Anisodamine freeze drying preparations for injections and preparation method
A technology of freeze-dried preparations and anisodamine, which can be used in freeze-dried transportation, anti-toxic agents, anti-inflammatory agents, etc., and can solve problems such as poor stability, inconvenient transportation and storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Screening of anisodamine freeze-dried preparation formulation process for injection
[0025] 1. Solubility of anisodamine:
[0026] Anisodamine is white crystal or crystalline powder, odorless and bitter. This product is soluble in ethanol or hydrochloric acid and dissolved in water.
[0027] 2. Determination of pH range
[0028] According to the quality standard of racemic anisodamine hydrochloride injection, the pH value range of the drug solution before freeze-drying of the product of this research is determined to be 4.0-6.0.
[0029] 3. Dissolution and investigation of the volume of the drug solution before freeze-drying
[0030] Weigh 89 mg of anisodamine, add water for injection to 10 ml, stir to dissolve, adjust the pH value to 4.87 with 1 mol / L hydrochloric acid, and the liquid medicine is colorless and clear. According to the dissolving situation of medicine, in order to make sub-dosing accurate, so determine that the filling volume is 2ml.
[0...
Embodiment 2
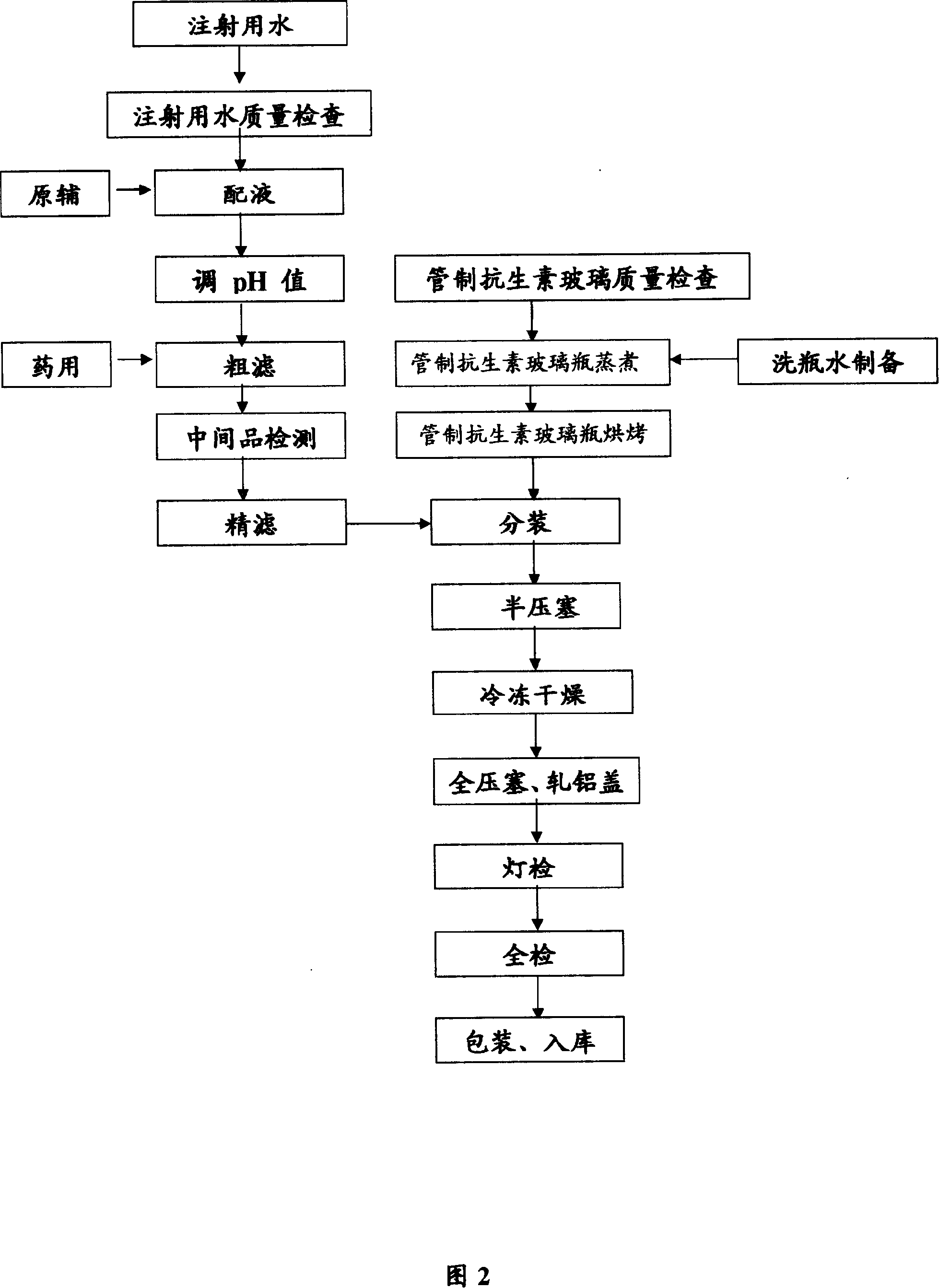
[0064] Embodiment 2 preparation technology of anisodamine freeze-dried preparation for injection
[0065] 1. Determination of the lowest eutectic point
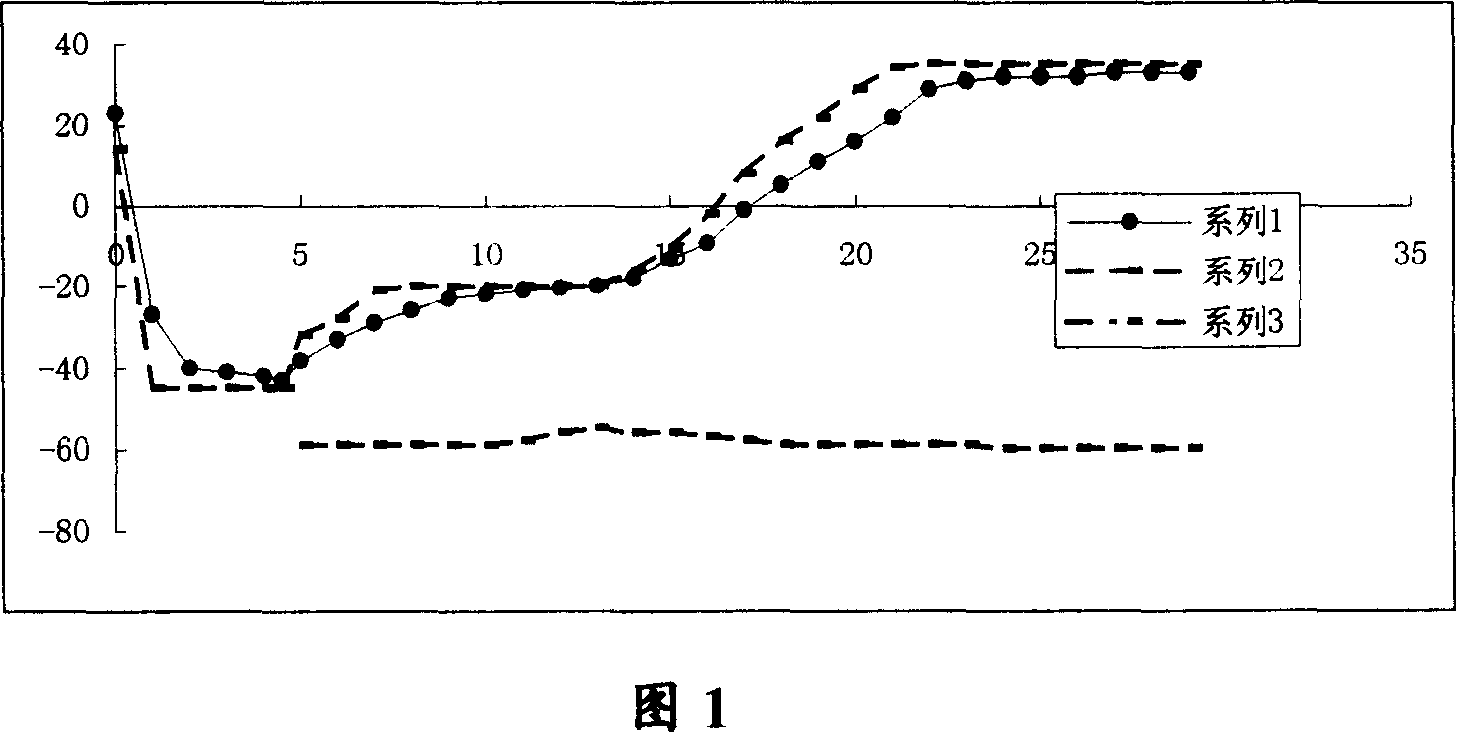
[0066] Prepare the liquid medicine according to the preliminarily determined formula, and measure its minimum eutectic point temperature by resistance method. Results Specification I was -19.0°C to -18.3°C; Specification II was -18.0°C to -17.5°C.
[0067] 2. Determination of freeze-drying process
[0068] According to the minimum eutectic point and the volume of the liquid medicine, we investigated the freeze-drying process, and determined the freeze-drying process conditions of the two specifications as shown in Table 9 according to the investigation results:
[0069] Table 9 freeze-drying process conditions
[0070] Freeze-drying conditions
freeze drying time
Pre-freezing period: start the freeze dryer, set the shelf temperature to -45
℃, after the shelf temperature drops to about -45℃, respect...
Embodiment 3
[0097] Example 3 Stability Test of Anisodamine Freeze-dried Preparation for Injection
[0098] The anisodamine freeze-dried preparation for injection was subjected to the influence factor test under the conditions of light (4500Lx), high temperature (60°C) and low temperature (4°C) for 10 days, and no significant change was seen in each investigation item; accelerated test (40°C) for 6 months , the properties of the sample, the clarity and color of the solution, clarity, acidity, related substances, and content have no obvious changes; , related substances, and content have no significant changes, and the results show that the quality is stable.
[0099] 1. Influencing factor test
[0100] Take a certain number of samples (batch number: 040301; 040307), remove the outer packaging, place them under the conditions of light (4500Lx), high temperature (60°C) and low temperature (4°C), take samples on 5 days and 10 days, and follow the quality standards ( Draft) method to measure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com