Cis-isomer of anisodamine and separation and detection method thereof
A technology of cis-isomers and anisodamine, which is applied in the field of drug separation and analysis, can solve the problems of low purity of optical isomers, and achieve the effects of improving purity, improving safety and effectiveness, and good peak shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] A kind of separation method of the cis isomer of anisodamine, comprising the following steps:
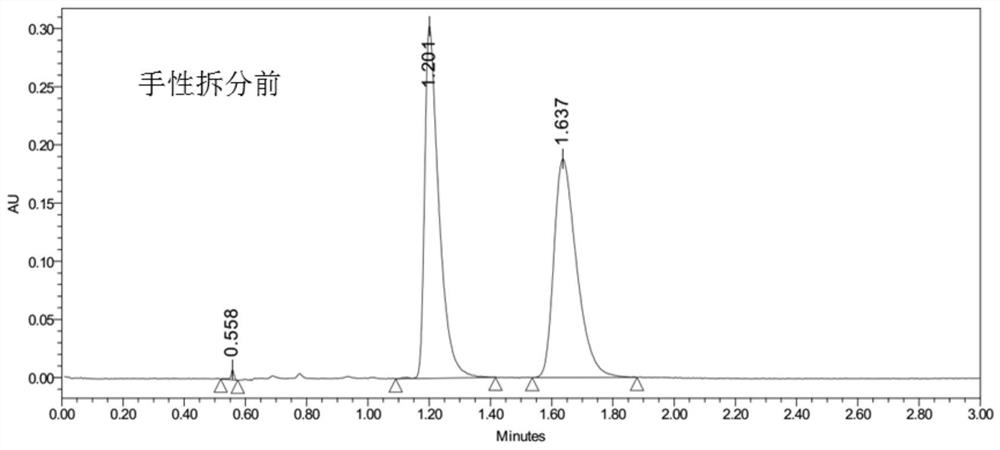
[0074] S1. Take 14 g of racemic anisodamine for separation by dynamic preparative HPLC to obtain cis-anisodamine containing 6R, 2'S and 6S, 2'R and trans-anisodamine containing 6R, 2'R and 6S, 2'S Alkali, 7g in total;
[0075] The chromatographic parameters that the preparative HPLC separates are as follows:
[0076] Chromatographic column: DAC80 dynamic preparation separation column;
[0077] Column packing: Huapu LD-2-C18;
[0078] Flow rate: 200ml / min;
[0079] Wavelength: 210nm;
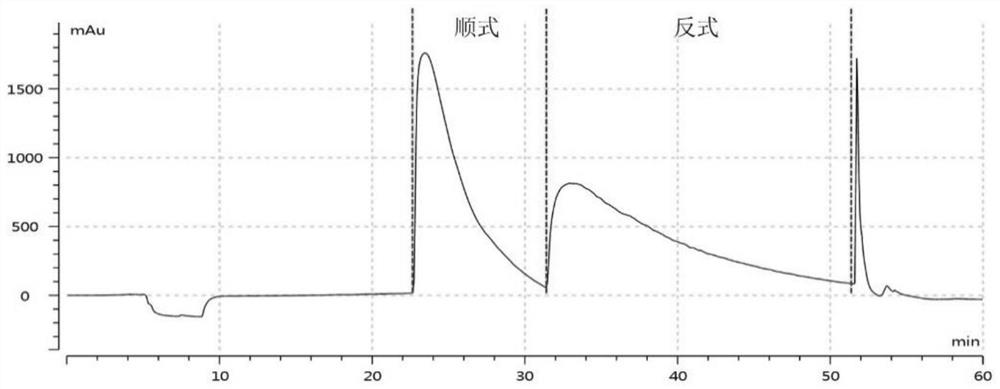
[0080] When the elution time is 0-45min, the mobile phase is a mixture of 0.1% formic acid and methanol in a mass ratio of 95:5; when the elution time is 45-60min, the mobile phase is 0.1% formic acid and methanol according to 30:5 70 mass ratio mixed mixture. The resulting cis-anisodamine and trans-anisodamine split map such as figure 1 shown.
[0081] S2. After adjusting the pH of the ...
Embodiment 2
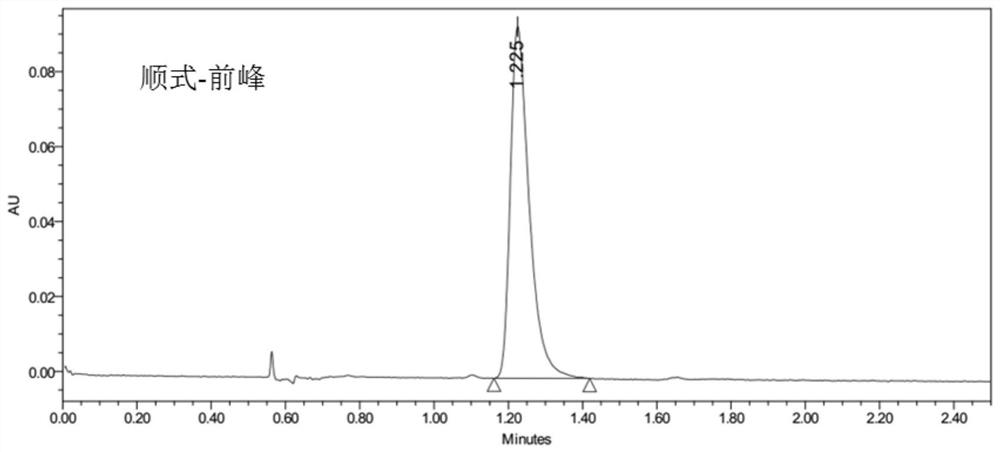
[0098] Get the anisodamine of 6R that step S3 obtains in embodiment 1, 2'S, adopt LCMS to detect, obtain the front peak of anisodamine cis-isomer as follows: Figure 5 shown, from Figure 5 It can be proved in Example 1 that the anisodamine of 6R, 2'S was successfully isolated, and the parameters of LCMS are as follows:
[0099] Chromatographic column: Waters X Bridge C18 column (50mm*4.6mm*3.5um);
[0100] Mobile phase: mobile phase A is 0.01mol / L NH 4 HCO 3 , mobile phase B is acetonitrile;
[0101] The flow rate is 2mL / min, the column temperature is 40℃, when the elution time is 0~1.6min, the mobile phase B is 5%~95%, and when the elution time is 1.6~3min, the mobile phase B is 95%.
Embodiment 3
[0103] Get the anisodamine of 6S that step S3 obtains in embodiment 1, the anisodamine of 2'R, adopt LCMS to detect, obtain the back peak of anisodamine cis-isomer as follows: Image 6 shown, from Image 6 It can be proved that the anisodamine of 6S and 2'R was successfully separated in Example 1, and the parameters of LCMS are as follows:
[0104] Chromatographic column: Waters X Bridge C18 column (50mm*4.6mm*3.5um);
[0105] Mobile phase: mobile phase A is 0.01mol / L NH 4 HCO 3 , mobile phase B is acetonitrile;
[0106] The flow rate is 2mL / min, the column temperature is 40℃, when the elution time is 0~1.6min, the mobile phase B is 5%~95%, and when the elution time is 1.6~3min, the mobile phase B is 95%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com