Patents
Literature
39 results about "Racanisodamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medicament for treating rhinitis
The invention relates to a medicinal preparation for treating rhinitis, wherein the formulation comprises astragalus root, xanthium. spp., white atractylodes rhizome, ledebouriella root, dahurian angelica root, asaryl, centipeda, wild chrysanthemum flower, grassleaved sweetflag rhizome, Ligusticum wallichii, flower bud of lily magnolia, Japanese yam, cuttle-bone, Chinese ephedra, cassia twig, keel, oyster shell, chlorpheniramine, racanisodamine hydrochloride, flower bud of lily magnolia, and asaryl.
Owner:刘益斌
Process for preparing racanisodamine hydrochloride used for injection
InactiveCN102988287AIncrease profitReduce manufacturing costAntipyreticAnalgesicsFiberRacanisodamine
The invention discloses a process (method) for preparing racanisodamine hydrochloride used for injection. The invention overcomes the defects that the traditional racanisodamine hydrochloride preparation process cannot effectively guarantee clarity and complete filtering of impurities such as fibers by utilizing high pressure ultrafiltration equipment and provides a preparation process with low cost, high yield and more stable quality of the finished product. The preparation process comprises the following steps: (1) placing water used for injection in a preparation tank, lowering water temperature to 39-41 DEG C; (2) dissolving sodium chloride with the water used for injection in the preparation tank for spare use, adding the racanisodamine hydrochloride into the preparation tank, opening a stirrer, adding 0.2mol / L of hydrochloric acid into the preparation tank, adding sodium chloride solution into the preparation tank, circulating and stirring until racanisodamine is completely dissolved; and (3) regulating pH value of liquid medicine obtained in step (2) to be 5.5-6.0 with 0.2mol / L of hydrochloric acid. The liquid medicine is filtered by a high pressure ultrafilter, then whether an intermediate product is qualified is inspected, a filling process is carried out, and the preparation process disclosed by the invention enhances market competitiveness of the racanisodamine hydrochloride product, is convenient to the marketing of the racanisodamine hydrochloride product and is worthy of popularization.
Owner:HENAN FUREN HUAIQINGTANG PHARMA
Navel-warming paste capable of warming spleen and stomach for dispelling cold and preparation method thereof
InactiveCN103110702ARelieve painSignificant effectHeavy metal active ingredientsAnthropod material medical ingredientsDiseaseNight sweat
The invention belongs to the technical field of a medicine, and in particular relates to a navel-warming paste capable of warming the spleen and the stomach for dispelling cold and a preparation method thereof. The navel-warming paste capable of warming the spleen and the stomach for dispelling cold is characterized by being mainly prepared from the following crude medicines: 20-25 parts of cinnamon, 10-15 parts of white pepper, 20-25 parts of pericarpium granati, 20-25 parts of fructus chebulae, 20-25 parts of Chinese gallnut, 5-7 parts of clove, 0.5-0.75 parts of a raceanisodamine hydrochloride tablet and 235-250 parts of yellow lead. The navel-warming paste disclosed by the invention has the beneficial effects of combining various medicines to warm the spleen and the stomach for dispelling cold as well as to relieve diarrhea and paint; the navel-warming paste has remarkable effect on treating acute and chronic abdominal pain and diarrhea, dyspepsia, inappetence and night sweat caused by pathogenic cold, and the cure rate is 90%; the navel-warming paste can cure generally by 1-2 courses of treatment, so that the course of disease is effectively shortened and paint of the patient is rapidly removed.
Owner:刘清池
Racanisodamine eye drops
InactiveCN1899286AThe content of the main drug is reducedGood treatment effectSenses disorderHeterocyclic compound active ingredientsTreatment effectIrritation
The present invention discloses one kind of racanisodamine eye drops, which consist of water for injection in 100 ml, racanisodamine in 0.051-0.999g, sodium hyaluronate in 0.012-0.049g, and isoosmotic regulating agent 0.4-2.5g. The racanisodamine eye drops have obvious curative effect on near sightness. Animal experiment proves less irritation on eye and stable performance. The racanisodamine eye has small dosage and low cost.
Owner:SHANGHAI SINE PHARMA LAB
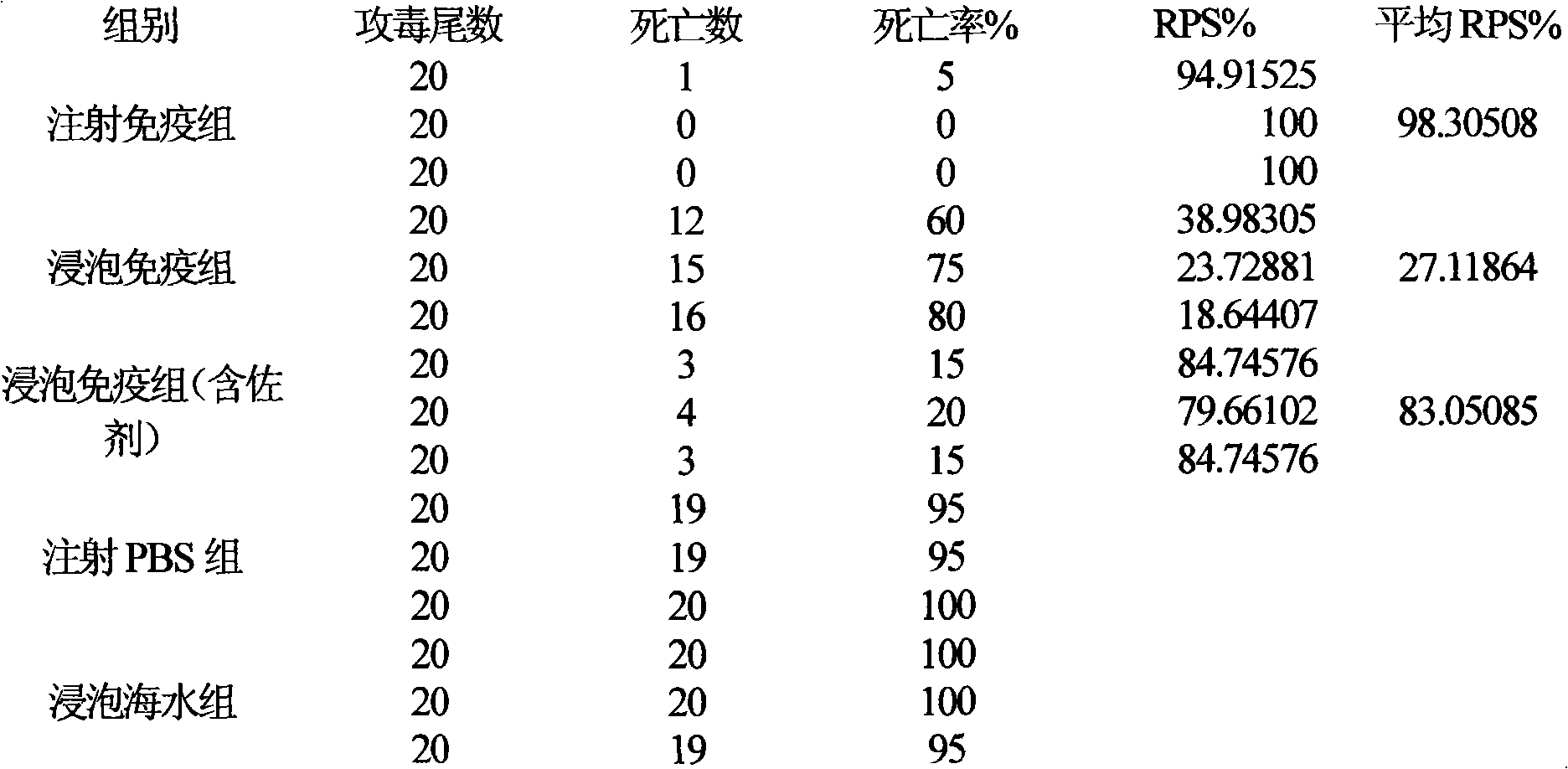
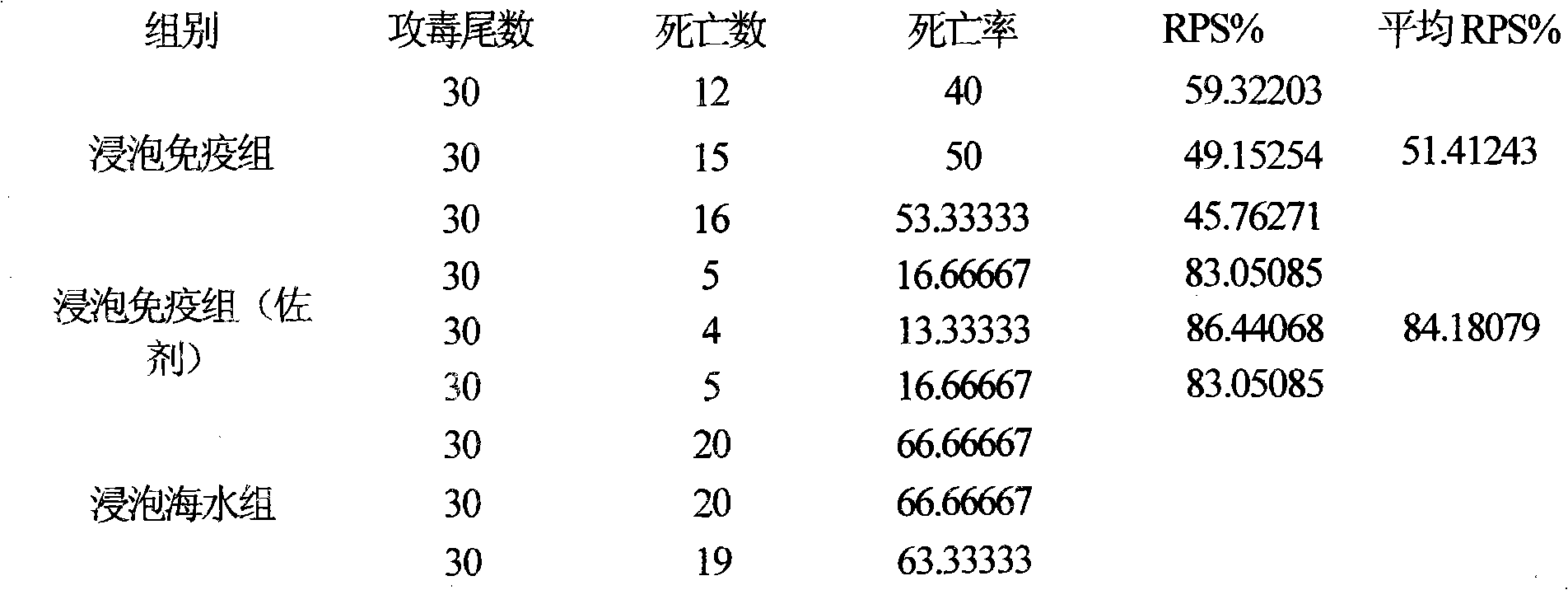
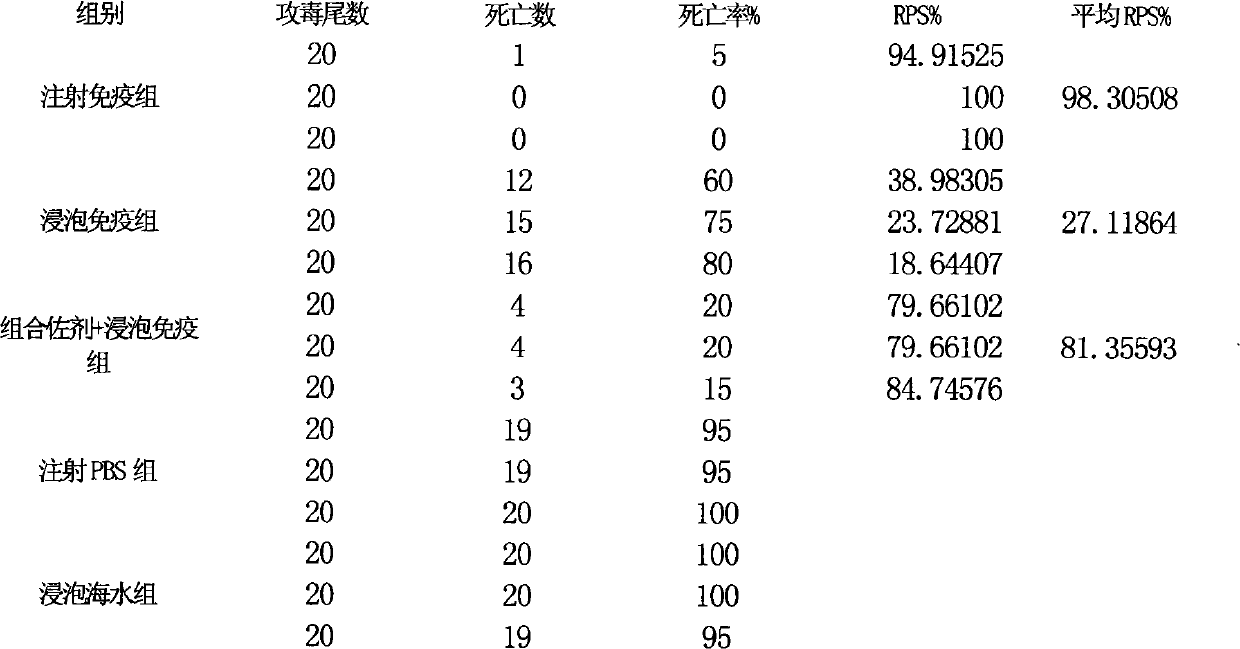
Fish soaking vaccine multiple-combination adjuvant and application and using method thereof
InactiveCN101791401AImprove immunityNo effect on activityAntibacterial agentsImmunological disordersImmune effectsAdjuvant
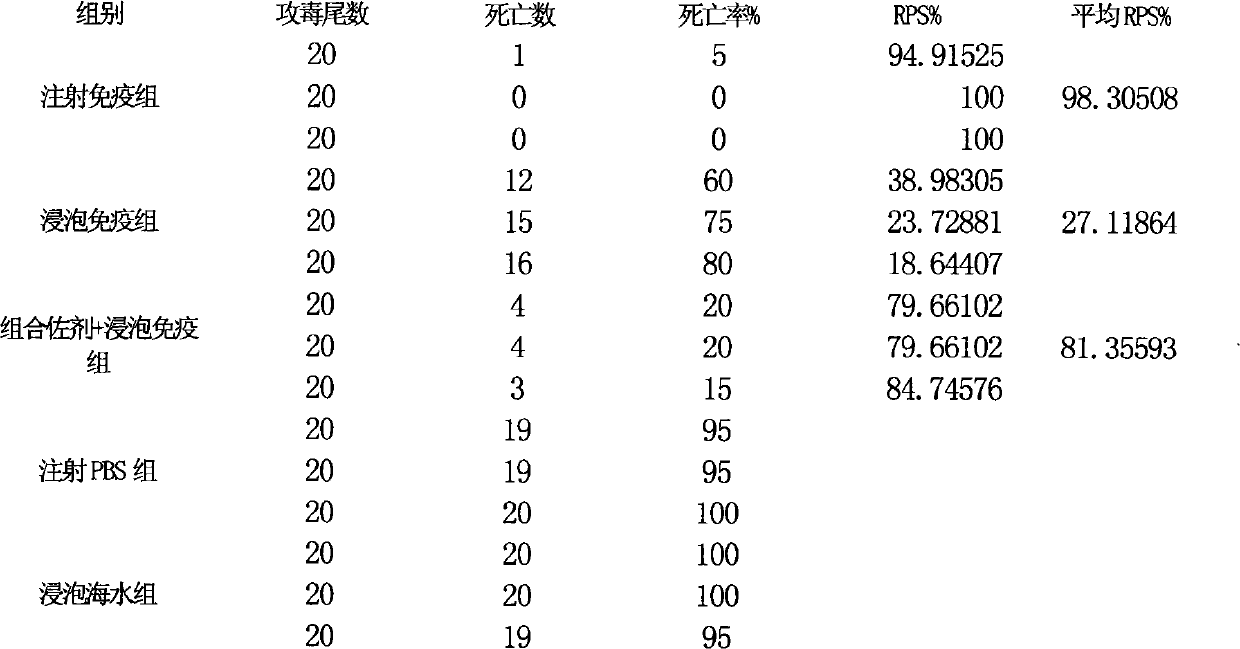
The invention relates to a fish soaking vaccine multiple-combination adjuvant and application and a using method thereof. The fish soaking vaccine multiple combination adjuvant comprises the combination of N-acetylcysteine, triton X-100, saponin and racanisodamine, wherein the using concentration of N-acetylcysteine, triton X-100, saponin and racanisodamine are respectively 0.1-10mg / L, 0.1-10mg / L, 0.1-50mg / L and 1-60mg / L. The fish soaking vaccine multiple-combination adjuvant is applied to the soaking vaccine comprising seawater and freshwater and the synergistic effect reaches the efficient immunity effect. The using method is as follows: firstly, selecting and immigrating the fish to be immunized into the solution of the N- acetylcysteine, triton X-100 and mixed aquaculture water to be presoaked for 1-20min, then adding saponin and the stand-by vaccine to be soaked for 1-20min, and finally adding racanisodamine into the vaccine soak solution for 1-10min, thus finishing the immunity. The adjuvant obviously increases the immune effect of the vaccine; the using method is simple convenient and safe, has low cost and does need additional facilities and working procedures, does not affect the cultivating livability and the growth speed of the fishes, and is simultaneously harmless to the environment and operators.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Medicinal powder for treating asthma and cough
Disclosed is a medicinal granule for treating cough and asthma, whose formulation comprises Chinese ephedra, mulberry twigs, apricot seeds, inula flower, pinellia tuber, baikal skullcap root, loquat leaves, Sichuan fritillary bulb, batryticated silkworm, aster, root of herbaceous peony, licorice root, corinth pink, safflower, clam shell and western pharmaceuticals including glycyrrhizin, chlorpheniramine maleate, diprophyllin, and racanisodamine hydrochloride.
Owner:刘益斌
Ointment for treating bedsore, diabetic wound and scald
ActiveCN102764342AEnhance tissue permeabilityPromote recoveryHydroxy compound active ingredientsInorganic active ingredientsSulfadiazineEvaporation
The embodiment of the invention discloses ointment for treating bedsore, diabetic wound and scald. The ointment comprises the following components in percentage by weight: 1.5 to 6.5 percent of sulfanilamide crystal, 1.5 to 6.5 percent of sulfadiazine, 2.0 to 10.0 percent of zinc oxide, 0.4 to 4.0 percent of cod liver oil, 0.1 to 3.0 percent of racanisodamine, 0.02 to 1.4 percent of borneol, 5.0 to 30.0 percent of herba violae, and the balance of medical vaseline. By adopting the ointment, tissue permeability at the afflicted part of a patient can be enhanced, moisture evaporation of the ointment is reduced, the acting time of the medicament at the afflicted part is prolonged, bacteria at the afflicted part are inhibited, local microcirculation is obviously improved, discontinuity during capillary blood perfusion is avoided, and healing of the afflicted part is promoted.
Owner:陈鼎汉
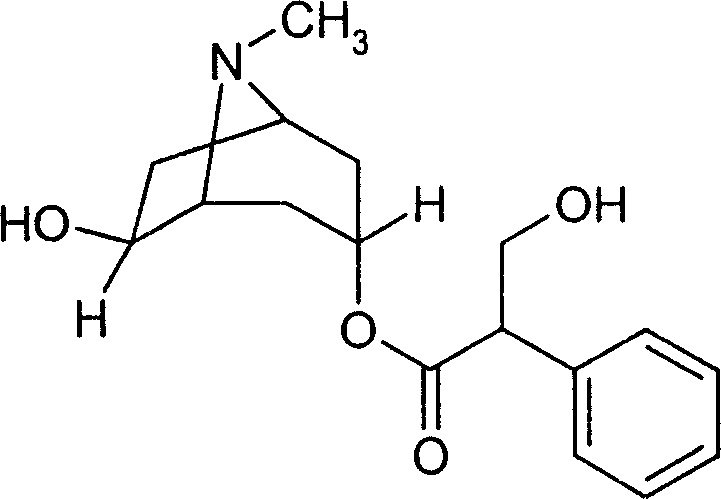
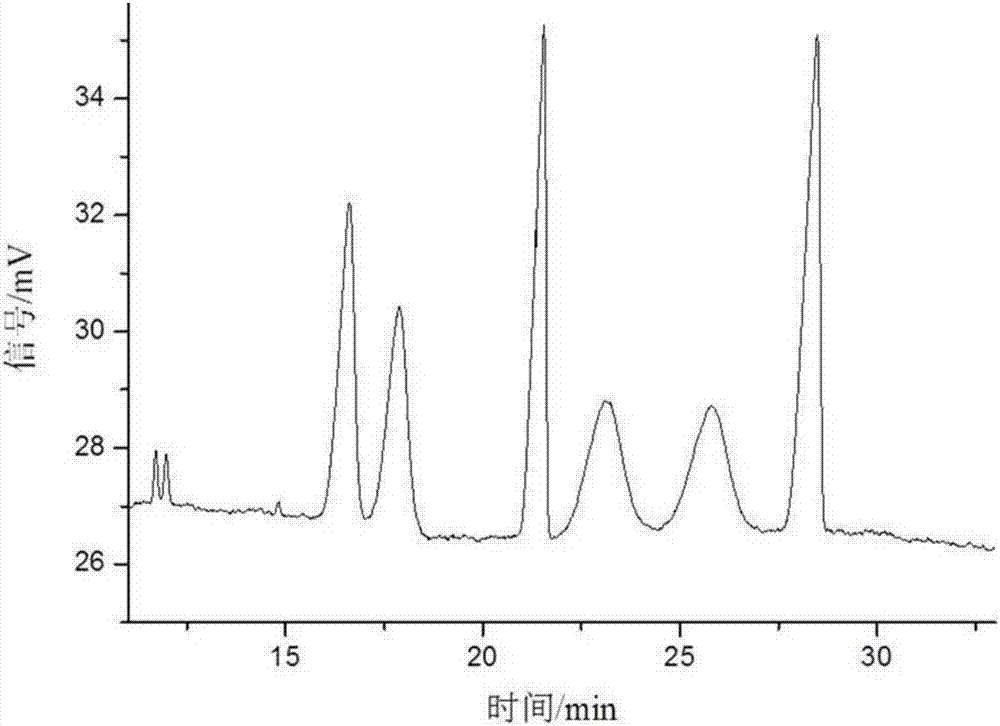
Separation method of anisodamine and enantiomers thereof
The invention discloses a separation method of anisodamine and enantiomers thereof. The anisodamine can be separated from a racanisodamine standard substance purchased from Nanjing Ze Lang Biotechnology Co., Ltd. The structural formulas of the anisodamine are represnted by formula I to formula IV shown in the description. The method is a capillary electrophoresis method, and is characterized in that an electrophoretic buffer solution contains a chiral resolving agent, and the chiral resolving agent is selected from sulfobutyl ether-beta-cyclodextrin, and / or a sulfobutyl ether-beta-cyclodextrin and hydroxypropyl-beta-cyclodextrin mixture; and 2, the separation voltage is -8 to -18 kV.
Owner:BEIJING UNIV OF CHINESE MEDICINE
Method for separating anisodamine and/or tropic acid mixed enantiomer
InactiveCN107991367AEfficient separationEasy to separateMaterial analysis by electric/magnetic meansCapillary electrophoresisElectrophoresis
The invention discloses a method for separating anisodamine and / or tropic acid mixed enantiomer. The method has the advantages that the anisodamine and tropic acid enantiomers can be respectively separated from a racemization anisodamine and tropic acid mixture; the structural formula is shown in formulas I to VI; the formulas I to VI are shown in the description. The method is a capillary electrophoresis method, and comprises the following steps that (1) an electrophoresis buffer solution contains a chiral resolution agent, and the chiral resolution agent is selected from a mixture of sulfobutyl-beta-cyclodextrin, and / or sulfobutyl-beta-cyclodextrin and hydroxypropyl-beta-cyclodextrin; (2) the separation voltage is -8kV to -18kV.
Owner:BEIJING UNIV OF CHINESE MEDICINE
Anisodamine freeze drying preparations for injections and preparation method
The present invention discloses a freezing stem preparation of the anisodamine and a preparation method, which is produced by concentrated preparation, carbon adsorption, crude filtration, dilution, intermediate good measurement, refined filtration, filling, half compression and plugging, freezing and drying with the active component of the nisodamine, the salt formation agent, the acid regulator, the isoosmotic adjusting agent, the excipient or the water for the injection.
Owner:张为群
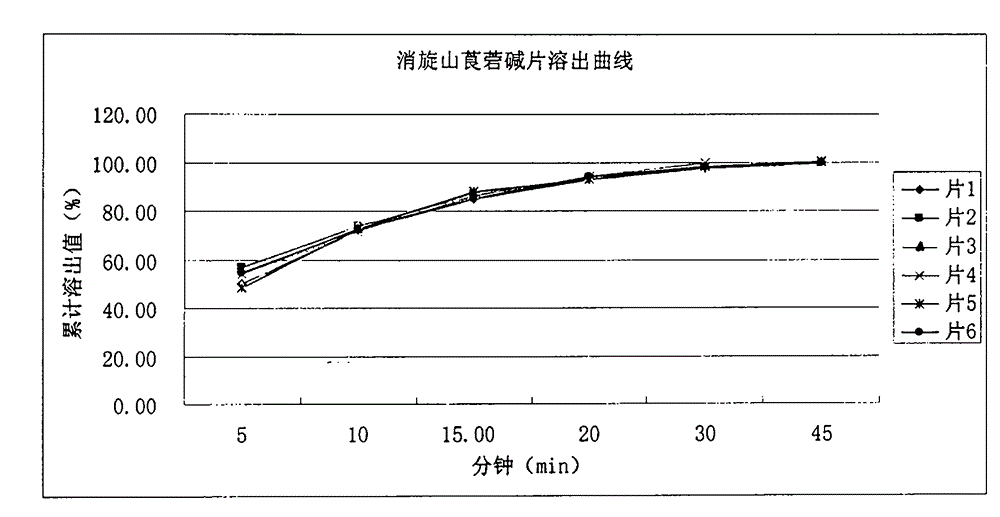
Medicinal composition for increasing dissolution rate of racanisodamine and preparation method thereof
InactiveCN104398513AHigh yieldHigh economic valueDigestive systemPharmaceutical non-active ingredientsOrganic solventDiluent
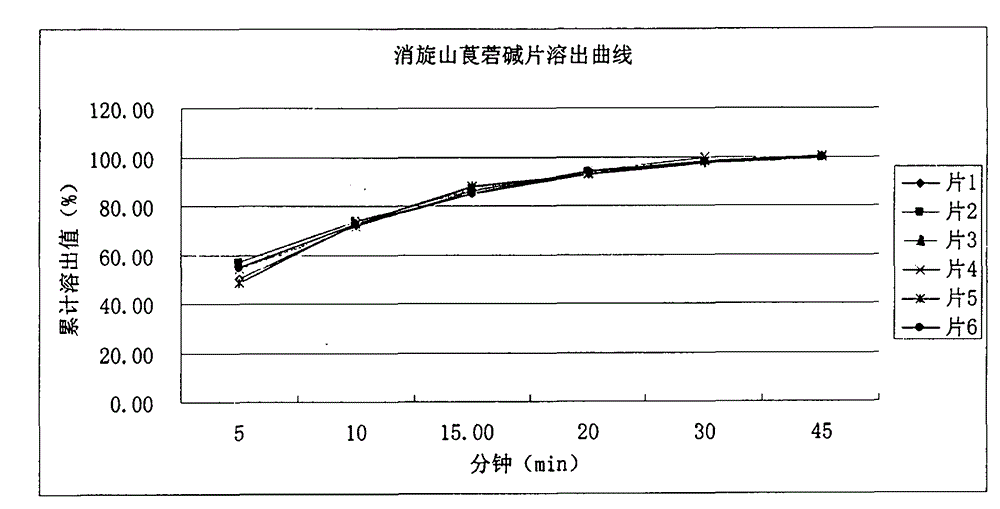
The invention relates to a medicinal composition for increasing dissolution rate of racanisodamine and a preparation method thereof. The medicinal composition and the preparation method thereof can effectively improve the dissolution rate of racanisodamine. Through a micronization technology and pharmaceutically acceptable diluents or carriers, a racanisodamine solid preparation, such as tablet, is prepared, so that the racanisodamine rapidly releases from the preparation to improve the bioavailability. The process of the invention is simple and easy, does not use organic solvent in the process, and has low production cost and little environmental pollution.
Owner:HENAN PURUI PHARMA
Novel plant acaridal application of racanisodamine hydrochloride
The invention discloses a novel plant acaridal application of racanisodamine hydrochloride and especially to application of racanisodamine hydrochloride in killing carmine spider mite. A method for preparation of an acaricide from racanisodamine hydrochloride comprises the following steps: adding 25 to 75 volume parts of commercially available racanisodamine hydrochloride injection into 20 to 60 volume parts of a solvent for dissolving, adding 5 to 15 volume parts of a solubilizer or / and 5 to 15 volume parts of an emulsifier to promote dissolving of racanisodamine hydrochloride and adding conventional adjuvants to prepare a spraying agent, missible oil, a suspending agent or pulvis according to a conventional process. The invention provides a novel harmonious pesticide which has good compatibility with the environment and is safe to mammals, which is of profound significance to long-term development of ecology, health of mankind and comprehensive control of pest mites.
Owner:BEIJING UNIV OF AGRI
Nasal drop and preparation method thereof
InactiveCN106668094AReasonable collocationFair usePharmaceutical delivery mechanismRespiratory disorderSide effectHormone
The invention relates to a nasal drop and a preparation method thereof. The nasal drop is prepared from the following raw material medicines in parts by weight: 1-2mL of a chlorpheniramine maleate injection which comprises 10-20mg of chlorpheniramine maleate, 1-3mL of a moroxydine hydrochloride injection which comprises 50-150mg of moroxydine hydrochloride, 0.1-0.5mL of a hydrochloric acid racanisodamine injection which comprises 1-5mg of hydrochloric acid racanisodamine, and 3-6mL of a radix bupleuri extract. The preparation method specifically comprises the following steps of: mixing the raw material medicines according to the ratio, and filling into a nasal drop container. The nasal drop is safe in medicinal formula, small in toxic and side effect, reasonable in matching of traditional Chinese medicines and western medicines, applicable to infants, free of hormone and free of drug dependency.
Owner:栗明峰
Eye drops for anti-eye fatigue and preparation method thereof
The invention discloses eye drops for resisting asthenopia. The eye drops comprise the following components: amiotide eye drops, inosine injection, adenosine disodium triphosphate injection, racanisodamine injection, vitamin B6 injection, taurine eye drops and an assistant for eyes, wherein the assistant for eyes is at least one of injection water or an isoosmotic adjusting agent; the isoosmotic adjusting agent is at least one of a sodium chloride physiological saline, glucose injection, sorbitol eye drops, borate saline buffer, mannitol injection or polyethylene glycol eye drops. The invention also discloses a preparation method of the eye drops for resisting asthenopia. The eye drops have the advantages that: 1, the overall efficacy of a compound preparation is obviously superior to a single drug effect; 2, the eye drops play roles in the whole process of the disease, the dose is reduced, and the side effect is reduced; 3, various medicines work in coordination with each other, are complementary in efficacy, and are taken as preconditions for playing roles; 4, the eye drops are obvious in pharmacological effect, applicable and convenient, and convenient to carry.
Owner:宁夏瑞视眼科研究所
Compound racanisodamine eye drops
InactiveCN112353757ASignificant and effective delayObvious and effective control effectSenses disorderPeptide/protein ingredientsThiomersalateDrug biological activity
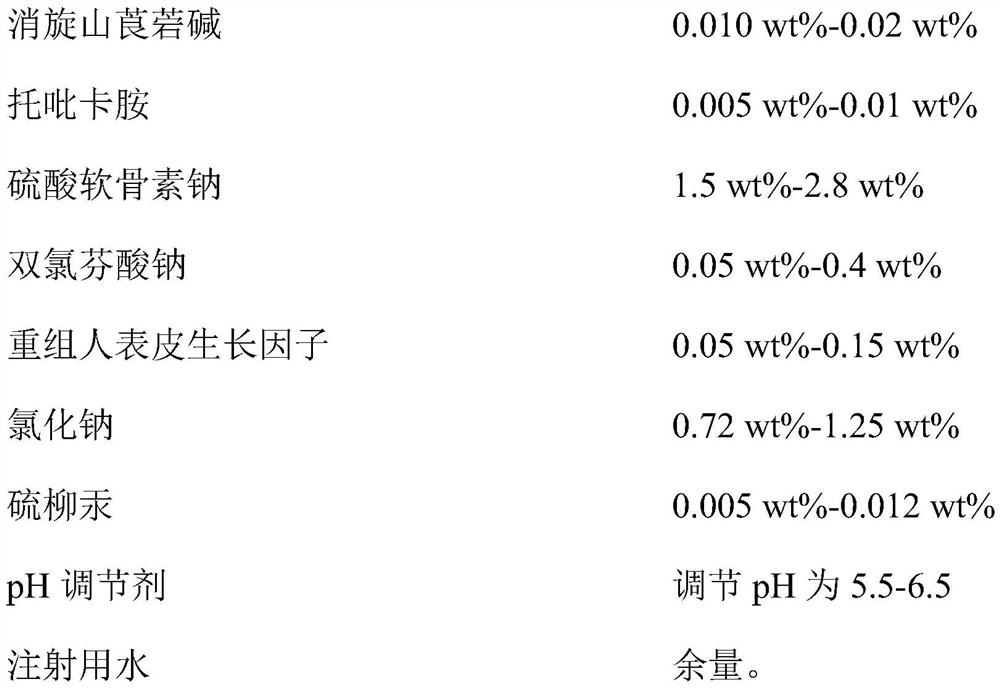
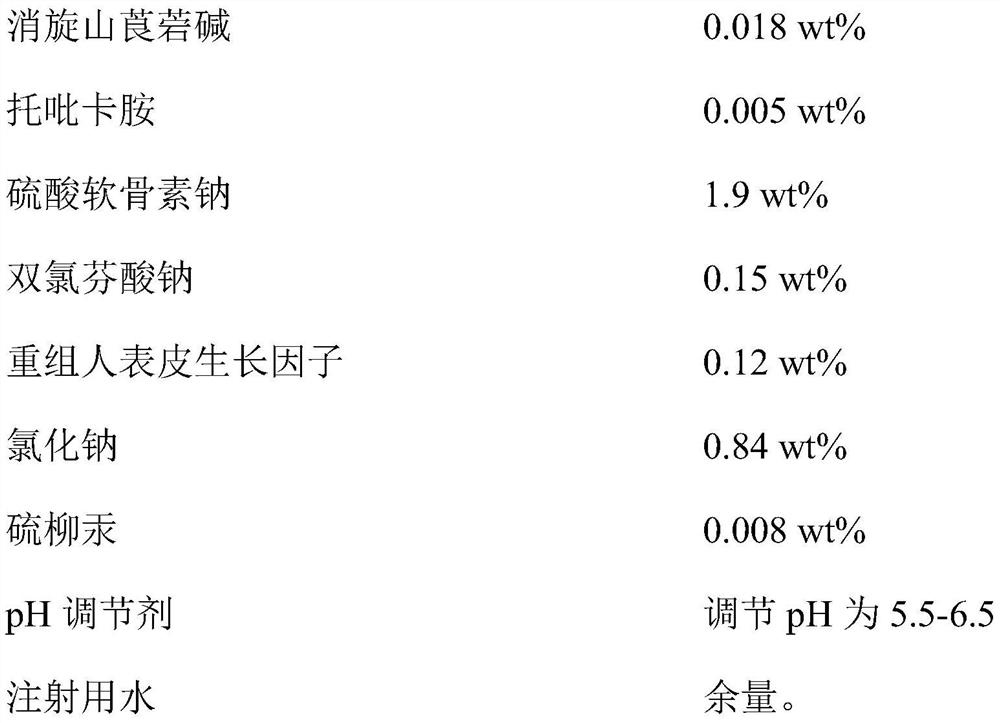
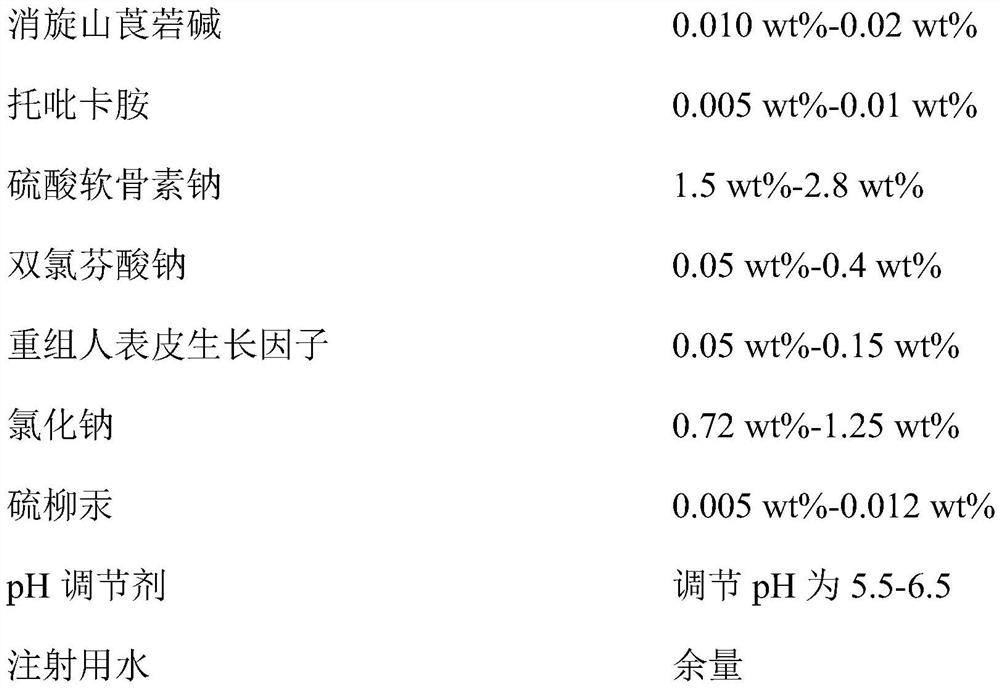
Provided is compound racanisodamine eye drops prepared from the following raw materials: 0.010-0.02 wt% of racanisodamine, 0.005-0.01 wt% of tropicamide, 1.5-2.8 wt% of sodium chondroitin sulfate, 0.05-0.4 wt% of diclofenac sodium, 0.05-0.15 wt% of a recombinant human epidermal growth factor, 0.72-1.25 wt% of sodium chloride, 0.005-0.012 wt% of thiomersalate, a pH regulator for adjusting pH to 5.5-6.5, and the balance being injection water. The compound racanisodamine eye drops disclosed by the invention are applied to adolescents, children and adults who often use eyes at a short distance, sothat the delaying and preventing effects on myopia of the adolescents and children are more obvious and effective after the compound racanisodamine eye drops are dripped for a long time, and for adults, visual fatigue can be significantly improved, and the elimination of eyeball discomfort and other symptoms can be accelerated; diclofenac sodium and racemic anisodamine are combined to achieve a good synergistic effect, diclofenac sodium is used as a ligand and matched with racemic anisodamine to generate a synergistic effect of biological activity, and meanwhile, diclofenac sodium and racemicanisodamine have the possibility of reducing toxicity, are low in price and easy to obtain and are worthy of popularization clinically.
Owner:ZHENJIANG HENGXIN PHARMA
Paste dressing formula for treating rhagadia of hands and feet and preparation method of paste dressing formula
InactiveCN112245483APromote healingPromote muscle and relieve painHydrocarbon active ingredientsAntimycoticsFormularyStearic acid
The invention discloses a paste dressing formula for treating rhagadia of hands and feet and a preparation method of the paste dressing formula, relates to the field of medicine and pharmacology, andaims at solving the problems that a traditional external medicine for treating the rhagadia of the hands and the feet is poor in curative effects and the rhagadia is prone to relapse. The paste dressing formula for treating the rhagadia of the hands and the feet comprises the following raw materials in percentage: 15%-35% of petrolatum, 10%-25% of glycerinum, 8%-18% of propylene glycol, 2%-8% of panthenol, 2%-10% of stearic acid, 3%-12% of cetostearyl alcohol, 0.1%-1% of racanisodamine, 10%-25% of horse oil, 1-5% of peach kernel extract and 1%-5% of bletilla striata gum. The petrolatum, the glycerol, the propylene glycol, the stearic acid, the panthenol and the cetostearyl alcohol are used as humectants, traditional Chinese medicines for promoting tissue regeneration and promoting skin regeneration including the horse oil, peach kernels, rhizoma bletillae and the like, and an analgesic component namely the racanisodamine are added, and through a series of operations of mixing, homogenizing, stirring and the like, products are made into ointment. Different from the traditional external medicine for treating the rhagadia of the hands and the feet which only has moisturizing and moistening effects on rhagadia wounds, the product also has the effects of promoting tissue regeneration, relieving pain and promoting wound healing and has the characteristic that the rhagadia is not easyto relapse after healing.
Owner:郑虹
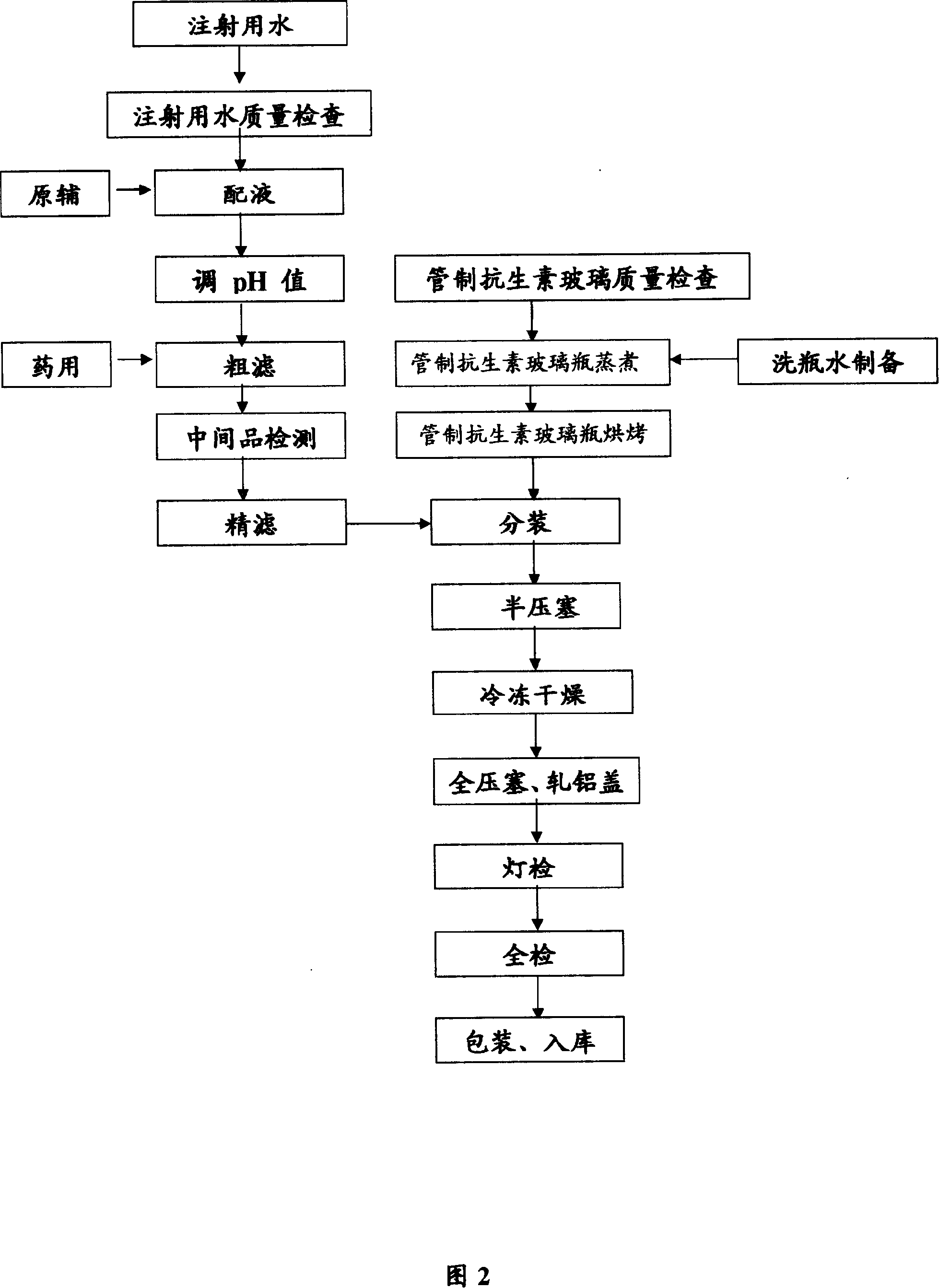
Preparation technology of racanisodamine hydrochloride injection
InactiveCN104288097AIncrease profitReduce manufacturing costDigestive systemMuscular disorderFiberUltrafiltration
The invention discloses a preparation technology of racanisodamine hydrochloride injection. A high pressure ultrafiltration device is used to overcome a defect that precious traditional preparation technologies of the racanisodamine hydrochloride injection cannot effectively ensure the clarity or the filtering safety of fibers or other impurities. The preparation technology disclosed in the invention has the advantages of low cost, high yield, and stable quality of the above finished product. The technology comprises the following steps: (1) putting injection water in a preparation tank, and decreasing the water temperature to 39-41DEG C; (2) taking the injection water in the tank, dissolving sodium chloride to prepare a solution for later use, adding racanisodamine into the preparation tank, starting a stirrer, adding 0.2mol / L of hydrochloric acid into the preparation tank, circulating, and stirring until racanisodamine is completely dissolved; and (3) adjusting the pH value of the soup obtained in step 2 by 0.2mol / L of hydrochloric acid to 5.5-6.0, filtering the soup through a high pressure ultrafiltration machine, inspecting, and canning qualified intermediate products. The preparation technology enhances the market competitiveness of the products, is convenient for the marketing of the products, and is worth to be popularized.
Owner:北京优瀚康科技有限公司
Raceanisodamine hydrochloride injection composition
The invention discloses a raceanisodamine hydrochloride injection composition with a Ph value less than 4, which contains raceanisodamine hydrochloride, hydrochloric acid, sodium chloride and water, and a preparation method of the composition.
Owner:TIANJIN JINYAO GRP
Rceanisodamine hydrochloride injection liquid stable in quality and preparation method of raceanisodamine hydrochloride injection liquid
PendingCN109528640AReduce productionLow impurity contentDigestive systemInorganic non-active ingredientsFiltrationTobramycin
The invention discloses raceanisodamine hydrochloride injection liquid stable in quality and a preparation method of the raceanisodamine hydrochloride injection liquid. The preparation method comprises the following steps of (1) adding hydrochloric acid to water for injection, and performing mixing so as to obtain a hydrochloric acid solution; (2) adding raceanisodamine and sodium chloride to thehydrochloric acid solution, and performing mixing until the pH value is 5.0-5.3 so as to obtain a raceanisodamine hydrochloride solution; (3) adding a glucose solution and tobramycin to the prepared raceanisodamine hydrochloride solution, and performing mixing so as to obtain mixed liquid M1; and (4) sequentially performing coarse filtration and fine filtration on the mixed liquid M1 so as to obtain the raceanisodamine hydrochloride injection liquid stable in quality. The effects that the product nature can be stable, the raceanisodamine hydrochloride injection liquid stable in quality still has favorable quality after long-term storage, and the impurity content of the prepared raceanisodamine hydrochloride injection liquid stable in quality is lower can be achieved.
Owner:江西润泽药业有限公司
Fish soaking vaccine combination adjuvant and application and using method thereof
InactiveCN101791403AImprove immunityNo effect on activityAntibody medical ingredientsImmune effectsAdjuvant
The invention relates to a fish soaking vaccine combination adjuvant and application and a using method thereof. The invention is characterized in that the combination adjuvant is the combination of three adjuvants which are respectively dithiothreitol, Vitamin D3 and racanisodamine, wherein the using concentrations of dithiothreitol, Vitamin D3 and racanisodamine are respectively 1-50mg / L, 0.1-20mg / L and 1-60mg / L; the fish soaking vaccine combination adjuvant is applied to the soaking vaccine comprising seawater and freshwater fishes, and the synergistic effect reaches the efficient immunity effect. The using method is as follows: firstly, selecting the fish with more than 2g weight and sound body surface to be immunized, and immigrating into dithiothreitol pretreatment solution with 1-50mg / L of the concentration range to be soaked for 1-20min, then adding VD3 with 1-20mg / L of the concentration range and vaccine to be soaked for 1-20min thereinto, and finally adding racanisodamine with 1-60mg / L of the concentration range to be soaked for 5-20 min. The fish soaking vaccine combination adjuvant obviously increases the immune effect of the vaccine; the using method is simple, convenient and safe, has low cost, does need additional facilities and working procedures, does not affect the cultivating livability and the growth speed of the fishes, and is simultaneously harmless to the environment and operating personnel.
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Compound raceanisodamine hydrochloride injection and preparation method thereof
InactiveCN104173274AImprove survival rateIncrease weightAntibacterial agentsDigestive systemMicronomicinGastrointestinal tract
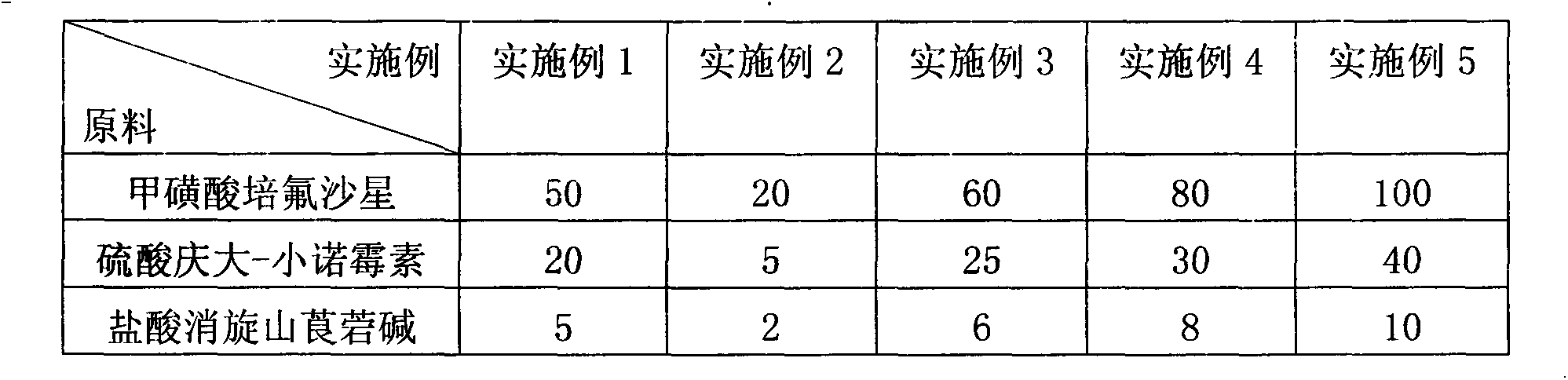
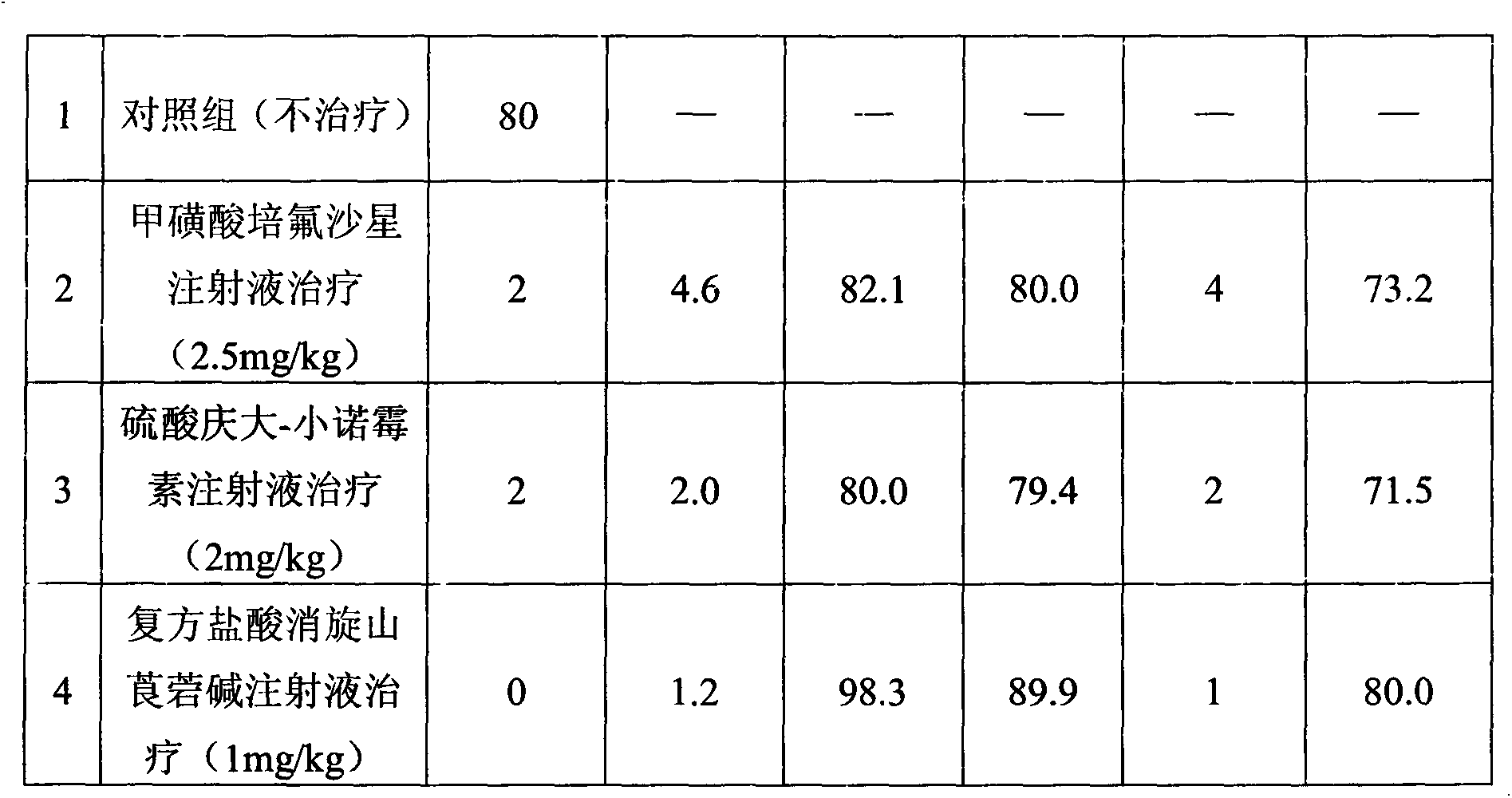
The invention discloses a compound raceanisodamine hydrochloride injection. The compound raceanisodamine hydrochloride injection comprises, by weight, 20-100 parts of pefloxacin mesylate, 1-40 parts of gentamycin sulfate-micronomicin, 1-10 parts of raceanisodamine hydrochloride and 800-1000 parts of injection water. The compound raceanisodamine hydrochloride injection has high effects on bacterial diarrhea of dogs, foxes and racoon dogs, can treat both symptoms and root causes and can relieve infected animal body stress reactions. The compound raceanisodamine hydrochloride injection comprises pefloxacin mesylate, gentamycin sulfate-micronomicin and raceanisodamine hydrochloride, has substantial effects on dogs, foxes and racoon dogs, and can relieve infected animal body stress reactions. An experiment proves that through combination of pefloxacin mesylate, gentamycin sulfate-micronomicin and raceanisodamine hydrochloride, the compound raceanisodamine hydrochloride injection can improve curative effects, has a wide clinical application range, can be widely used in dog, fox and racoon dog gastrointestinal tracts infected by bacteria, can improve dog, fox and racoon dog survival rates and can improve dog, fox and racoon dog body weights.
Owner:TIANJIN BIJIA BIOTECH
Medicament for treating rhinitis
InactiveCN100335097CSafe and effectiveInanimate material medical ingredientsRespiratory disorderTwigRacanisodamine
Owner:刘益斌
Ointment for treating bedsore, diabetic wound and scald
ActiveCN102764342BEnhance tissue permeabilityPromote recoveryHydroxy compound active ingredientsInorganic active ingredientsViola yedoensisSulfadiazine
The embodiment of the invention discloses ointment for treating bedsore, diabetic wound and scald. The ointment comprises the following components in percentage by weight: 1.5 to 6.5 percent of sulfanilamide crystal, 1.5 to 6.5 percent of sulfadiazine, 2.0 to 10.0 percent of zinc oxide, 0.4 to 4.0 percent of cod liver oil, 0.1 to 3.0 percent of racanisodamine, 0.02 to 1.4 percent of borneol, 5.0 to 30.0 percent of herba violae, and the balance of medical vaseline. By adopting the ointment, tissue permeability at the afflicted part of a patient can be enhanced, moisture evaporation of the ointment is reduced, the acting time of the medicament at the afflicted part is prolonged, bacteria at the afflicted part are inhibited, local microcirculation is obviously improved, discontinuity during capillary blood perfusion is avoided, and healing of the afflicted part is promoted.
Owner:陈鼎汉
Anti-allergic inflammation drops
InactiveCN107970236AQuick and effective controlGood effectImmunological disordersHeterocyclic compound active ingredientsDiseaseInnovative Therapies
The invention provides anti-allergic inflammation drops. The anti-allergic inflammation drops are prepared from sodium cromoglycate, diclofenac sodium, racanisodamine hydrochloride, dexamethasone, triamcinolone acetonide, glycyrrhizin and B-vitamins; various medicines are combined to have a synergistic action and have a treatment effect in various links; the anti-allergic inflammation drops have aremarkable pharmaceutical effect and are rapid and effective in disease control; the anti-allergic inflammation drops integrate different functions of various medicines so that the dosage of the single medicine is reduced, the total pharmaceutical effect is improved, the side effect is remarkably reduced, the effect of getting twofold results with half the effort is realized and the curative effect is very satisfactory; the whole scheme is created for allergic diseases, has the characteristics of comprehensively treating, treating both symptoms and root cause of diseases, striving for qualityand accurately positioning and is an innovative therapy based on brand-new acquaintance of a disease theory.
Owner:宁夏瑞视眼科研究所
Lubricating gel capable of relieving spasm and pain
InactiveCN107998463ARelieve body and mindRelieve physical painSurgeryInflammatory edemaChlorhexidine Acetate
The invention relates to lubricating gel capable of relieving spasm and pain. The gel is prepared from 0.5-0.8% of hydroxypropyl methyl cellulose, 0.8-1.2% of tetracaine hydrochloride, 1.0-2.0% of polyethylene glycol, 0.01-5% of chlorhexidine acetate, 0.01-5% of triamcinolone, 0.01-5% of racanisodamine and the balance purified water. Meanwhile, the gel has multiple effects including that a lubricating function is achieved when cannulas are introduced into human body cavities and endoscopic surgical dry tools enter the human body, local anesthesia is conducted on skin mucosae, and foam of the cavities is removed to relieve the spasm and pain and alleviate mucosa inflammatory edema caused by cannula insertion and other operation. Not only can the examination and surgery time be saved, but also the psychological pain and physical pain of a patient can be alleviated, the success rates of examination and surgery are increased at the same time, damage caused by surgery or instrument operation to the mucosae is reduced, and the lubricating gel has a significant clinical meaning.
Owner:张楷乐
Chinese and western medicine composition for treating porcine diarrhea and preparation method thereof
InactiveCN106668140AAchieve full recoveryQuick effectHydroxy compound active ingredientsDigestive systemSide effectGenipin
The invention discloses a Chinese and western medicine composition for treating porcine diarrhea, prepared from the following main raw materials in parts by weight: 16-20 parts of longhairy antenoron herb, 20-25 parts of wild chrysanthemum flowers, 8-10 parts of narirutin, 2-9 parts of fargesin, 6-12 parts of d-tetrahydropalmatine, 26-30 parts of nerolidol, 8-15 parts of sulforaphane, 6-10 parts of genipin-1-beta-D-gentiobioside, 17-20 parts of polygalaxanthone III and 18-24 parts of racanisodamine. The raw components of the Chinese and western medicine are selected strictly according to the cognitive mechanism of porcine diarrhea, thereby achieving the purpose of overall recovery, and the Chinese and western medicine has the characteristics of high effect-taking speed, stable effects, convenience in carrying and administration, and no toxic or side effects after long-term administration.
Owner:ZHENGZHOU RENHONG PHARMA CO LTD
Drug formula used for promoting skin wound healing
The invention provides a drug formula used for promoting skin wound healing, which belongs to the field of a drug formula. The formula is characterized in that the formula includes, by weight, 40-60 parts of a vitamin C injection (5 ml:1 g), 10-20 parts of a vitamin B1 injection (1 ml:50 mg), 10-20 parts of a vitamin B6 injection (1 ml:50 mg), and 10-20 parts of a raceanisodamine hydrochloride injection (1 ml:10 mg). The drug formula is mainly used for treatment of diabetic foot and bedsore, and has good effects of being resistant to inflammation, relieving pain, controlling infection and ulceration, improving microcirculation, and promoting wound healing.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Combined adjuvant for fish soaked vaccine and its application and use method
Owner:YELLOW SEA FISHERIES RES INST CHINESE ACAD OF FISHERIES SCI
Compound oral liquid for treating esophageal carcinoma and cardiac carcinoma dysphagia
InactiveCN106109589AEasy to prepareLow priceDispersion deliveryHydroxy compound active ingredientsVolume concentrationRacanisodamine
The invention relates to compound oral liquid for effectively treating esophageal carcinoma and cardiac carcinoma dysphagia. According to the technical scheme, the compound oral liquid is prepared by evenly mixing 30-250ml of mannitol injection with the volume concentration being 20%, 3-15ml of lidocaine hydrochloride injection with the volume concentration being 2%, 5-30mg of racanisodamine hydrochloride injection and 30-300ml of fresh lotus root juice. The compound oral liquid is simple to prepare, easy in raw material obtaining, low in price, good in taste, convenient to take and evident in effect and is innovation of medicine for treating esophageal carcinoma and cardiac carcinoma dysphagia.
Owner:HENAN UNIV OF CHINESE MEDICINE
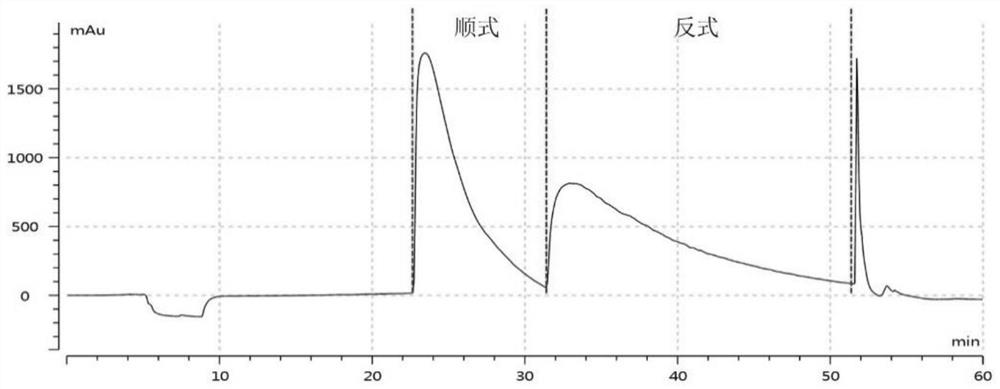
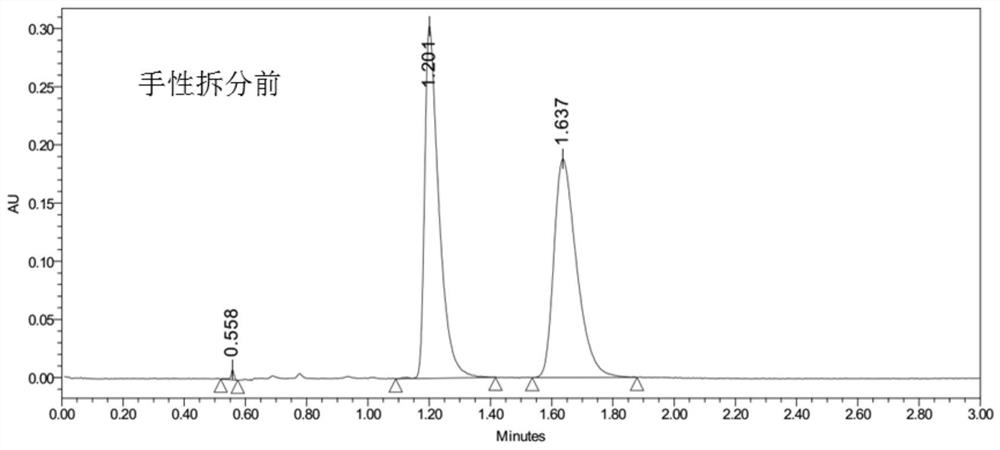
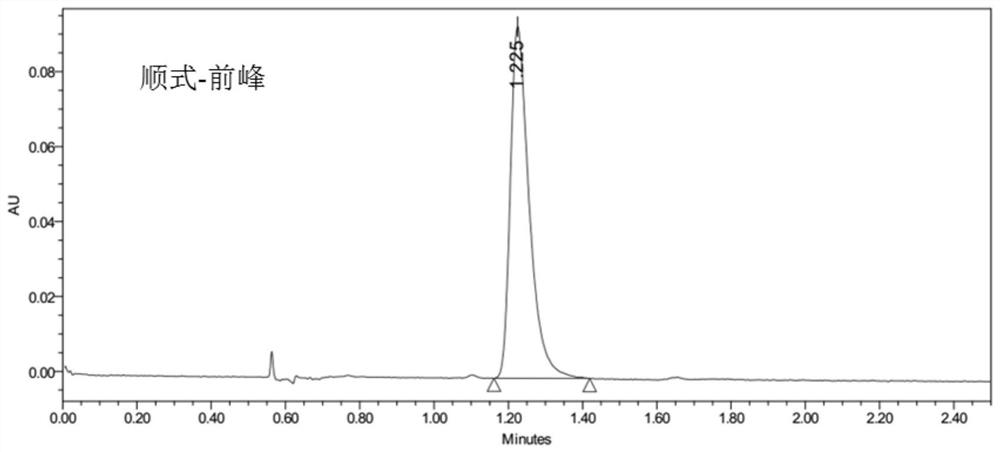
Cis-isomer of anisodamine and separation and detection method thereof
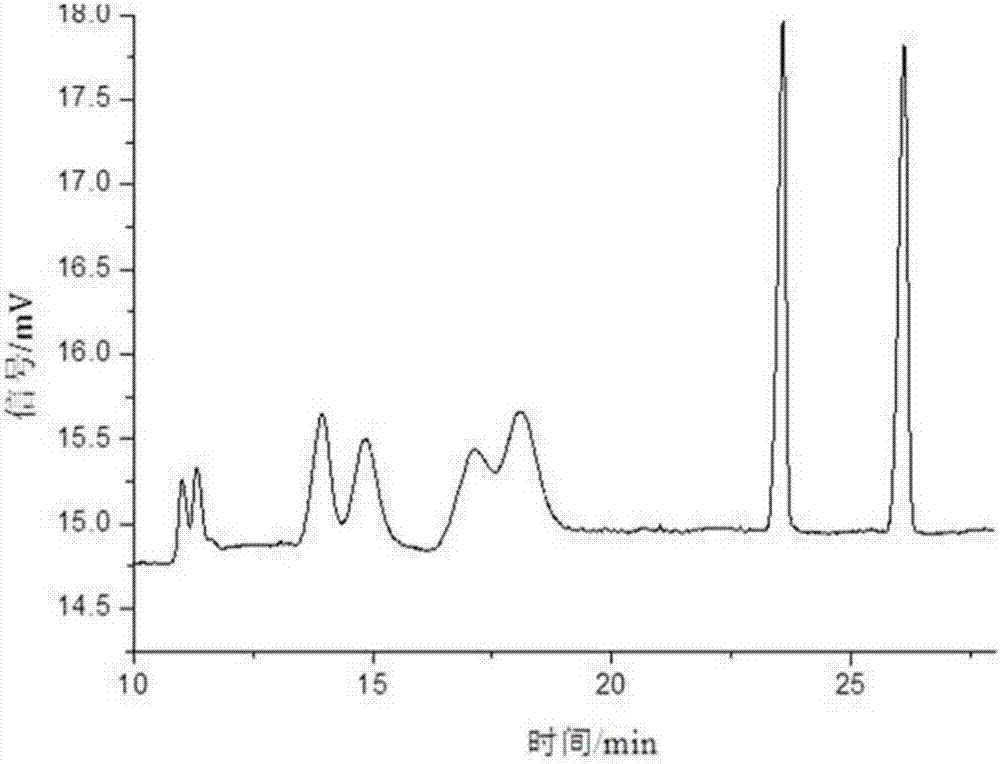
PendingCN114874206AImplement extractionHigh purityComponent separationOrganic chemistry methodsPharmaceutical drugCombinatorial chemistry
The invention belongs to the field of medicine separation and analysis, and particularly relates to a cis-isomer of anisodamine and a separation and detection method of the cis-isomer. Through combination of HPLC and supercritical chromatography, the cis-isomer of racanisodamine can be effectively separated, and then through creative chromatographic parameter selection in HPLC and supercritical chromatography, the separation degree is improved, so that the purity of the two finally obtained cis-isomer monomers of anisodamine is high, and the purity of the two obtained cis-isomer monomers is high. And a foundation is laid for large-scale industrial application.
Owner:CHENGDU FIRST PHARMACEDTICAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com