n+x chamber according to the opening sequence, the opening time interval between the adjacent chambers in an n+1 chamber to the n+x chamber is setas 4-12 hours; the nchamber contains compound polyethylene glycol electrolyte powder or mannitol injection; the n+1 chamber contains microecological preparation agents with group A and B; ann+2 chamber contains the microecological preparation agents with group B and C; an n+3 chamber to an n+33 chamber contain the microecological preparation agent with group A or B; and an n+34 chamber to the n+x chamber contains the microecological preparation agent with group B. Taking different preparation agents by washing intestines and taking the agents in different sequences is conducive to the rapid establishment of the intestinal flora. The invention further discloses an application of the kit in the reconstruction of diabetic intestinal flora, the flora reconstruction is conducive to regulating the blood glucose of patients, so that the reconstruction of intestinal flora is feasible in regulating the blood glucose of the diabetic patients.
Owner:HEBEI UNIVERSITY
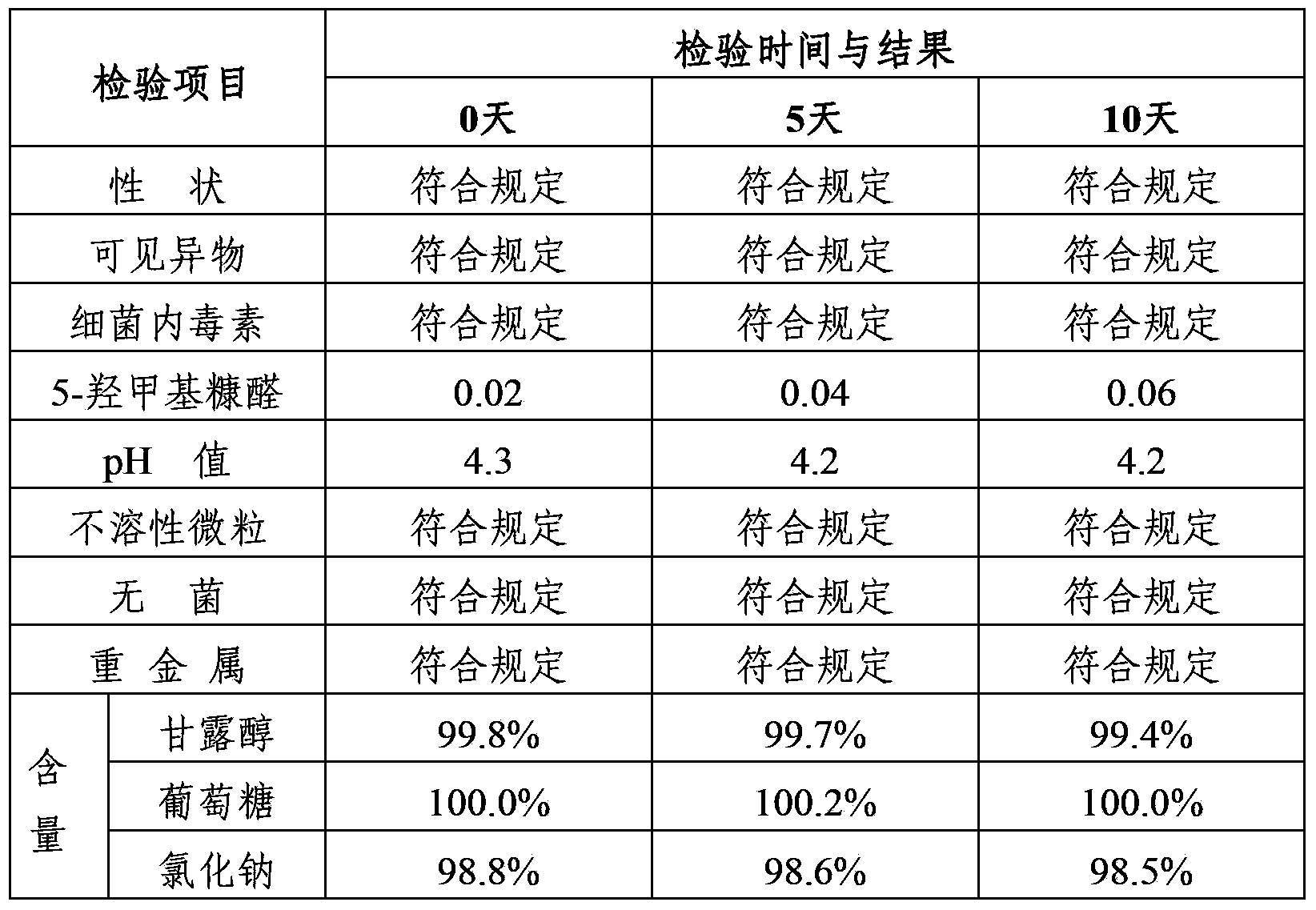
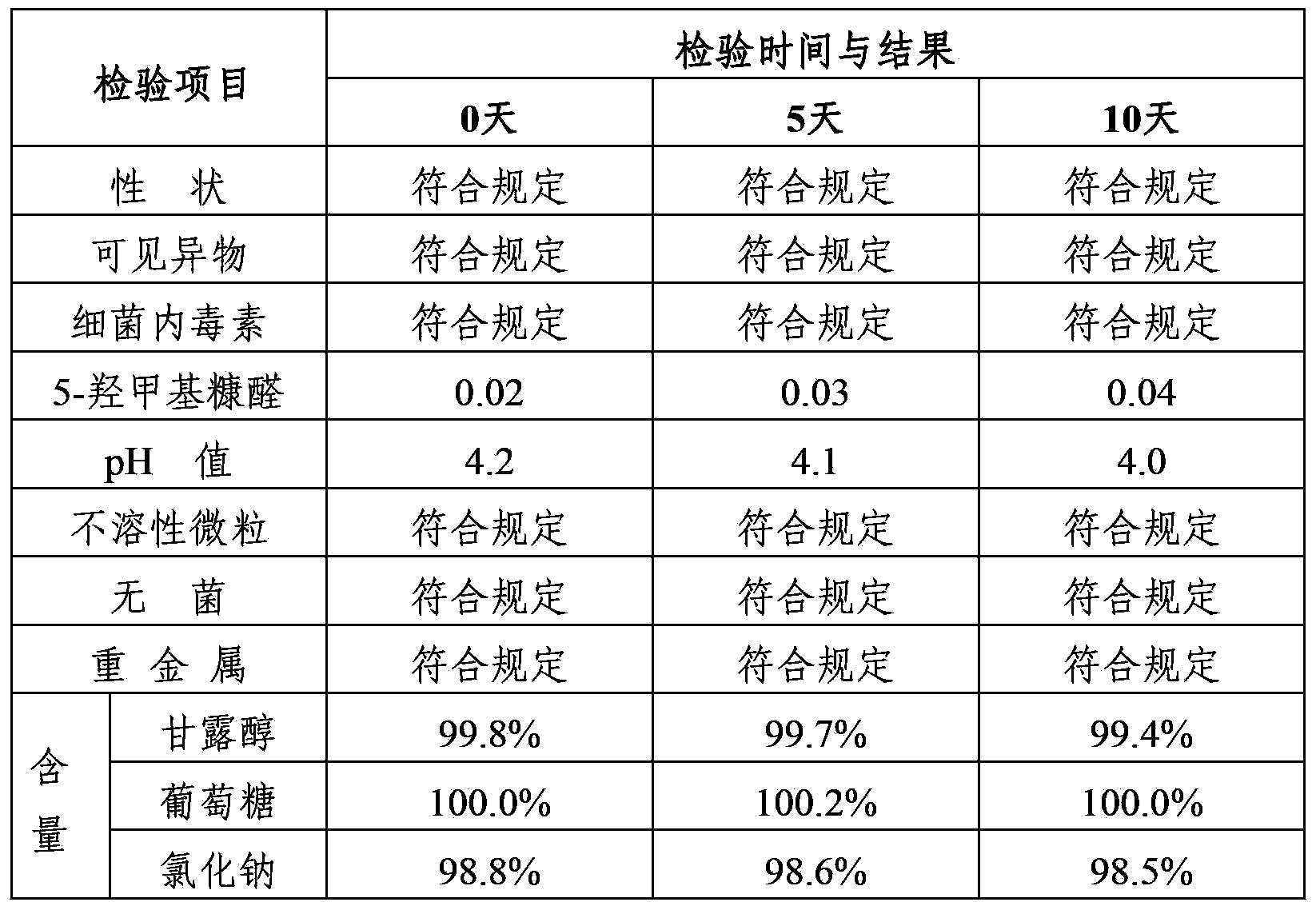
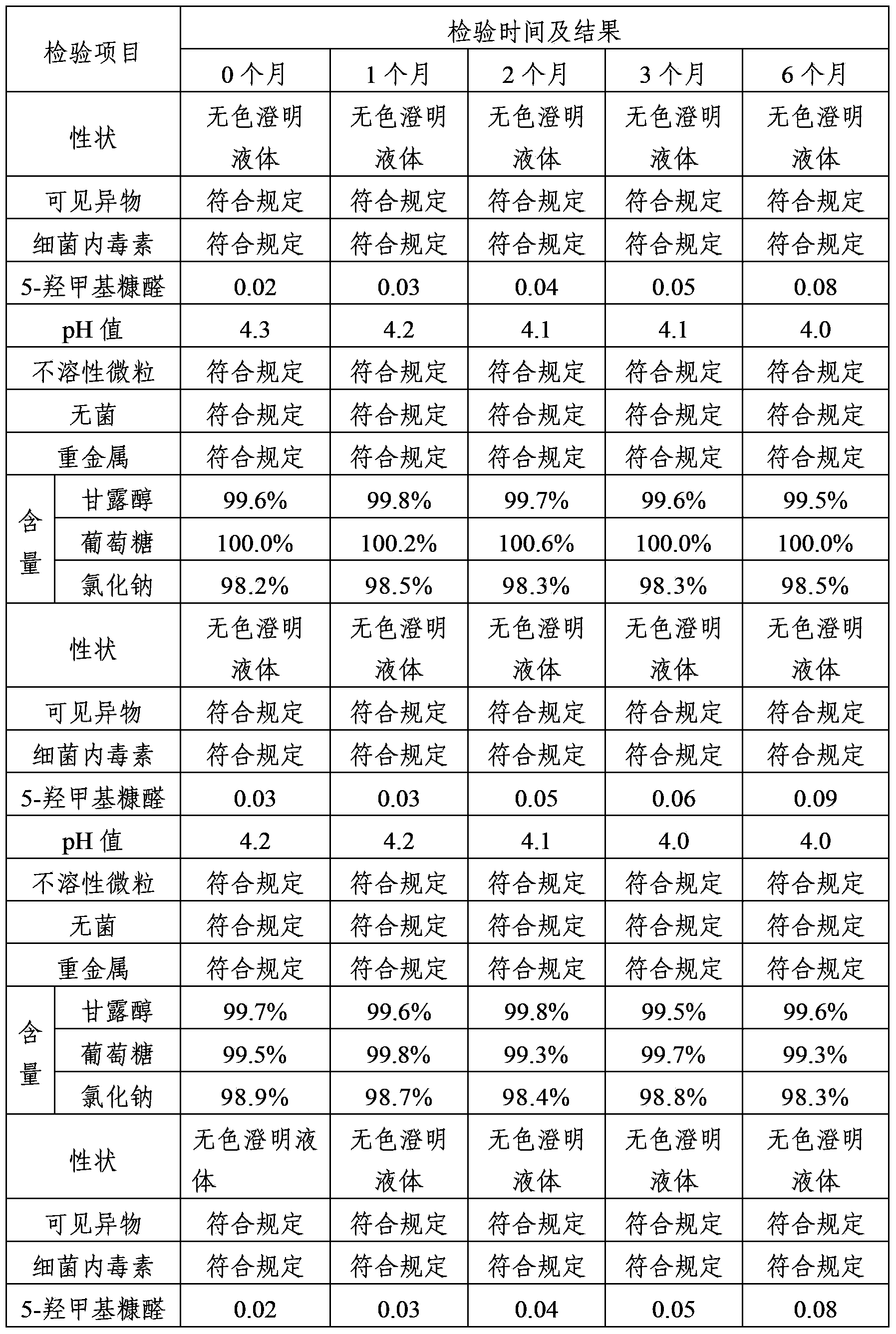
Mannitol injection
ActiveCN104027305AImprove stabilityQuality improvementHydroxy compound active ingredientsPharmaceutical delivery mechanismPharmacologyMannitol Injection
Owner:安庆回音必制药股份有限公司
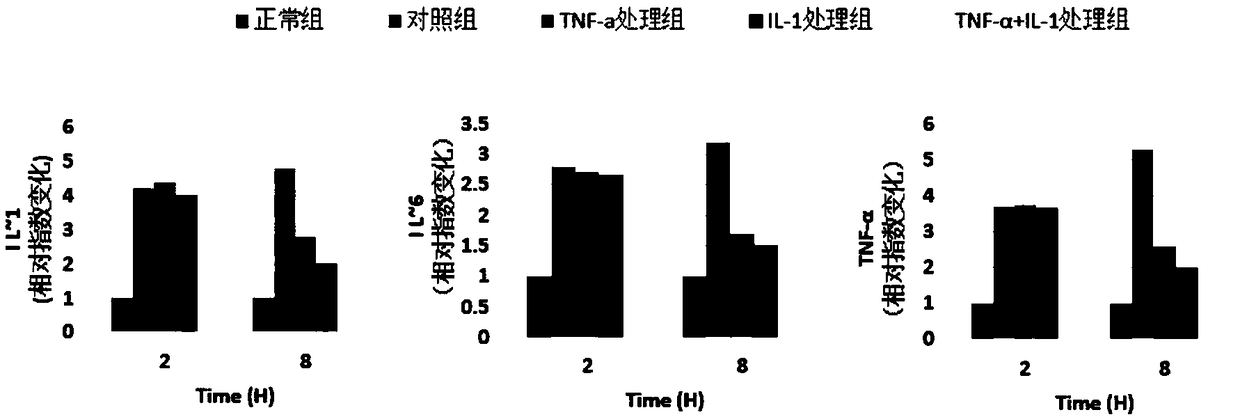
Mesenchymal stem cell injection for treating cerebral ischemic stroke and preparation method
InactiveCN108619169AIncrease vitalityEnsure safetyAntipyreticAnalgesicsInflammatory factorsVitamin injection
The invention discloses mesenchymal stem cell injection for treating cerebral ischemic stroke and a preparation method. The injection is prepared from activated mesenchymal stem cells with the quantity of 5*10<5> to 2*10<6> / mL, human serum albumin with the mass / volume ratio of 1 percent to 5 percent, mannitol injection with the mass / volume ratio of 1 percent to 8 percent, compound vitamin injection with the mass / volume ratio of 1 percent to 5 percent, 50 percent glucose injection with the mass / volume ratio of 1 percent to 5 percent and the balance of normal saline. The mesenchymal stem cell injection for treating the cerebral ischemic stroke, disclosed by the invention, releases pro-inflammatory factors including TNF (Tumor Necrosis Factor)-alpha, IL (Interleukin)-1, IL-6 and the like in an inflammatory damage process after cerebral ischemia; the mesenchymal stem cell injection is injected to treat the cerebral ischemic stroke.
Owner:北京山龟干细胞生物科技有限公司
Application of mannitol in preparation of anti- pancreatic cancer drug
InactiveCN103006625AHydroxy compound active ingredientsAntineoplastic agentsWilms' tumorCancer cell apoptosis
The invention discloses an application of mannitol in preparation of an anti-pancreatic cancer drug, and in particular relates to a use of a mannitol injection with a specific concentration of 10%-19% for treating pancreatic cancer. Traditionally, mannitol is only used as a diuretic, a high-permeability hypotensor and the like, while the inventor discovers through scientific experiments that mannitol has anti-tumor activity, and through several cell apoptosis detection technologies and by detecting apoptosis-related genes bcl-1, and bax, proves that the specific-concentration mannitol can induce various human cancer cell strains to generate obvious cancer cell apoptosis, grasps the effect relation with concentration and time, and first illustrates the possible mechanism; furthermore, the inventor has used the research result for tumor patient treatment and has achieved obvious curative effect, which provides theoretical basis and practical cases for the application of mannitol in preparation of the anti-tumor drug and the clinical generalization thereof.
Owner:广西壮族自治区肿瘤防治研究所
Method for measuring content of mannitol injection
InactiveCN105158167ASimplify detection stepsEasy to operatePolarisation-affecting propertiesPreparing sample for investigationMedicineEconomic benefits
The invention discloses an improved method for measuring the content of mannitol injection. The content of mannitol injection is measured through an optical rotation measuring method, operation is easy, time is saved, production cost is reduced, economic benefits are improved, medicine is effectively prevented from being contaminated, and safety risks of medicine (intermediate products) are reduced. Compared with the prior measuring art, the method is more suitable for measuring the content of intermediate products in the production process of medicine preparing enterprises.
Owner:GUANGXI YUYUAN PHARMA
Production and preparation process capable of lowering mannitol injection crystallization
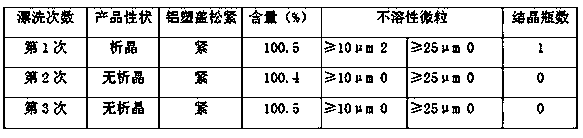
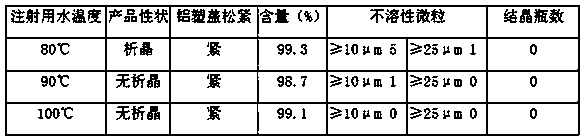
ActiveCN106389324AImprove filtering effectIncrease temperatureHydroxy compound active ingredientsPharmaceutical delivery mechanismMaterials preparationMannitol Injection
The invention relates to a production and preparation process capable of lowering mannitol injection crystallization. The production and preparation process is characterized in that material preparation is operated under Grade-B environment conditions under a background Grade-C, and raw materials are enabled to pass a 200-mesh screen, namely a screen of 75um+ / -4.1um; a super filter element terminal of 0.1um is adopted for filtering; a preparation method of a PH value adjuster includes: weighing 180ml of concentrated hydrochloric acid, adding water for injection to 1000ml, shaking well, and using a filter membrane of 0.1um to perform suction filtering to obtain a hydrochloric acid adjuster of 2mol / L; a PH value adjusting method includes: weighing 2mol / L hydrochloric acid in a needed amount, pouring into a clean measuring cup, linearly adding into a diluting tank while stirring, and stirring well; 90-100 DEG C is determined as using temperature of the water for injection, number of intensive washing times is 10 times of flushing, and water pressure for flushing each time is not lower than 0.15Mpa. By the production and preparation process, proportion of mannitol crystallization during selling is lowered, and high-quality products are provided for clinical medication.
Owner:JIANGXI KELUN MEDICINE IND
Drug composition for treating radiation-induced oral mucositis and application thereof
InactiveCN109512806AReduce damageImprove the quality of lifeHydroxy compound active ingredientsDigestive systemVITAMIN B12 INJECTIONClinical therapy
The invention relates to a drug composition for treating radiation-induced oral mucositis and application thereof. The drug composition is prepared from the following crude drugs: 20% of mannitol injection, lidocaine hydrochloride injection, vitamin B12 injection, dexamethasone sodium phosphate for injection and raceanisodamine hydrochloride injection. The invention also provides the application of the drug composition in preparation of a drug for treating radiation-induced acute oral mucositis. The drug composition and the application thereof have the advantages that (1) compared with the traditional drug for clinically treating the radiation-induced oral mucositis, the injury degree of the radiation-induced oral mucositis can be obviously relieved, the symptom relieving time can be obviously shortened, and the survival quality in the radiotherapy process of a patient can be improved; (2) the preparation process of a formula is simple, special process and conditions are not needed, the drugs needed by composition of the formula are clinically-common drugs respectively, the drug source is sufficient, the price is low, the preparation is convenient, the storage and transportation are convenient, the economic burden of the patient is low and the drug composition can be well applied on clinic.
Owner:JINSHAN HOSPITAL FUDAN UNIV
Fast detection method of intermediate mannitol content of compound mannitol injection
InactiveCN108593646AStrong specificityGood repeatabilityMaterial analysis by optical meansMANNITOL/SORBITOLPhysical chemistry
The invention discloses a fast detection method of the intermediate mannitol content of a compound mannitol injection. Firstly, the optical rotation alpha 1 of glucose in the compound mannitol injection is measured under the conditions of using sodium lamp D rays with the wavelength being 589 nm as a light source and the measuring temperature being 20+ / -0.5 DEG C; then, the total optical rotationalpha 2 of glucose and mannitol in the compound mannitol injection is measured under the same conditions; the mannitol content in the compound mannitol injection is calculated by using a differentialrotation method. The detection method provided by the invention has the advantages that the interference of the glucose on the mannitol detection result is avoided; the differential rotation method isused, so that the content of the mannitol can be accurately obtained; ammonium molybdate is used as a rotation increasing agent, so that the optical rotation performance of a solution to be tested can be obviously increased, and the detection result is more accurate. The detection method has the advantages of strong specificity, high accuracy, good middle precision and good repeatability. The method has the advantages that the practicability is high; the detection environment temperature and the sample standing time do not have obvious influence on the detection result.
Owner:SICHUAN KELUN PHARMA CO LTD
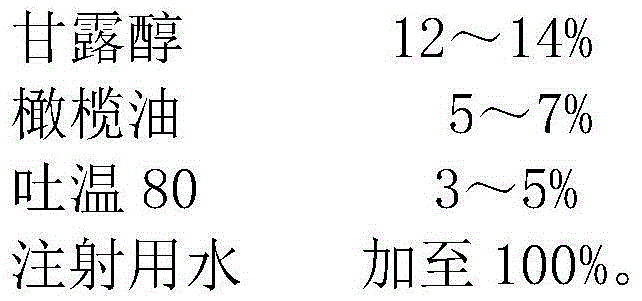
Mannitol injection
ActiveCN105147602ALower intracranial pressureAchieve brain protectionHydroxy compound active ingredientsPharmaceutical delivery mechanismHemolysisMANNITOL/SORBITOL
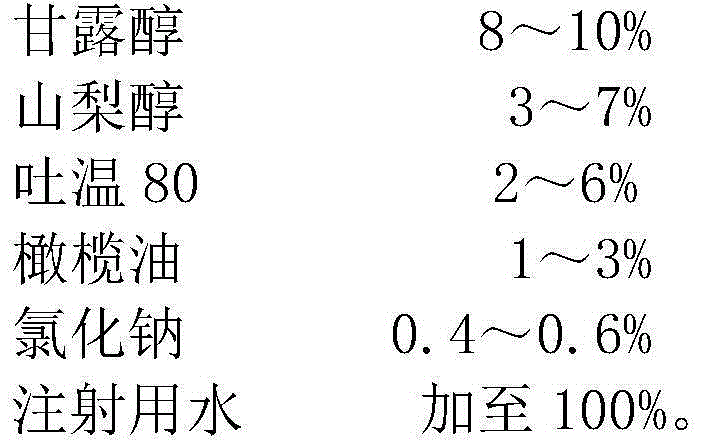
The invention relates to the technical field of pharmaceutic preparations, in particular to a mannitol injection. The mannitol injection is prepared from, by weight, 14%-20% of mannitol, 3%-9% of olive oil, 1%-7% of tween 80 and the balance of water for injection. According to the mannitol injection, the curative effect is good, crystals do not occur at low temperature, the use safety is high, and the phenomena of allergies and hemolysis do not occur.
Owner:GUANGXI YUYUAN PHARMA
Mannitol injection
InactiveCN105943495AReduce the chance of crystallizationImprove stabilityHydroxy compound active ingredientsPharmaceutical delivery mechanismGINSENG EXTRACTTherapeutic effect
The invention relates to the technical field of medicinal preparations, in particular to a mannitol injection. The mannitol injection is prepared from the following raw materials in percentage by weight: 12 to 19 percent of mannitol, 0.5 to 1.3 percent of a jasmine extract, 0.8 to 1.6 percent of a pseudo-ginseng extract, and the balance of injection water, and the total percent is 100. Compared with the prior art, the mannitol injection provided by the invention has a good treatment effect and a good effect on treating high intracranial pressure caused by cerebral hemorrhage, does not crystallize at a low temperature, and is high in use safety and convenient to use clinically; hypersensitivity is avoided; adverse reactions, such as an infusion reaction, are remarkably reduced.
Owner:苏远芳
Application of mannitol in preparation of anti-breast cancer drug
InactiveCN103006626AHydroxy compound active ingredientsPharmaceutical delivery mechanismWilms' tumorCancer research
The invention discloses an application of mannitol in preparation of an anti-breast cancer drug, and in particular relates to a use of a mannitol injection with a specific concentration of 10%-19% for treating breast cancer. Traditionally, mannitol is only used as a diuretic, a high-permeability hypotensor and the like, while the inventor discovers through scientific experiments that mannitol has anti-tumor activity, and through several cell apoptosis detection technologies and by detecting apoptosis-related genes bcl-2, and bax, proves that the specific-concentration mannitol can induce various human cancer cell strains to generate obvious cancer cell apoptosis, grasps the effect relation with concentration and time, and first illustrates the possible mechanism; furthermore, the inventor has used the research result to tumor patient treatment and has achieved obvious curative effect, which provides theoretical basis and practical cases for the application of mannitol in preparation of the anti-tumor drug and the clinical generalization thereof.
Owner:广西壮族自治区肿瘤防治研究所
Application of mannitol in preparation of anti-stomach cancer drug
InactiveCN103006623AHydroxy compound active ingredientsAntineoplastic agentsWilms' tumorStomach cancer
The invention discloses an application of mannitol in preparation of an anti-stomach cancer drug, and in particular relates to a use of a mannitol injection with a specific concentration of 10%-19% for treating stomach cancer. Traditionally, mannitol is only used as a diuretic, a high-permeability hypotensor and the like, while the inventor discovers through scientific experiments that mannitol has anti-tumor activity, and through several cell apoptosis detection technologies and by detecting apoptosis-related genes bcl-2, and bax, proves that the specific-concentration mannitol can induce various human cancer cell strains to generate obvious cancer cell apoptosis, grasps the effect relation with concentration and time, and first illustrates the possible mechanism; furthermore, the inventor has used the research result for tumor patient treatment and has achieved obvious curative effect, which provides theoretical basis and practical cases for the application of mannitol in the preparation of the anti-tumor drug and the clinical generalization thereof.
Owner:广西壮族自治区肿瘤防治研究所
Compound mannitol injection
ActiveCN105147600ALower intracranial pressureInhibit apoptosisHydroxy compound active ingredientsPharmaceutical delivery mechanismTreatment effectMANNITOL/SORBITOL
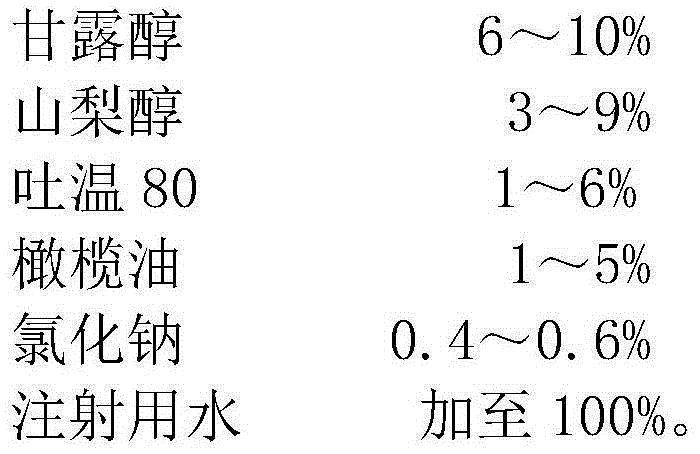
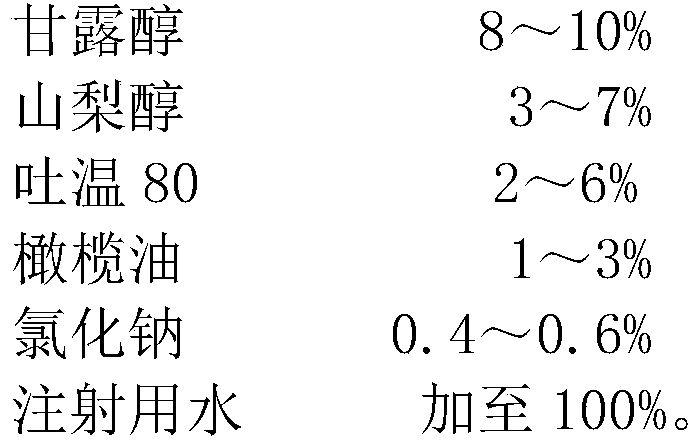
The invention relates to the technical field of pharmaceutic preparations, in particular to compound mannitol injection. The compound mannitol injection is prepared from, by weight, 6-10% of mannitol, 3-9% of sorbitol, 1-6% of Tween 80, 1-5% of olive oil, 0.4-0.6% of sodium chloride and the balance injection water. The compound mannitol injection is good in treatment effect, does not crystallize at low temperatures, is high in using safety performance, can not cause allergies, can be used conveniently clinically, and is capable of reducing adverse reactions including infusion reactions remarkably.
Owner:GUANGXI YUYUAN PHARMA
A kind of compound mannitol injection
ActiveCN105147600BLower intracranial pressureInhibit apoptosisHydroxy compound active ingredientsInorganic non-active ingredientsMANNITOL/SORBITOLCurative effect
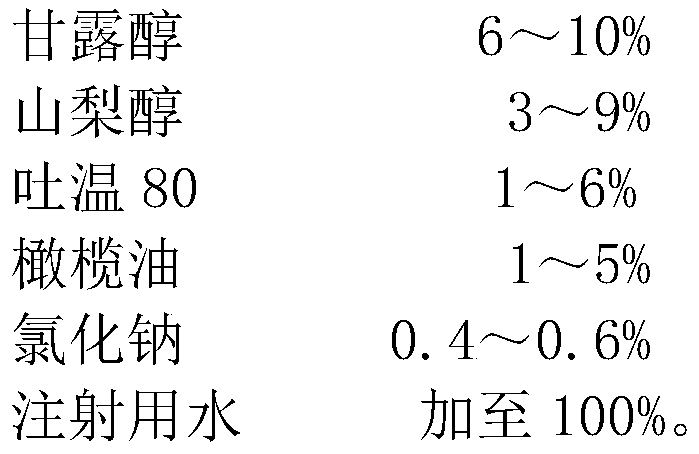
The invention relates to the technical field of pharmaceutical preparations, in particular to a compound mannitol injection, which is made of the following raw materials in percentage by weight: mannitol 6-10%, sorbitol 3-9%, Tween 80 1-6%, olive Add 1-5% oil, 0.4-0.6% sodium chloride, and water for injection to 100%. The compound mannitol injection of the invention has good curative effect, no crystallization at low temperature, high use safety, no allergy phenomenon, convenient clinical use, and significantly reduces adverse reactions such as infusion reactions.
Owner:GUANGXI YUYUAN PHARMA
Mannitol injection
ActiveCN105287368ALower intracranial pressureAchieve brain protectionNervous disorderHydroxy compound active ingredientsCod liver oilMANNITOL/SORBITOL
The invention relates to the technical field of pharmaceutical preparations, in particular to mannitol injection. The mannitol injection is prepared from, by weight, 9-16% of mannitol, 2-8% of cod-liver oil, 1-7% of Tween-80 and the balance water for injection. The mannitol injection is good in treatment effect, non-crystallizable at lower temperature and high in safety.
Owner:GUANGXI YUYUAN PHARMA
Eye drops for anti-eye fatigue and preparation method thereof
The invention discloses eye drops for resisting asthenopia. The eye drops comprise the following components: amiotide eye drops, inosine injection, adenosine disodium triphosphate injection, racanisodamine injection, vitamin B6 injection, taurine eye drops and an assistant for eyes, wherein the assistant for eyes is at least one of injection water or an isoosmotic adjusting agent; the isoosmotic adjusting agent is at least one of a sodium chloride physiological saline, glucose injection, sorbitol eye drops, borate saline buffer, mannitol injection or polyethylene glycol eye drops. The invention also discloses a preparation method of the eye drops for resisting asthenopia. The eye drops have the advantages that: 1, the overall efficacy of a compound preparation is obviously superior to a single drug effect; 2, the eye drops play roles in the whole process of the disease, the dose is reduced, and the side effect is reduced; 3, various medicines work in coordination with each other, are complementary in efficacy, and are taken as preconditions for playing roles; 4, the eye drops are obvious in pharmacological effect, applicable and convenient, and convenient to carry.
Owner:宁夏瑞视眼科研究所
Application of mannitol in preparation of anti-stomach cancer drug
InactiveCN103006623BHydroxy compound active ingredientsAntineoplastic agentsWilms' tumorStomach cancer
The invention discloses an application of mannitol in preparation of an anti-stomach cancer drug, and in particular relates to a use of a mannitol injection with a specific concentration of 10%-19% for treating stomach cancer. Traditionally, mannitol is only used as a diuretic, a high-permeability hypotensor and the like, while the inventor discovers through scientific experiments that mannitol has anti-tumor activity, and through several cell apoptosis detection technologies and by detecting apoptosis-related genes bcl-2, and bax, proves that the specific-concentration mannitol can induce various human cancer cell strains to generate obvious cancer cell apoptosis, grasps the effect relation with concentration and time, and first illustrates the possible mechanism; furthermore, the inventor has used the research result for tumor patient treatment and has achieved obvious curative effect, which provides theoretical basis and practical cases for the application of mannitol in the preparation of the anti-tumor drug and the clinical generalization thereof.
Owner:广西壮族自治区肿瘤防治研究所
Intestinal flora reconstruction kit and its application
ActiveCN109303698BEasy to useImprove securityPharmaceutical containersMedical packagingJoint painSpondarthritis
Owner:HEBEI UNIVERSITY
Injectable drug for treating varicosity
ActiveCN103156866BEasily damagedNon-destructiveHydroxy compound active ingredientsCardiovascular disorderSide effectTissue fibrosis
The invention discloses an injectable drug for treating varicosity, and the drug is prepared by mixing medical mannitol injection with dextrose injection according to a weight ratio of 15:5. The drug disclosed by the invention is injected to local diseased vein, acts on the inside part of the diseased blood vessel and activates the blood vessel to form aseptic inflammation so as to implement fibrillation of the tissues, close the blood vessel and improve blood circulation of legs, no destructive effect is caused to the function of the body, all cirsoid veins are retracted 10 days later, the skin is smooth as before and varicosity never recurs. The drug disclosed by the invention is widely used by people, stable in curative effect, low in cost and free of toxic and side effects. So far, thousands of clinically cured patients never suffer from varicosity again; the drug is low in cost and is only 1 / 4 of the surgery cost; the patients feel no pain and are easy to accept the drug, so the drug obtains good social benefit and economic benefit.
Owner:曲洋志
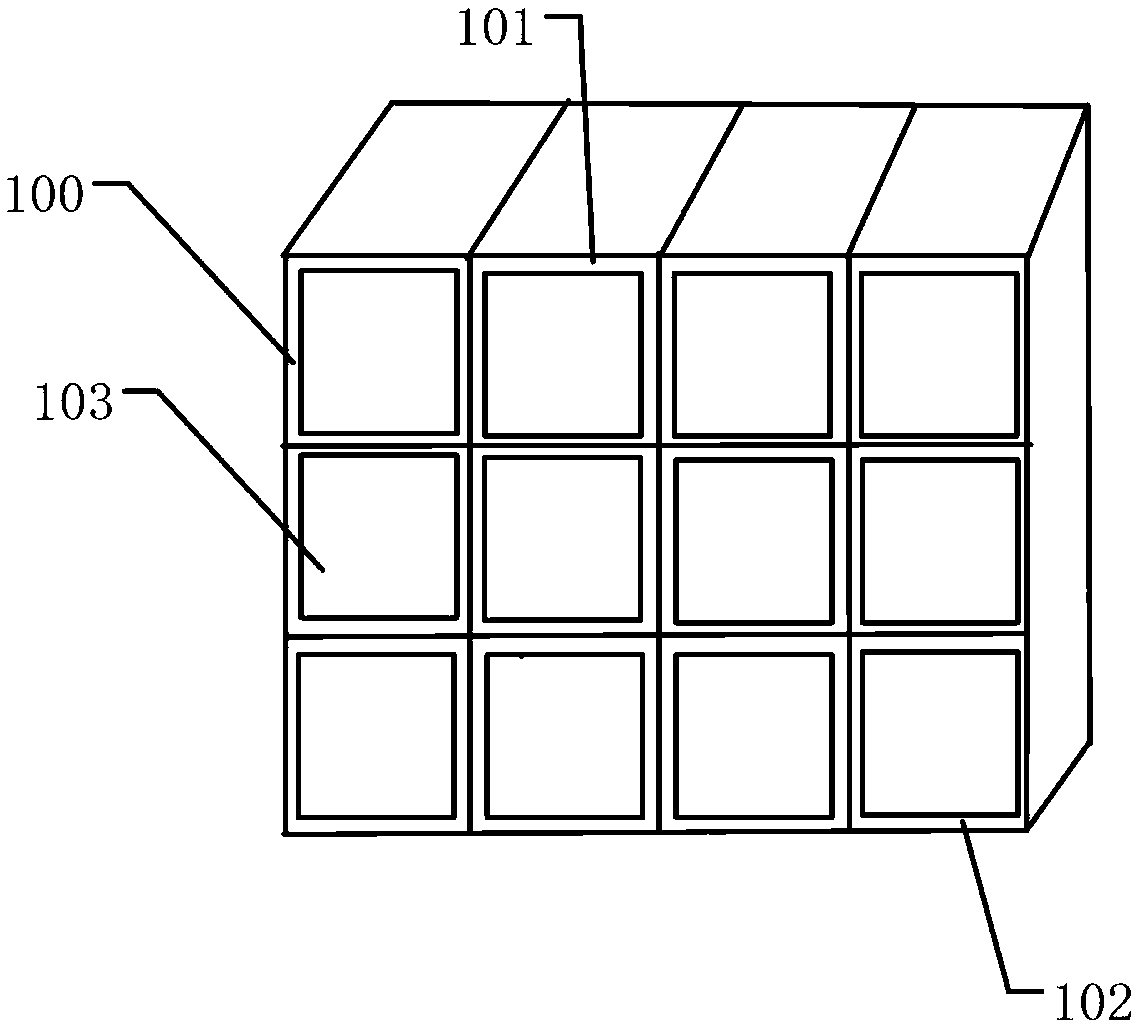
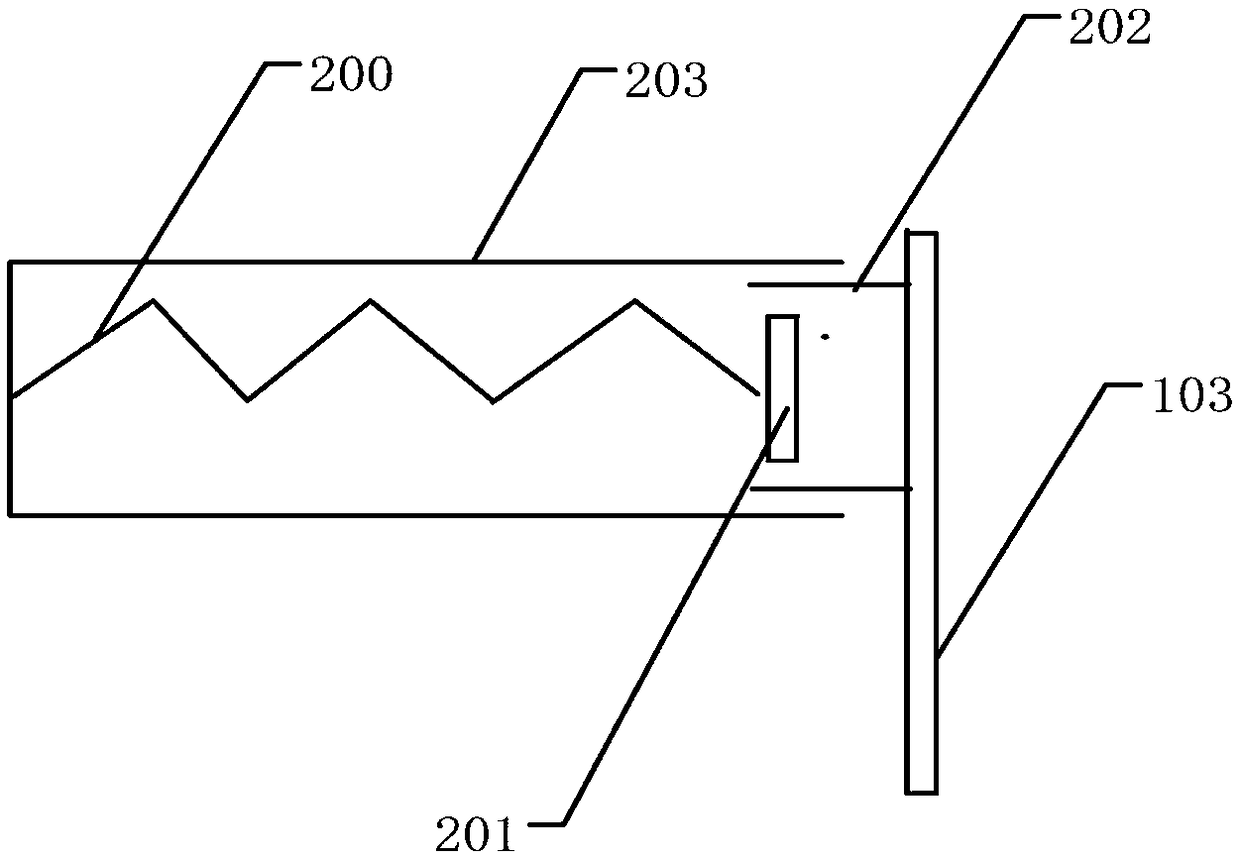
Device for preventing mannitol injection from crystallizing
InactiveCN111572957AEffective lifting isolationImprove odorContainers preventing decayContainers to prevent mechanical damageActivated carbonStructural engineering
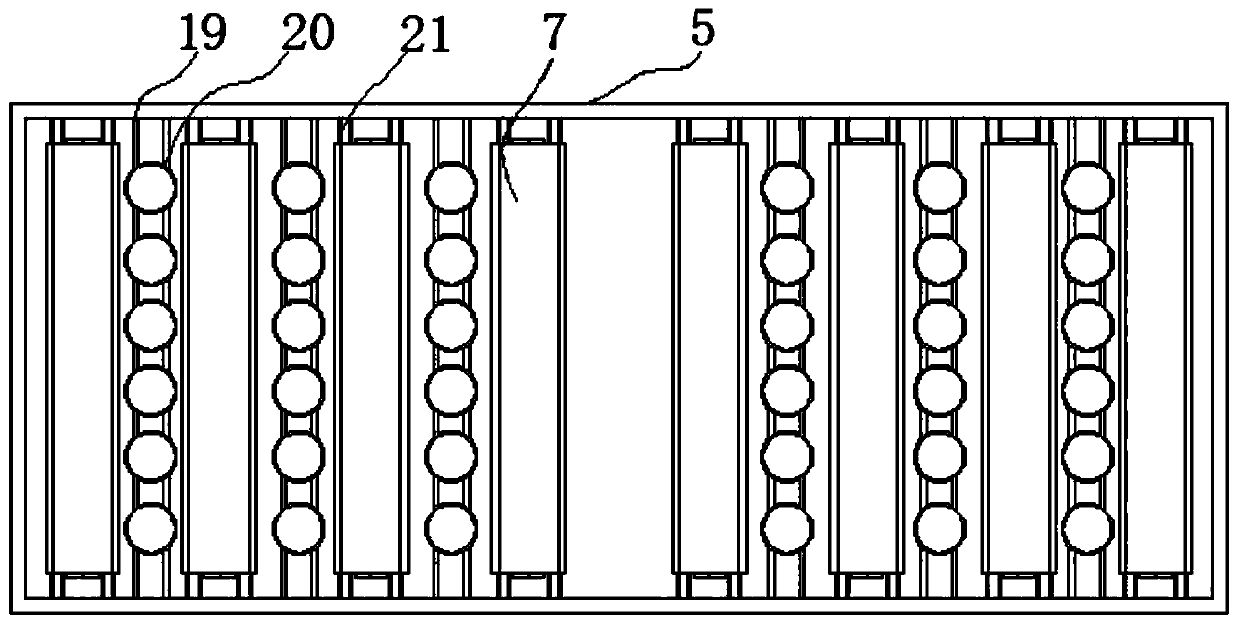
The invention relates to a device for preventing mannitol injection from crystallizing. The device comprises a mannitol thermotank, pin caps, and hinge movable rods, sliding groove rods are formed inthe two sides of the mannitol thermotank, clamping grooves are connected to the inner sides of the sliding groove rods, a bearing frame is mounted on the inner sides of the clamping grooves, the two sides of the outer portion of the mannitol thermotank are connected with closing doors, hinge columns are arranged on one sides of the interiors of the closing doors, a ventilation frame is connected with the top end of the interior of the mannitol thermotank, the top end of the ventilation frame is provided with a top cover frame, the top end of the bearing frame is provided with a mannitol bottleplacing frame, and the bottom end of the ventilation frame is connected with an active carbon layer frame. The device has the beneficial effects that a user can push the active carbon layer frame todrive a sliding block to do lifting motion along the position between the sliding groove rods, the active carbon layer frame can be effectively lifted to be isolated between storage frames, peculiar smell between walls of the mannitol thermotank can be absorbed between layers of the active carbon layer frame, and the peculiar smell of the mannitol thermotank can be purified.
Owner:马丽芳
Mannitol injection
ActiveCN104027305BImprove stabilityQuality improvementHydroxy compound active ingredientsPharmaceutical delivery mechanismPharmacologyMannitol Injection
Owner:安庆回音必制药股份有限公司
Compound oral liquid for treating esophageal carcinoma and cardiac carcinoma dysphagia
InactiveCN106109589AEasy to prepareLow priceDispersion deliveryHydroxy compound active ingredientsVolume concentrationRacanisodamine
The invention relates to compound oral liquid for effectively treating esophageal carcinoma and cardiac carcinoma dysphagia. According to the technical scheme, the compound oral liquid is prepared by evenly mixing 30-250ml of mannitol injection with the volume concentration being 20%, 3-15ml of lidocaine hydrochloride injection with the volume concentration being 2%, 5-30mg of racanisodamine hydrochloride injection and 30-300ml of fresh lotus root juice. The compound oral liquid is simple to prepare, easy in raw material obtaining, low in price, good in taste, convenient to take and evident in effect and is innovation of medicine for treating esophageal carcinoma and cardiac carcinoma dysphagia.
Owner:HENAN UNIV OF CHINESE MEDICINE
Intestinal flora reestablishment kit and application thereof
ActiveCN109303698AEasy to useImprove securityPharmaceutical containersMedical packagingJoint arthralgiaAnkylosing spondylitis
The invention discloses an intestinal flora reestablishment kit. The kit comprises a kit body; the kit body comprises multiple cavities which are sequentially and regularly opened, the cavities are from the n th cavity to the (n+x) th cavity according to the opening sequence, and the time interval of opening time of adjacent cavities from the n th cavity to the (n+x) th cavity is 4-12 h; the n thcavity comprises compound polyethylene glycol electrolyte powder or mannitol injection, the (n+1) th cavity comprises A and B microecologics, the (n+2) th cavity comprises B microecologics, the cavities from the (n+3) th cavity to the (n+21) th cavity comprise B and A microecologics, and the cavities from the (n+22) th cavity to the (n+x) th cavity comprise B microecologics. Through intestinal lavage and sequential taking of different preparations, quick reestablishment of the intestinal flora is facilitated. The invention further discloses application of the kit to reestablishment of ankylosing spondylitis and rheumatoid intestinal florae. Through the reestablishment of the intestinal flora, arthralgia of a patient is relieved, joints move freely, so that the kit is feasible in the aspectof relieving arthralgia and discomfort of the patient.
Owner:HEBEI UNIVERSITY
Application of mannitol in preparation of anti-gastric adenocarcinoma drug
InactiveCN103006624AHydroxy compound active ingredientsAntineoplastic agentsWilms' tumorCancer research
The invention discloses an application of mannitol in preparation of an anti-gastric adenocarcinoma drug, and in particular relates to a use of a mannitol injection with a specific concentration of 10%-19% for treating gastric adenocarcinoma. Traditionally, mannitol is only used as a diuretic, a high-permeability hypotensor and the like, while the inventor discovers through scientific experiments that mannitol has anti-tumor activity, and through several cell apoptosis detection technologies and by detecting apoptosis-related genes bcl-1, and bax, proves that the specific-concentration mannitol can induce various human cancer cell strains to generate obvious cancer cell apoptosis, grasps the effect relation with concentration and time, and first illustrates the possible mechanism; furthermore, the inventor has used the research result for tumor patient treatment and has achieved obvious curative effect, which provides theoretical basis and practical cases for the application of mannitol in preparation of the anti-tumor drug and the clinical generalization thereof.
Owner:广西壮族自治区肿瘤防治研究所
A production and preparation process for reducing crystallization of mannitol injection liquid
ActiveCN106389324BReduce the crystallization ratioControl quantityHydroxy compound active ingredientsPharmaceutical delivery mechanismMaterials preparationMembrane configuration
Owner:JIANGXI KELUN MEDICINE IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com