Mannitol injection
A technology of mannitol and injection, applied in the field of mannitol injection, can solve the problems of large nephrotoxicity, inconvenience, crystallization and the like, and achieve the effects of improving the quality, stability and safety of the injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
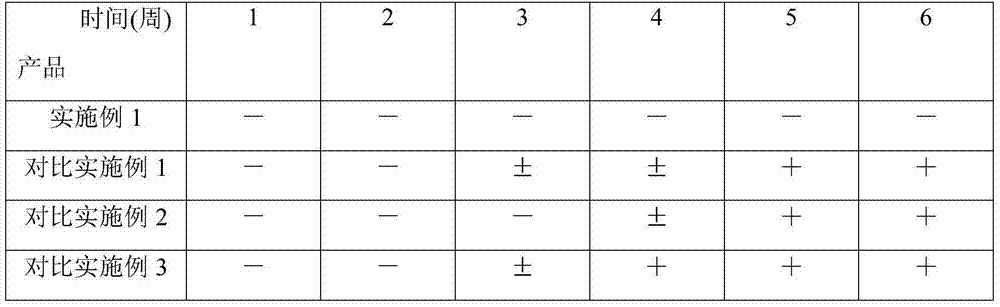
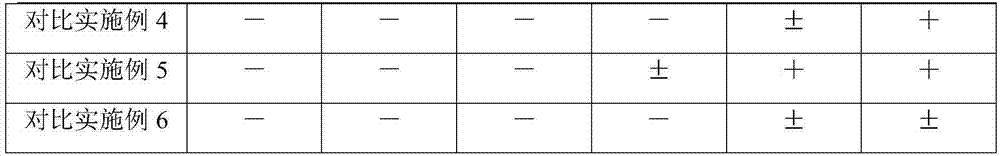
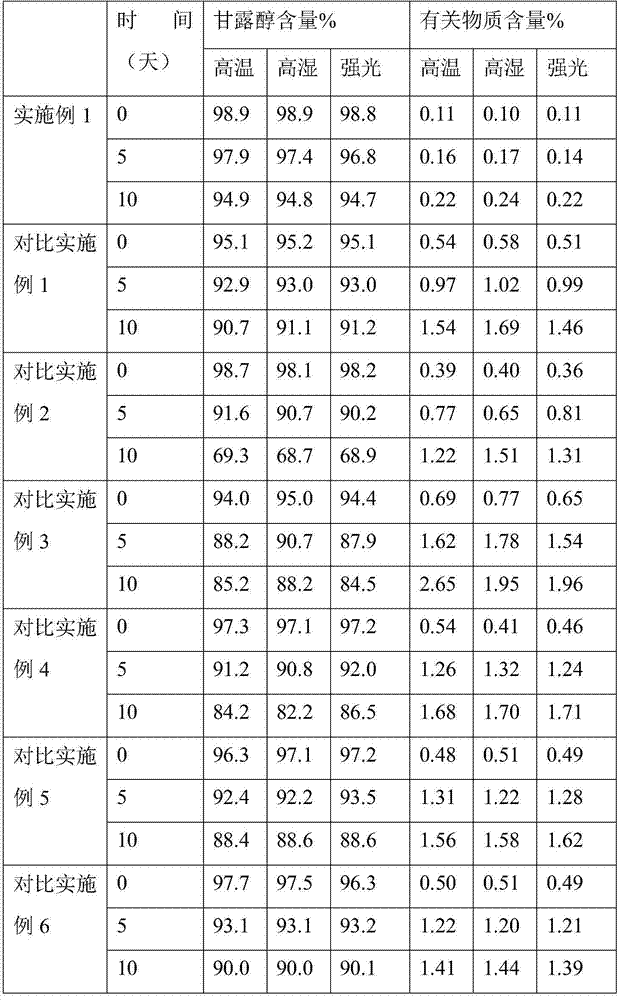
Examples
Embodiment 1
[0026] Mannitol injection has the following components (by weight):
[0027] Mannitol 20%
[0028] Glycine 6%
[0029] Lecithin 2%
[0030] The balance is water for injection.
[0031] Preparation:
[0032] (1) Mix mannitol, glycine and lecithin with 1 / 3 weight of water for injection in proportion, add activated carbon, absorb, filter and decarbonize, and obtain a concentrated solution.
[0033] (2) Mix the above-mentioned concentrated solution with the remaining amount of water for injection, then add activated carbon for secondary adsorption, filter and decarbonize, and obtain a diluted solution.
[0034] (3) Filter the diluted solution in step (2) with a 0.22 μm filter, and fill it.
[0035] (4) Sterilize the injection obtained in step (3) at a temperature of 120-130° C. to obtain the mannitol injection of the present invention.
[0036] The amount of activated carbon added in the above preparation method is 0.02% (w / v), and the carbon removal is filtered with a 0.45 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com