Medicinal composition for increasing dissolution rate of racanisodamine and preparation method thereof
A technology of anisodamine and its composition, which is applied in the field of pharmaceutical composition and its preparation for improving the dissolution rate of racemic anisodamine, which can solve the problem of poor dissolution rate and stability, non-compliance of drug dissolution rate, and influence on bioavailability issues such as high yield and clinical use effect, to achieve the effect of high yield and high economic value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The components and the proportioning ratio of the improved racemic anisodamine pharmaceutical composition of the present invention are as follows:
[0046] Formula quantity (1000 tablets / batch), the specification is 5mg tablet.
[0047] components particle size Proportion Feeding amount racemic anisodamine 50μm 6.25% 5g lactose 115μm 27.5% 22g microcrystalline cellulose 115μm 27.5% 22g starch 115μm 28.6% 22.9g
[0048] Croscarmellose Sodium 115μm 5% 4g purified water ---- 0 0 (dry removal) Croscarmellose sodium (step e plus) 115μm 5% 4g Magnesium stearate 115μm 0.1% 0.08g total ---- 100% 80g
[0049] The pharmaceutical composition containing the dissolution rate of 6.25% of the total component weight in the table above is prepared into a tablet by the following method:
[0050] (a) the active compound racemic anisodamine is micronized to control particle size;
...
Embodiment 2
[0061] The components and the proportioning ratio of the improved racemic anisodamine pharmaceutical composition of the present invention are as follows:
[0062] Tablet production (1000 tablets / batch), the specification is 10mg tablet,
[0063] components particle size Proportion Feeding amount racemic anisodamine 25μm 12.5% 10g sucrose 75μm 7.5% 6g Mannitol 75μm 17.5% 14g pregelatinized starch 75μm 12.5% 10g Crospovidone 75μm 15% 12g syrup ---- 10% 8g Croscarmellose sodium (step e plus) 75μm 15% 12g Calcium stearate 75μm 10% 8g
[0064] total ---- 100% 80g
[0065] The pharmaceutical composition of the dissolution rate of racemic anisodamine in the above table is compressed into a tablet by the preparation method of Example 1.
[0066] Experimental Test 2:
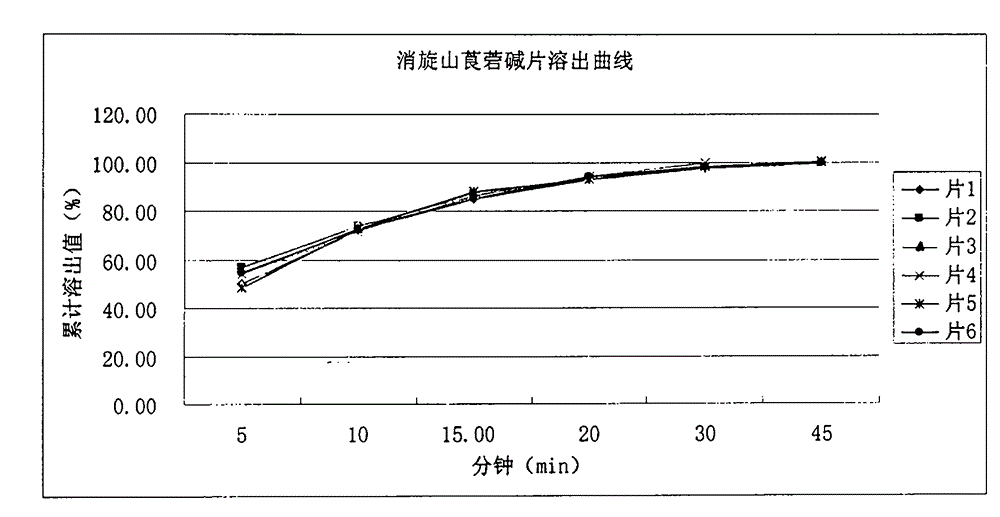
[0067] Under simulated gastrointestinal conditions, 6 tablets were randomly selected from the tablets prepared...
Embodiment 3
[0071] The components and the proportioning ratio of the improved racemic anisodamine pharmaceutical composition of the present invention are as follows:
[0072] Tablet production (10000 tablets / batch), the specification is 5mg tablets,
[0073] components particle size Proportion Feeding amount racemic anisodamine 75μm 6.25% 50g lactose 150μm 22% 176g calcium carbonate 150μm 22% 176g dextrin 150μm 23.75% 190g Sodium carboxymethyl starch 150μm 8% 64g Starch slurry ---- 5% 40g Low-substituted hydroxypropyl cellulose (step e added) 150μm 8% 64g Micropowder silica gel 150μm 5% 40g total ---- 100% 800g
[0074] The pharmaceutical composition of the dissolution rate of racemic anisodamine in the above table is compressed into a tablet by the preparation method of Example 1.
[0075] Experimental Test 3:
[0076] Under simulated gastrointestinal conditions, 6 tablets were randomly se...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com