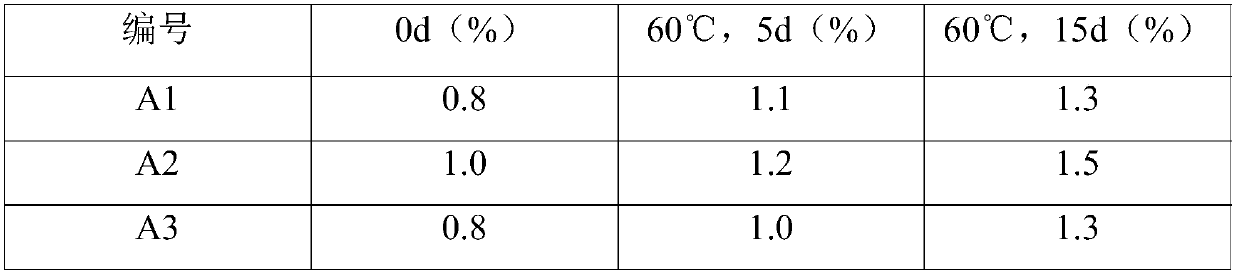
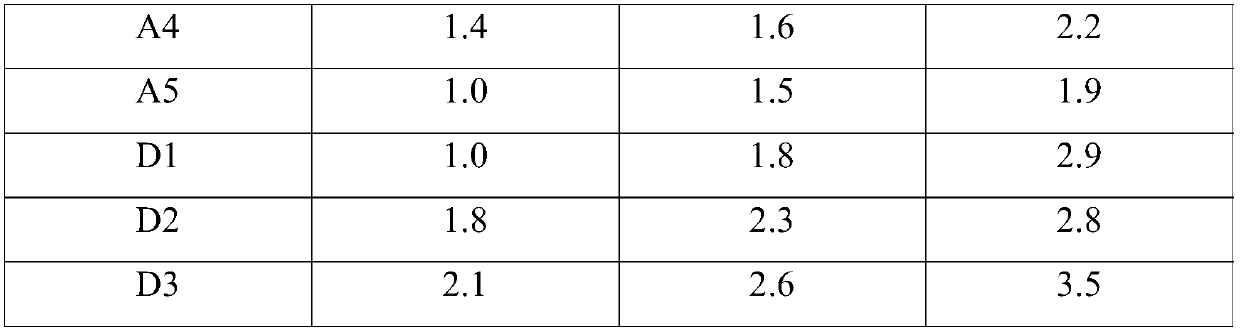
Rceanisodamine hydrochloride injection liquid stable in quality and preparation method of raceanisodamine hydrochloride injection liquid
A technology for anisodamine and injection, which is applied in the field of racemic anisodamine hydrochloride injection and its preparation, and can solve problems such as unstable production process, multiple impurities, and decreased content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0015] The invention provides a preparation method of racemic anisodamine hydrochloride injection with stable quality, wherein the preparation method comprises:
[0016] 1) adding hydrochloric acid to water for injection and mixing to obtain a hydrochloric acid solution;
[0017] 2) adding racemic anisodamine and sodium chloride to the hydrochloric acid solution and mixing until the pH value is 5.0-5.3 to obtain racemic anisodamine hydrochloride solution;
[0018] 3) adding glucose solution and tobramycin to the above-prepared racemic anisodamine hydrochloride solution and mixing to obtain a mixed solution M1;
[0019] 4) After the mixed solution M1 is subjected to coarse filtration and fine filtration in sequence, racemic anisodamine hydrochloride injection with stable quality is obtained.
[0020] The present invention prepares by first mixing hydrochloric acid and water for injection, then adding racemic anisodamine and sodium chloride therein, and further adjusting the pH...
Embodiment 1
[0032] 1) adding hydrochloric acid to water for injection and mixing to obtain a hydrochloric acid solution (so that the concentration of the hydrochloric acid solution is 0.1mol / L);
[0033] 2) Add 8 g of racemic anisodamine and 3 g of sodium chloride to 1 L of hydrochloric acid solution, place it at a temperature of 50° C., stir at a stirring rate of 100 r / min for 20 min, mix and adjust the pH value to 5.0 to obtain racemic hydrochloric acid Anisodamine solution;
[0034] 3) Add 300 mL of glucose solution with a concentration of 5% by weight and 0.3 g of tobramycin to 1 L of the above-prepared racemic anisodamine hydrochloride solution and mix to prepare a mixed solution M1;
[0035] 4) After the mixed solution M1 was sequentially filtered through a filter device with a filter hole of 0.45 μm and finely filtered through a filter device with a filter hole of 0.22 μm, racemic anisodamine hydrochloride injection A1 with stable quality was obtained.
Embodiment 2
[0037] 1) adding hydrochloric acid to water for injection and mixing to obtain a hydrochloric acid solution (so that the concentration of the hydrochloric acid solution is 0.15mol / L);
[0038] 2) Add 15 g of racemic anisodamine and 10 g of sodium chloride to 1 L of hydrochloric acid solution, place it at a temperature of 70° C., stir for 50 min at a stirring rate of 300 r / min, mix and adjust the pH value to 5.3 to obtain racemized hydrochloric acid Anisodamine solution;
[0039] 3) Add 600mL of glucose solution with a concentration of 5% by weight and 1g of tobramycin to 1L of the above-prepared racemic anisodamine hydrochloride solution and mix to prepare a mixed solution M1;
[0040] 4) After the mixed solution M1 was sequentially filtered through a filter device with a filter hole of 0.45 μm and finely filtered through a filter device with a filter hole of 0.22 μm, racemic anisodamine hydrochloride injection A2 with stable quality was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com