A production and preparation process for reducing crystallization of mannitol injection liquid
A preparation process, mannitol technology, applied in blood diseases, extracellular fluid diseases, pharmaceutical formulations, etc., can solve problems such as unsatisfactory experimental results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Quality control of raw and auxiliary materials and packaging materials:
[0034] (1) Mannitol, injection grade;
[0035] (2) Activated carbon, pharmaceutical grade;
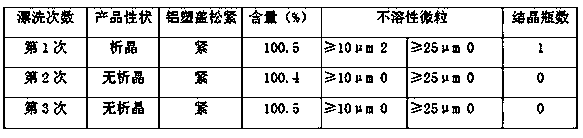
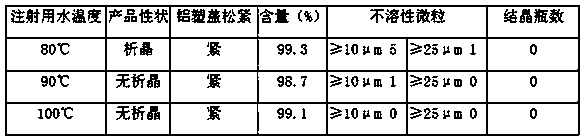
[0036] (3) Water for injection is made of polyethersulfone and filtered by a super filter element with a pore size of 0.1 μm. The smaller the pore size, the more fine insoluble particles will be retained. The insoluble particles and quantity in the bottle control the formation of crystal nuclei from the source to prevent the induction of crystallization;
[0037] (4) PH regulator (2mol / L hydrochloric acid), pharmaceutical grade;
[0038] (5) Glass infusion bottle, made of strong pharmaceutical grade material, the thickness of the thinnest part (bottle body thickness ≥ 1.5mm, bottle bottom thickness ≥ 2.5mm), smooth inner surface, no stones, no uneven surface;
[0039] (6) Butyl rubber stopper, pharmaceutical grade 22.8;
[0040] (7) Aluminum cover, pharmaceutical grade 22.8 type;
[0041] Preparation ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com