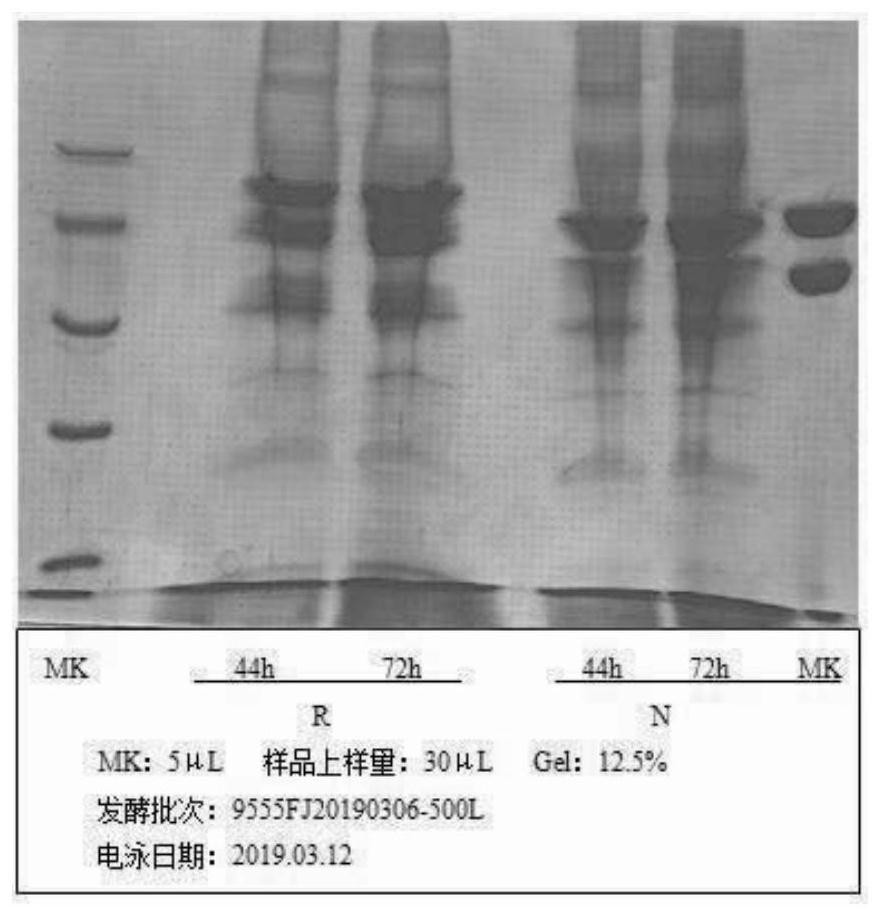
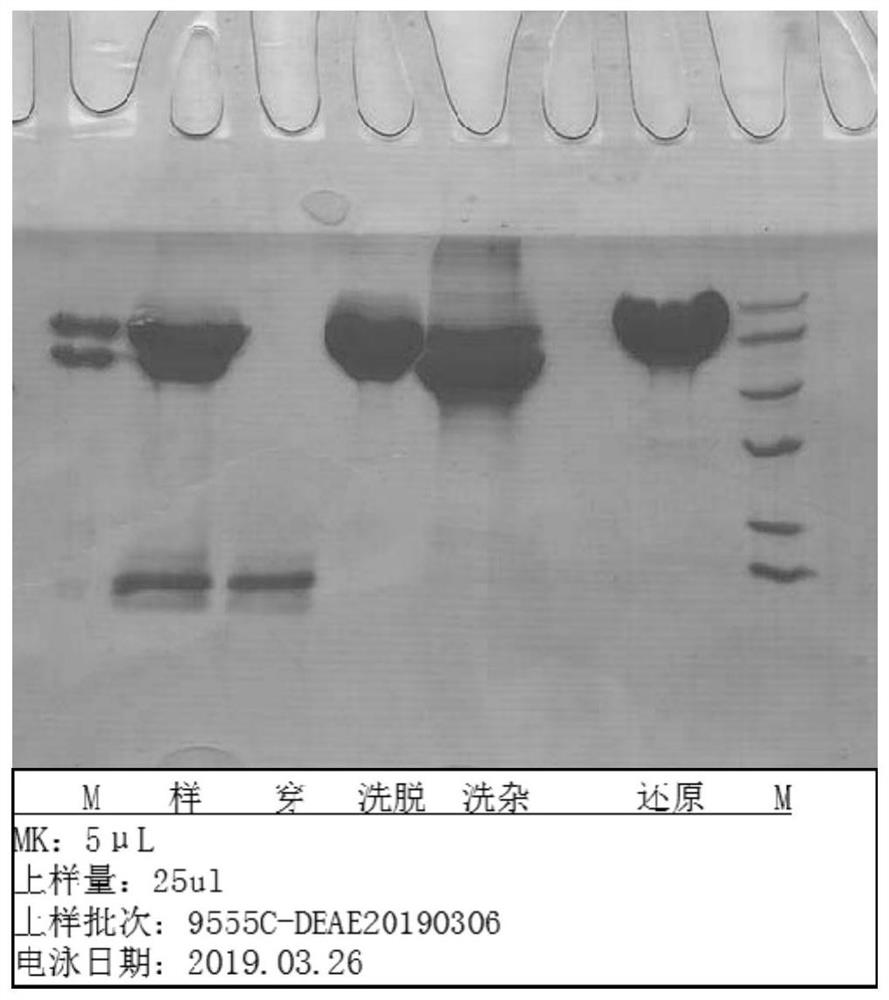
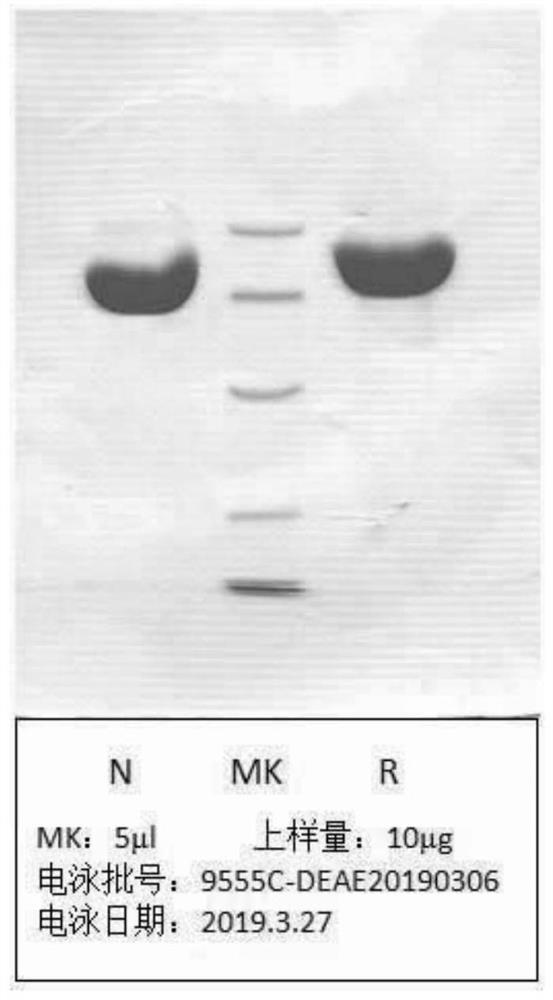
Fusion protein of human serum albumin and interleukin 2 and application thereof
A technology of human serum albumin and human interleukin, which is applied in the field of fusion proteins and can solve problems such as side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Embodiment 1: Human serum albumin (HSA) gene expression and the construction of carrier plasmid
[0108]Using the total RNA extracted from human fetal liver as a template, the HSA gene was synthesized and amplified by reverse transcription polymerase chain polymerization. 5 micrograms of total RNA were synthesized into corresponding DNA strands under the action of reverse transcriptase (purchased from GIBCO / BRL). The reaction condition was 45°C for 20 minutes, then the temperature was raised to 55°C, and the temperature was maintained for 40 minutes.
[0109] The DNA sequences of the oligonucleotide primers used are: SEQ ID No.11 and SEQ ID No.12.
[0110] Two EcoRI recognition sites were added to the two ends of the HSA gene and cloned into an expression vector. The PCR reaction conditions were 94° C. for 4 minutes, and the DNA encoding HSA was further amplified for 35 cycles: 94° C. for 30 seconds; 58° C. for 30 seconds; 72° C. for 2 minutes and 30 seconds. At the ...
Embodiment 2
[0112] Example 2: Expression of recombinant human serum albumin / interleukin-2 fusion protein and its analog fusion protein and construction of vector plasmid
[0113] According to the GeneBank registration number: NM_000586, NP_000577.2, the amino acid sequence SEQ ID No.10 of human interleukin-2 (wild type) mature peptide was obtained, the inventor designed the molecular structure of recombinant human serum albumin fusion protein, and interleukin-2 mature peptide In a "seamless connection" manner, no connecting peptides are placed between the C-terminals of human serum albumin mature peptides, SEQ ID No.1, and the corresponding nucleotide sequence is SEQ ID No.2, or interleukin- 2 The mature peptides are connected in a "seamless connection" mode, and no connecting peptides are placed between the N-terminals of the mature peptides of human serum albumin, SEQ ID No.3, and the corresponding nucleotide sequence is SEQ ID No.4.
[0114] According to the known computer analysis and...
Embodiment 3
[0117] Embodiment 3: Transformation and preparation of Pichia pastoris expression engineering bacteria
[0118] Pichia pastoris strain X33 colony was inoculated in a 50ml centrifuge tube containing 5ml YPD culture medium, and cultured overnight at 30°C at a speed of 250 rpm. The next day, take 0.2ml of the overnight culture and transfer it into 500ml of YPD culture solution, and place it in a 2-liter Erlenmeyer flask. Incubate with rotation at 30°C for 2-3 hours to bring the cell density to OD 600 =1.3-1.5. The yeasts were collected by centrifugation, resuspended in 500ml ice-cold sterile water and washed twice. Then the yeast was suspended in 20ml of ice-cold 1M sorbitol solution and washed once.
[0119] Taking the pYZ-rhIL-2v5 / SA plasmid DNA constructed in Example 2 as an example, the plasmid DNA is treated with Pme I restriction endonuclease to form a linear plasmid molecule. 5 μg of linearized plasmid DNA was mixed with 80 μl of treated yeast and placed in a 0.2 cm th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com