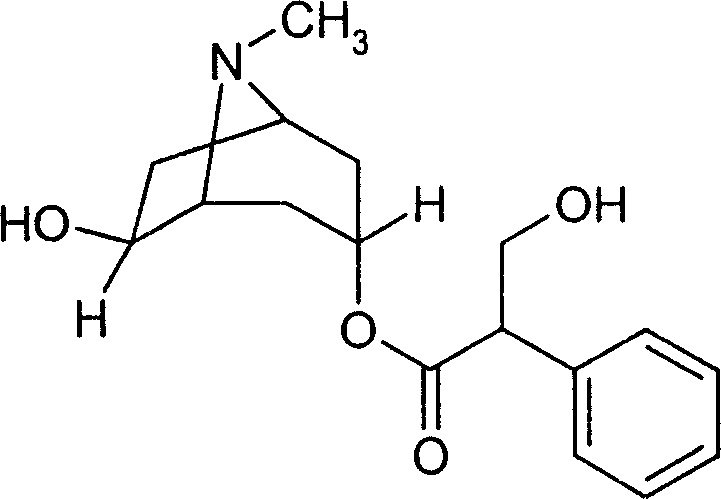
Racanisodamine eye drops
A technology of anisodamine and eye drops, applied in sensory diseases, active ingredients of heterocyclic compounds, drug combinations, etc., can solve the problems of large dosage of racemic anisodamine, poor reducibility, inconvenient use, etc., and achieve significant therapeutic effect , cost saving, and the effect of reducing the main drug content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] 100ml water for injection:
[0047] Racemic anisodamine 0.051g
[0048] Sodium hyaluronate 0.048g
[0050] Benzalkonium chloride 0.01g
[0051] Process:
[0052] Sodium hyaluronate is added to the 1 / 4 (v / v) water for injection, and it swells at room temperature.
[0053] Dissolve sodium chloride and benzalkonium chloride in 1 / 2 (v / v) water for injection, then add the fully swollen sodium hyaluronate solution, stir evenly, and set aside.
[0054]After dissolving racemic anisodamine with 2mol / L dilute hydrochloric acid, add it to the above-mentioned mixed solution and stir evenly.
[0055] Adjust the pH to 4.0 with hydrochloric acid.
[0056] Add water for injection to the full amount, stir evenly, filter and fill.
Embodiment 2
[0058] 100ml water for injection:
[0059] Racemic anisodamine 0.06g
[0060] Sodium hyaluronate 0.045g
[0061] Boric acid 1.5g
[0062] Borax 0.3g
[0063] Methyl paraben 0.03g
[0064] Process:
[0065] 1. Add sodium hyaluronate to the 1 / 4 (v / v) water for injection, and swell at room temperature.
[0066] 2. Dissolve boric acid, borax, and benzalkonium chloride in 1 / 2 (v / v) water for injection, then add the fully swollen sodium hyaluronate solution, stir evenly, and set aside.
[0067] 3. Dissolve racemic anisodamine with 2mol / L dilute hydrochloric acid and add it to the above-mentioned mixed solution and stir evenly.
[0068] 4. Add water for injection to the full amount, stir evenly, filter and fill. The pH is 4.5.
Embodiment 3
[0070] 100ml water for injection:
[0071] Racemic anisodamine 0.10g
[0072] Sodium hyaluronate 0.039g
[0073] Sodium chloride 0.9g
[0074] Ethyl paraben 0.01g
[0075] Process:
[0076] 1. Add sodium hyaluronate to the 1 / 4 (v / v) water for injection, and swell at room temperature.
[0077] 2. Dissolve sodium chloride and benzalkonium chloride in 1 / 2 (v / v) water for injection, then add to the fully swollen sodium hyaluronate solution, stir evenly, and set aside.
[0078] 3. Dissolve racemic anisodamine with 2mol / L dilute hydrochloric acid and add it to the above-mentioned mixed solution and stir evenly.
[0079] 4. Adjust the pH to 5.8 with hydrochloric acid.
[0080] 5. Add water for injection to the full amount, stir evenly, filter and fill.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com