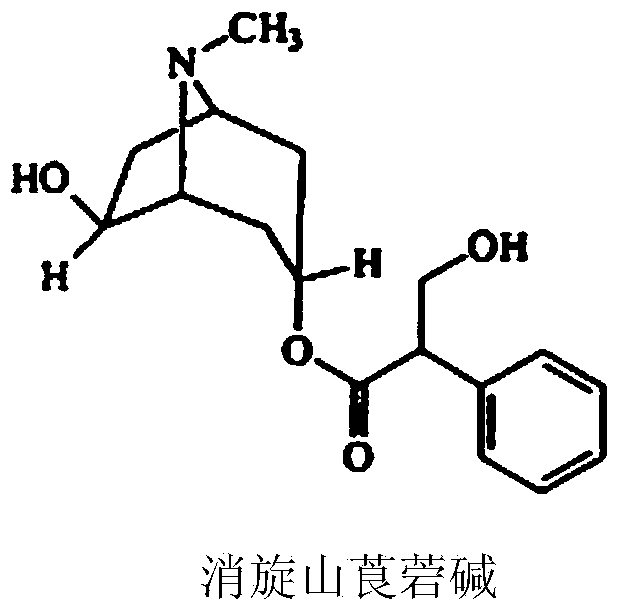
Raceanisodamine hydrochloride injection composition
An anisodamine and composition technology, which is applied in the field of racemic anisodamine hydrochloride injection composition, can solve the problems of easy precipitation of small white spots, yellowish solution color, inability to determine the prescription and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
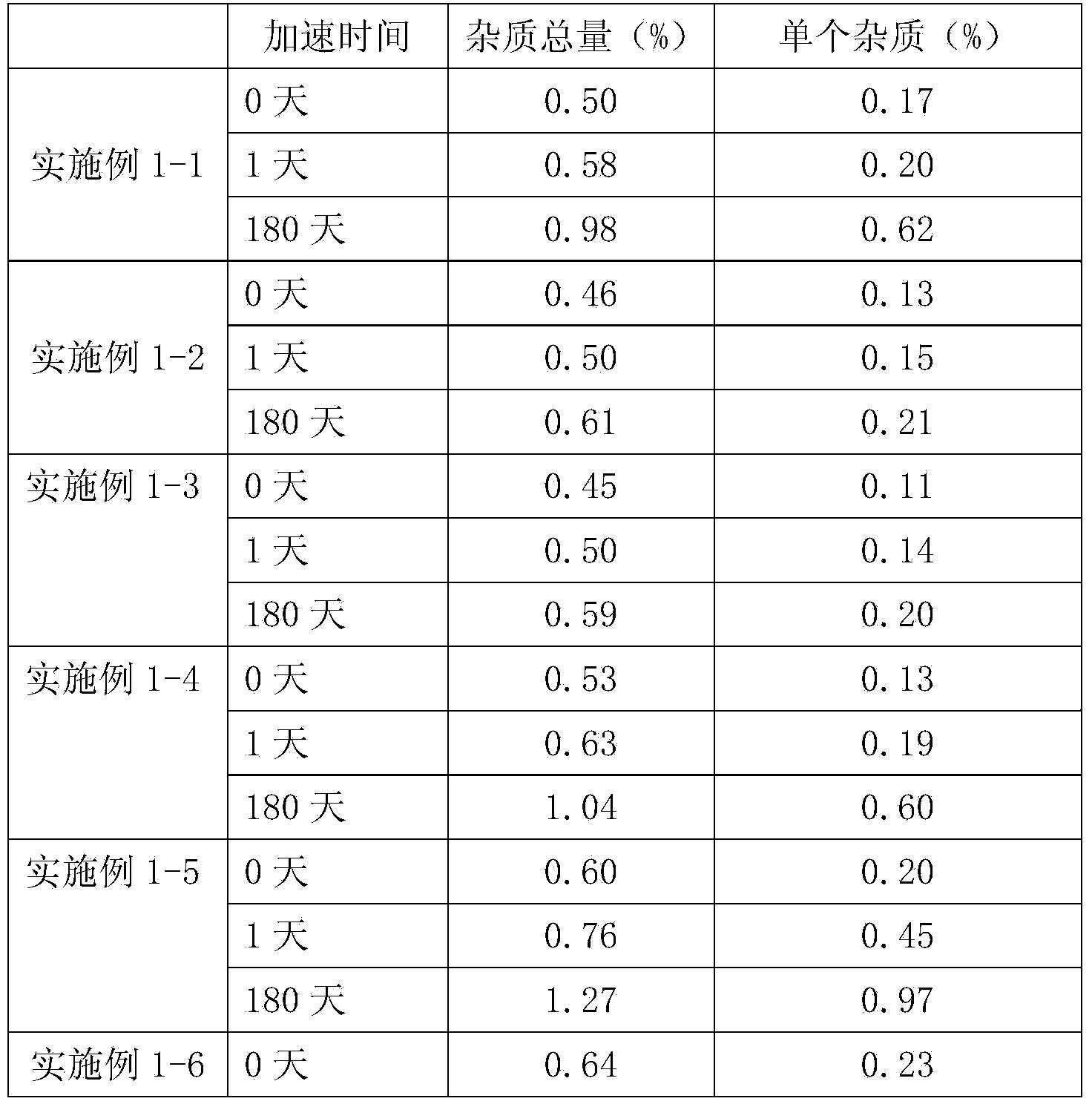
Embodiment 1
[0026] Example number
[0028] Mix racemic anisodamine, part of hydrochloric acid, sodium chloride and water evenly, adjust the pH value with the rest of hydrochloric acid and water for injection, and set the volume to 1000ml, then filter through a terminal of 0.22μm and pot it. Sterilize by steam at 100°C for 30 minutes. Nitrogen protection can be used during preparation and potting.
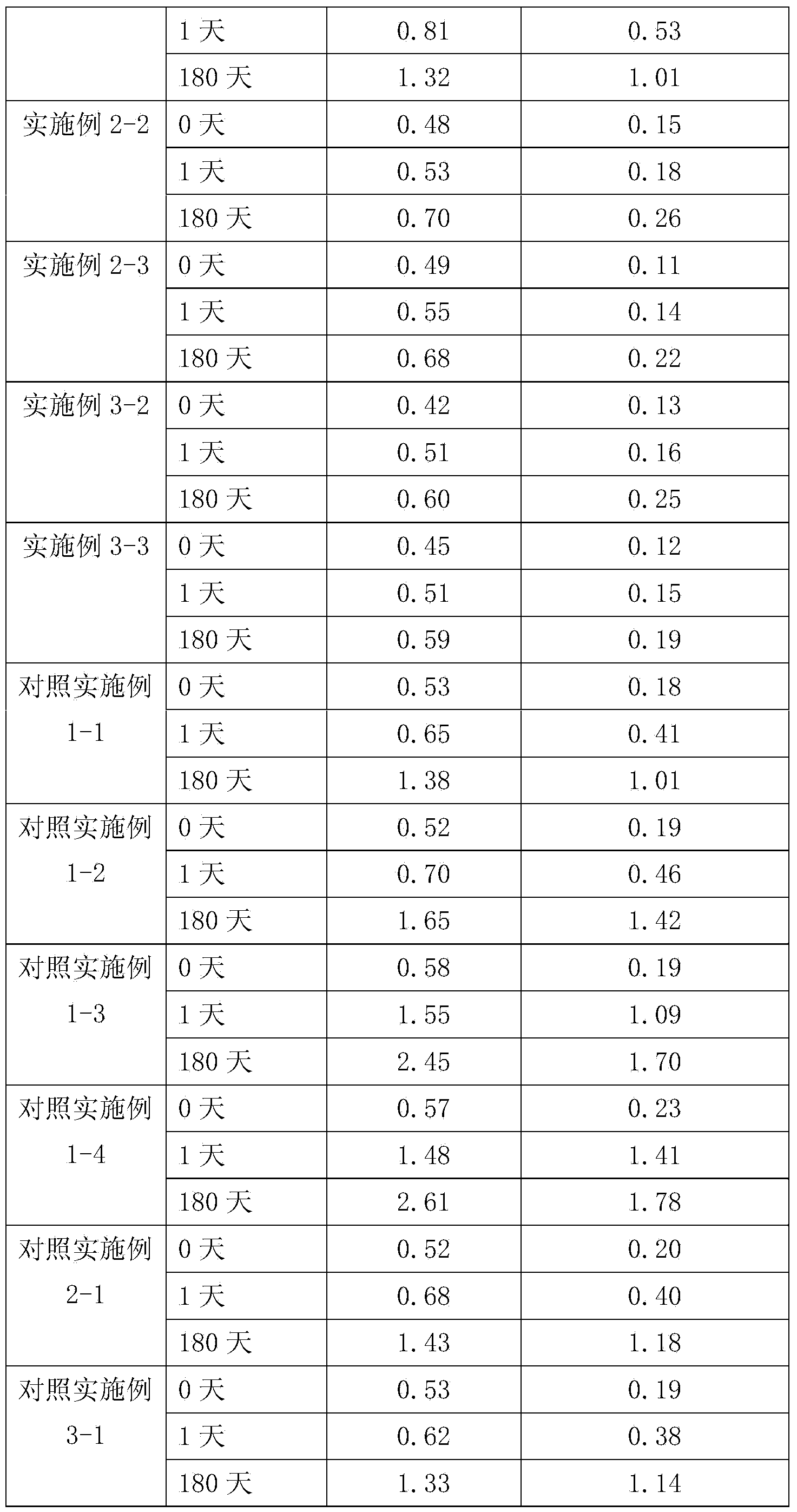
Embodiment 2
[0030] Example number
[0031] The preparation method is the same as in Example 1.
Embodiment 3
[0033] Example number
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com