Adipose tissue cryopreservation liquid at clinical application level and cryopreservation method
A fat tissue, clinically applied technology, applied in the field of biomedicine, can solve problems such as adverse reactions, achieve the effect of reducing the risk of cell DNA distortion and protein denaturation, avoiding side effects and reducing functional impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
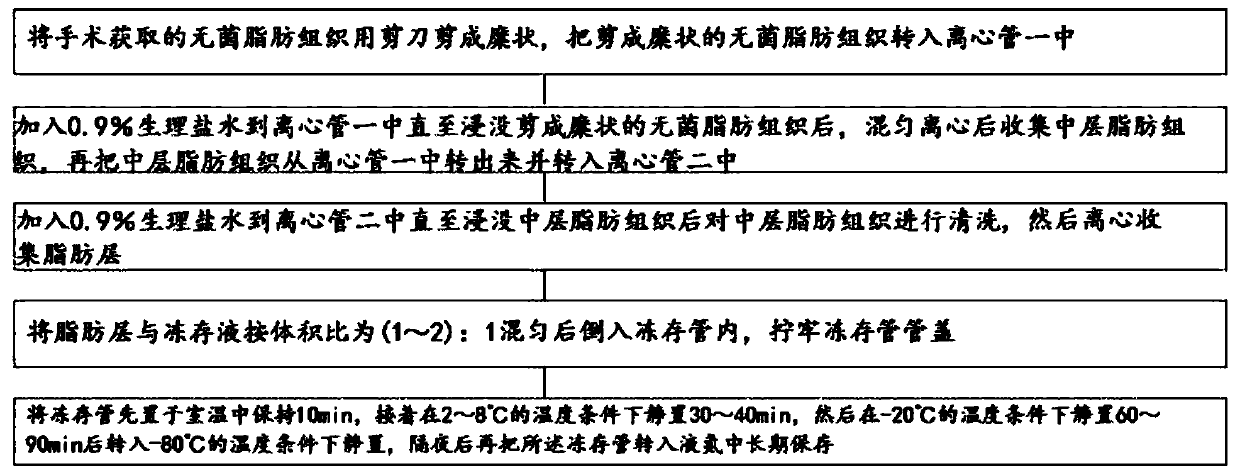
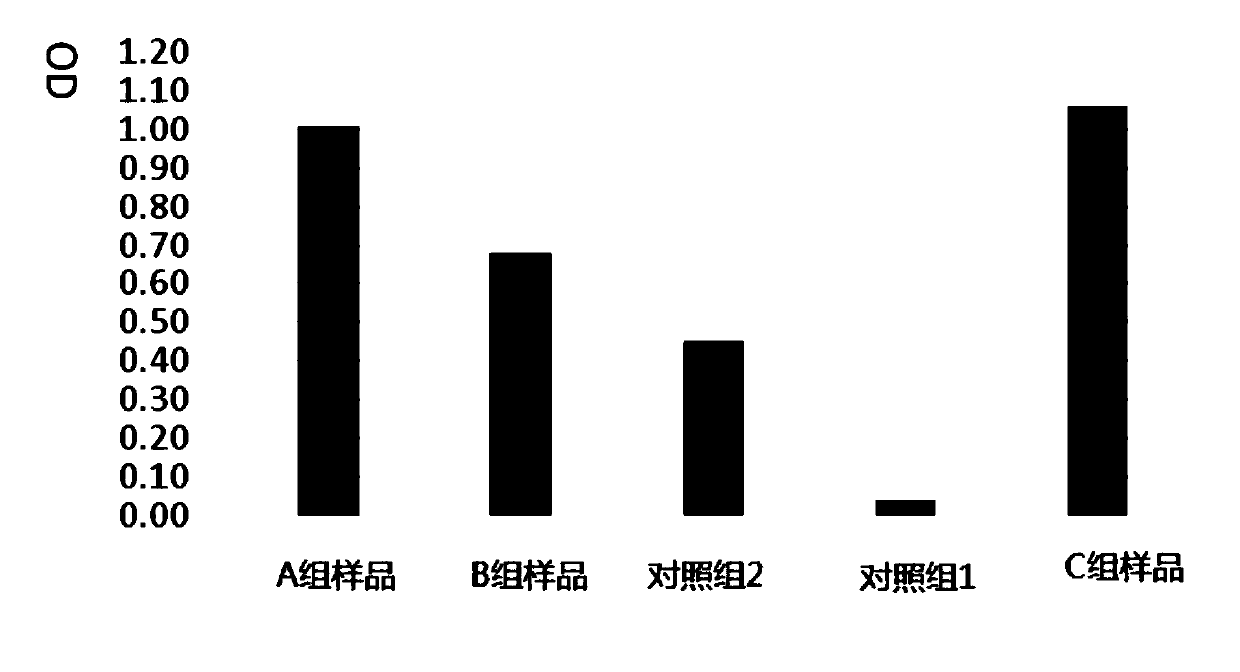
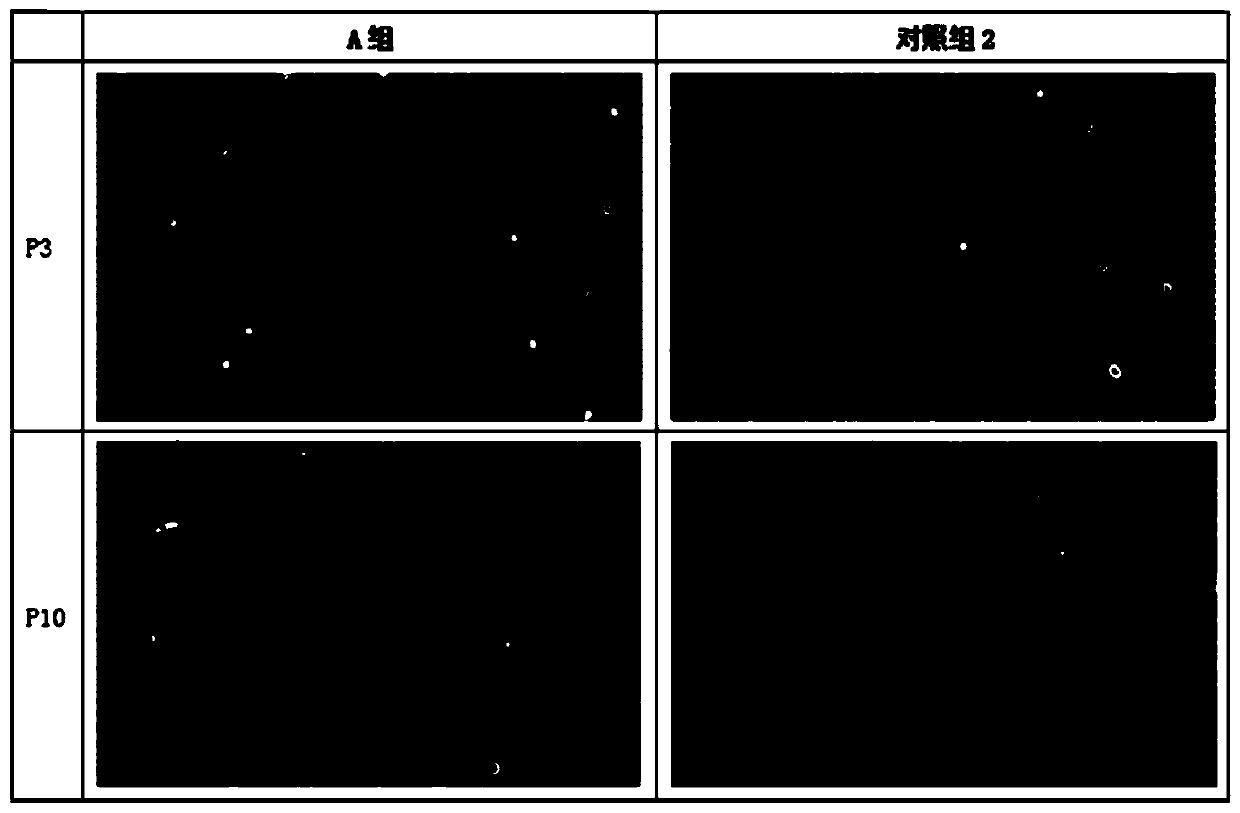
[0055] Such as Figure 1-Figure 6 As shown, clinical application grade adipose tissue cryopreservation solution, including:
[0056] 20mmol / L injectable grade sucrose;
[0057] 20mmol / L mannitol injection;
[0058] 5mmol / L glucose injection;
[0059] 2mol / L adenosine injection;
[0060] 3mmol / L reduced glutathione;
[0061] 1.2mol / L trehalose;
[0062] 0.3mmol / L of EDTA-2Na;
[0063] The injectable grade sucrose of described 20mmol / L, the mannitol injection of 20mmol / L, the glucose injection of 5mmol / L, the adenosine injection of 2mol / L, the reduced glutathione of 3mmol / L, 1.2mol Trehalose / L and EDTA-2Na of 0.3mmol / L are mixed to form a mixed solution, which is a clinical application level adipose tissue cryopreservation solution, and the solvent or diluent of the clinical application level adipose tissue cryopreservation solution Lactated Ringer's Injection. The component of described Sodium Lactated Ringer's Injection is: under the condition that the full amount of d...
Embodiment 2
[0078] Clinical application grade adipose tissue cryopreservation solution, including:
[0079] 50mmol / L injectable grade sucrose;
[0080] 50mmol / L mannitol injection;
[0081] 5mmol / L glucose injection;
[0082] 3mol / L adenosine injection;
[0083] 5mmol / L reduced glutathione;
[0084] 4.5mol / L trehalose;
[0085] 1mmol / L of EDTA-2Na;
[0086] The injectable grade sucrose of described 50mmol / L, the mannitol injection of 50mmol / L, the glucose injection of 5mmol / L, the adenosine injection of 3mol / L, the reduced glutathione of 5mmol / L, 4.5mol The trehalose / L and the EDTA-2Na of 1mmol / L are mixed to form a mixed solution, and this mixed solution is adipose tissue cryopreservation liquid of clinical application level, and the solvent or diluent of the adipose tissue cryopreservation liquid of described clinical application level is Sodium Lactated Ringer's Injection, the components of the Sodium Lactated Ringer's Injection are: under the condition that the total amount of t...
Embodiment 3
[0101] Clinical application grade adipose tissue cryopreservation solution, including:
[0102] 15mmol / L injectable grade sucrose;
[0103] 15mmol / L mannitol injection;
[0104] 1mmol / L glucose injection;
[0105] 1mol / L adenosine injection;
[0106] 0.5mmol / L reduced glutathione;
[0107] 1mol / L trehalose;
[0108] 0.1mmol / L of EDTA-2Na;
[0109] The injectable grade sucrose of described 15mmol / L, the mannitol injection of 15mmol / L, the glucose injection of 1mmol / L, the adenosine injection of 1mol / L, the reduced glutathione of 0.5mmol / L, 1mol Trehalose / L and EDTA-2Na of 0.1mmol / L are mixed to form a mixed solution, which is a clinical application level adipose tissue cryopreservation solution, and the solvent or diluent of the clinical application level adipose tissue cryopreservation solution Lactated Ringer's Injection. The component of described Sodium Lactated Ringer's Injection is: under the condition that the full amount of described Sodium Lactated Ringer's Inje...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com