Separation method of anisodamine and enantiomers thereof
A kind of technology of anisodamine and enantiomer, applied in the field of separation of anisodamine and its enantiomer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Selection of Cyclodextrin Derivatives
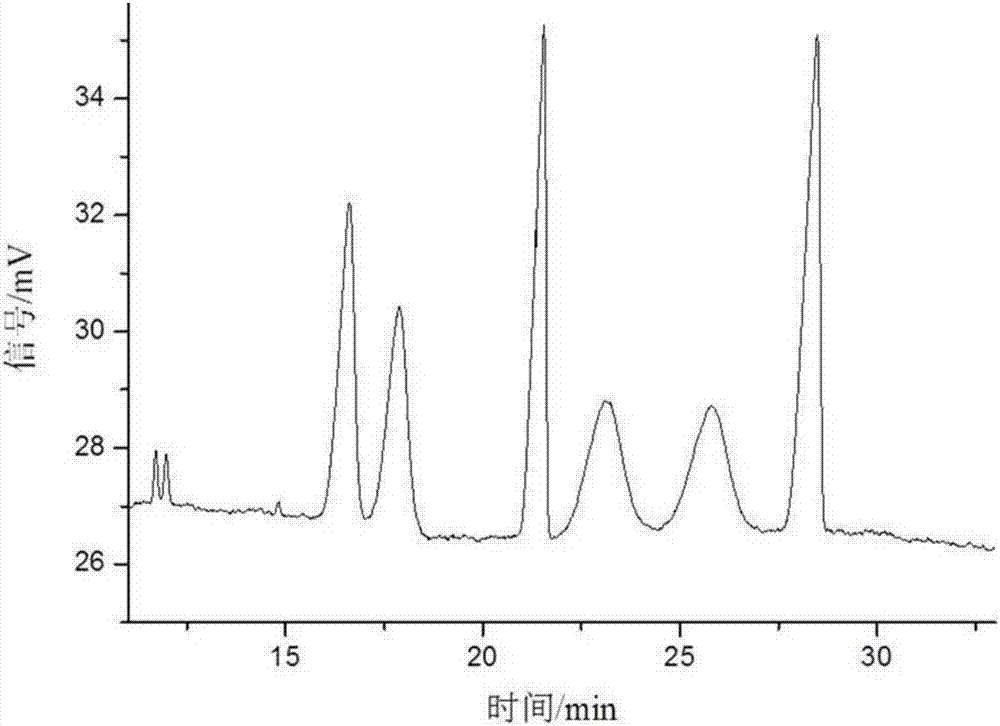
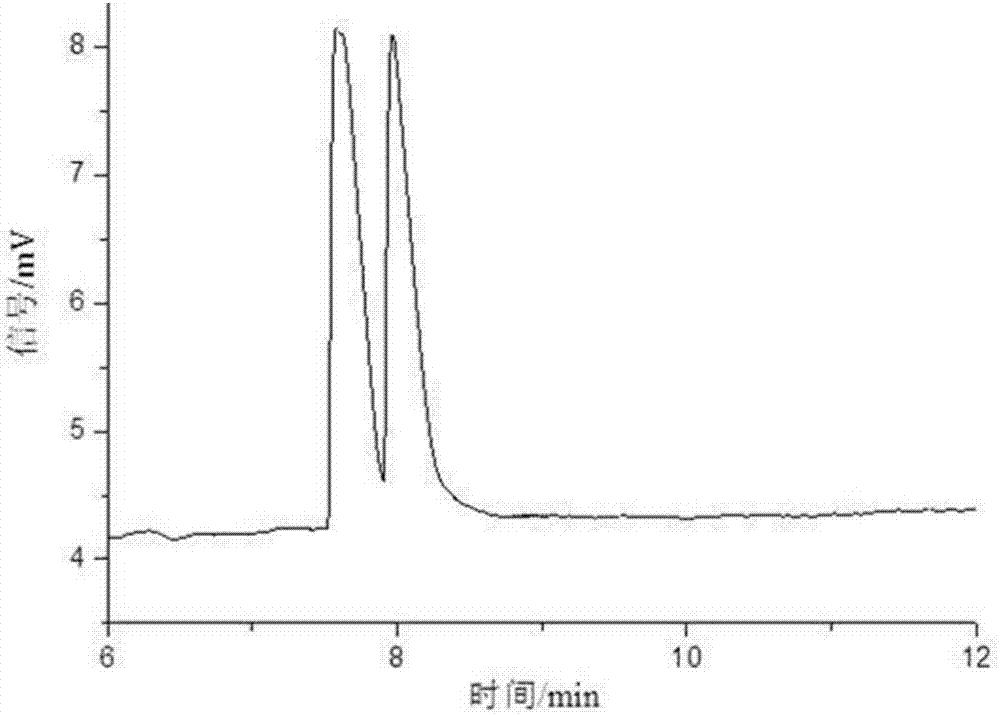
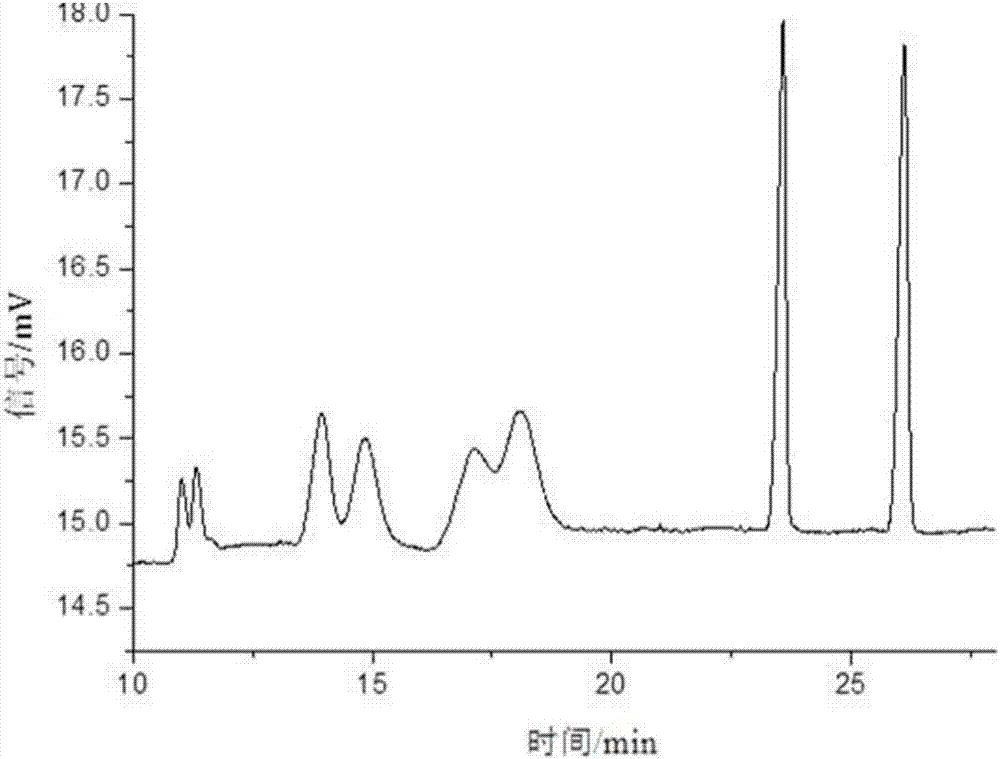
[0061] Take sulfonated-β-dextrin polymer, HP-β-CD, Me-β-CD, SE-β-CD, mixture of SE-β-CD and Me-β-CD, SE-β-CD and The mixture of HP-β-CD and the mixture of SE-β-CD and sulfonated-β-dextrin polymer were used as resolving agents to resolve anisodamine. The concentration of the resolving agent in the electrophoresis buffer is 10mg / mL, the pH of the electrophoresis buffer is 3, the temperature is room temperature, the voltage is -15kV, and the gravity injection is 5s. The result is as Figure 1-7 shown. Among them, the use of sulfonated-β-dextrin polymer, HP-β-CD, Me-β-CD alone, and the combination of SE-β-CD and Me-β-CD did not resolve it well Effect; SE-β-CD can split it and achieve baseline separation. When using SE-β-CD as a chiral resolving agent, adding HP-β-CD or sulfonated-β-dextrin polymer, although the resolution has no higher improvement, it can be mixed with SE-β-CD . Therefore, the electrophoresis buffer contains SE-...
Embodiment 2
[0063] Effect of buffer pH value on the separation degree of anisodamine
[0064] The influence of the chiral separation when the pH value changed from 2.0 to 9.0 was investigated. The concentration of SE-β-CD in the electrophoresis buffer is 10mg / mL, the temperature is room temperature, and the voltage is -15kV. The result is as Figure 8-10 shown. In the experiment, it was found that when the pH was 2.0-5.0, due to the small electroosmotic flow, the sample could not be driven to migrate to the negative electrode, so the sample peak could not be detected under the positive voltage. Using reverse voltage in the pH range of 2.0-5.0, relying on the electrophoresis of anisodamine and cyclodextrin inclusion complex, anisodamine is brought from the negative electrode to the positive electrode, and the enantiomers are separated in different degrees. At pH=2.5, the 4 pairs of enantiomers of anisodamine can be separated. At pH=3, the 4 pairs of enantiomers of anisodamine were comp...
Embodiment 3
[0066] Effect of SE-β-CD Concentration on the Resolution of Anisodamine
[0067] In the experiment, the change of the separation degree of anisodamine was investigated when the temperature was room temperature, the pH was 3.0, the operating voltage was -15kV, and the concentration of SE-β-CD was 2.5-15mg / mL. The result is as Figure 11 shown. Anisodamine can achieve different degrees of separation within the selected range. When the concentration of SE-β-CD is 2.5mg / mL, there are 3 pairs of enantiomers separated; when the concentration of SE-β-CD is 5-7.5mg / mL , 4 pairs of enantiomers were separated, but the chromatographic peaks of the third and fourth pairs of enantiomers overlapped; when the concentration of SE-β-CD was 10 mg / mL, the resolution of anisodamine was the best , 4 pairs of enantiomers were completely resolved; when the concentration of SE-β-CD was 11.5 mg / mL, the 4 pairs of enantiomers of anisodamine could be separated, but the last two peaks could not be comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com