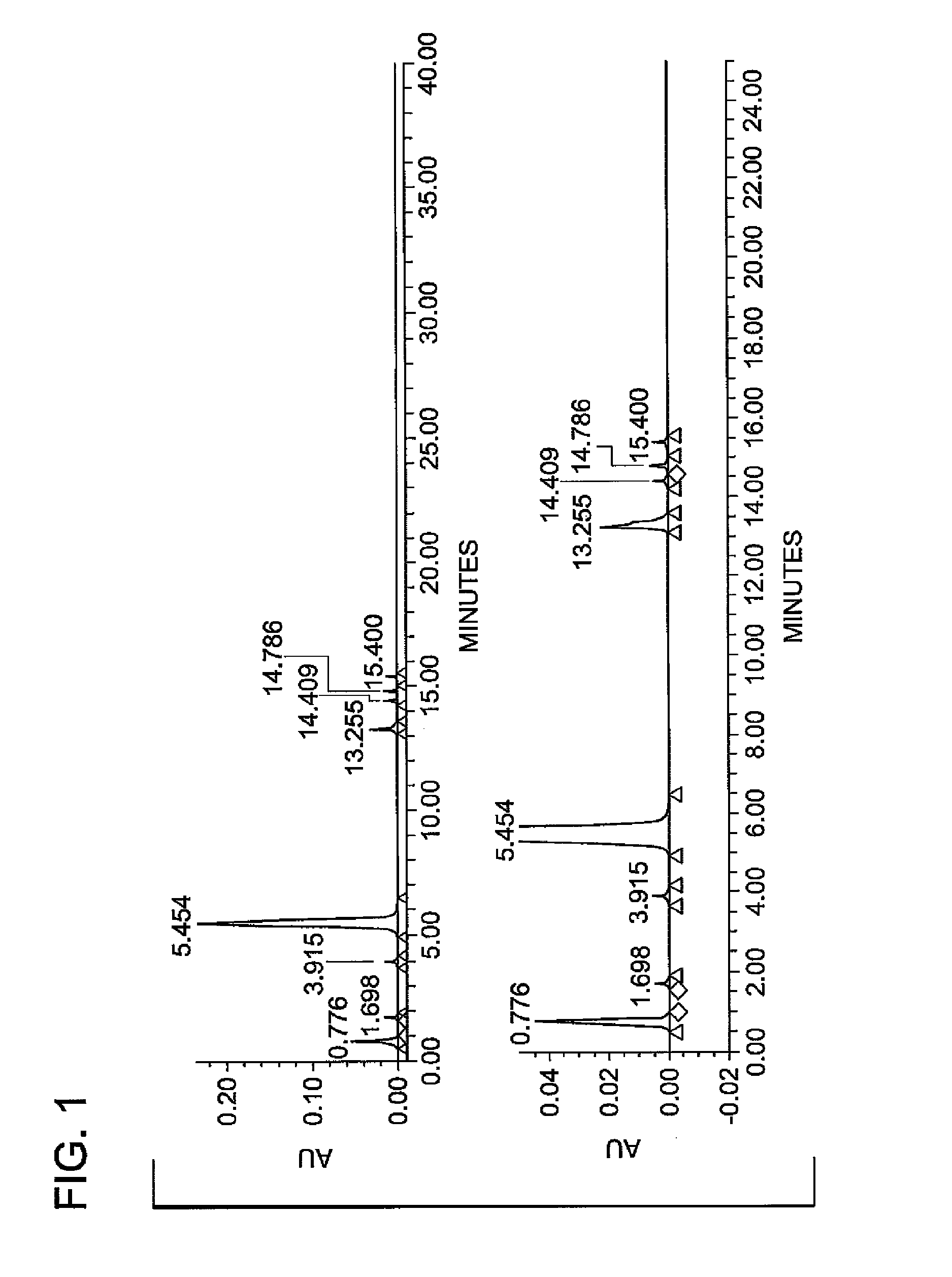
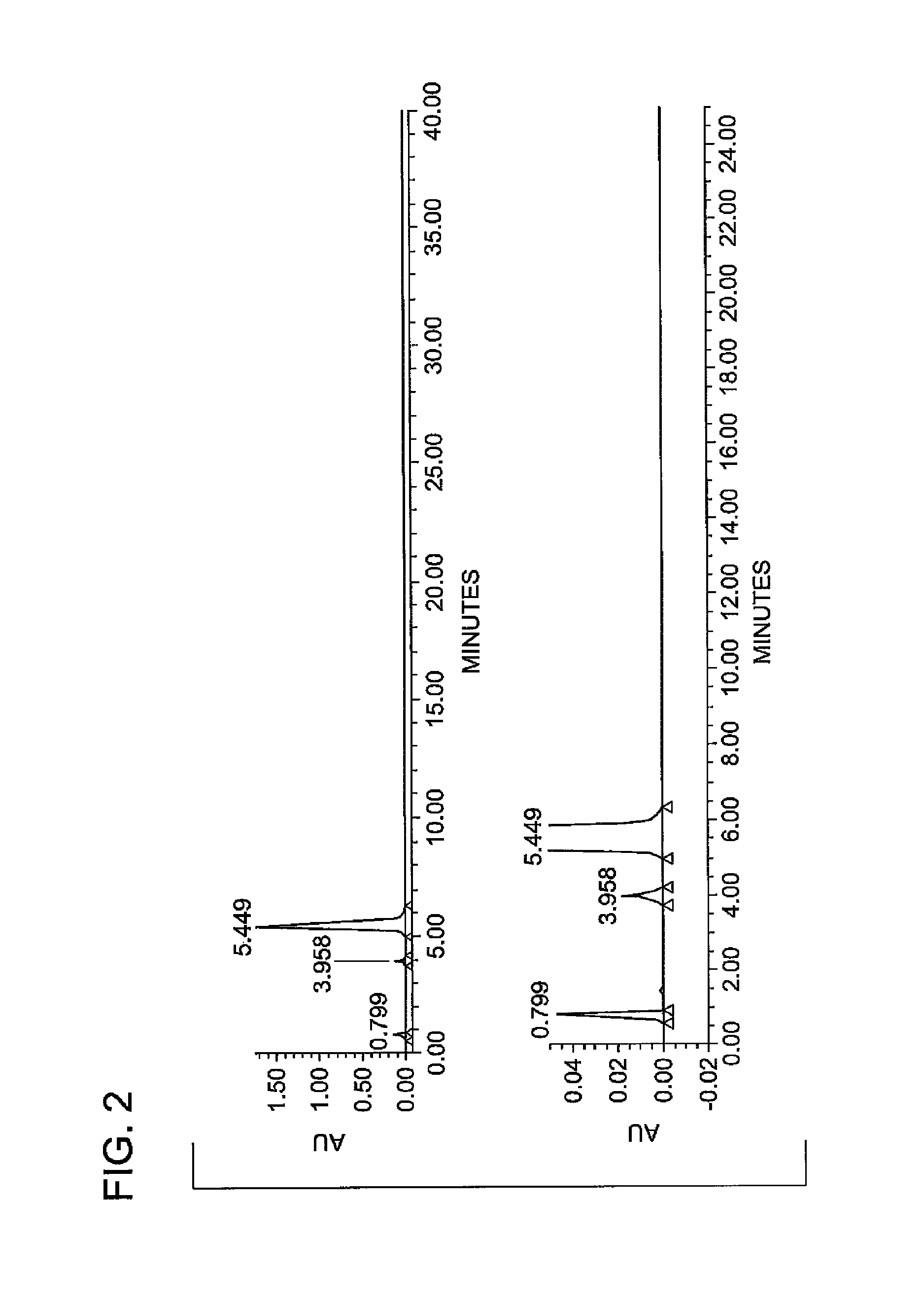
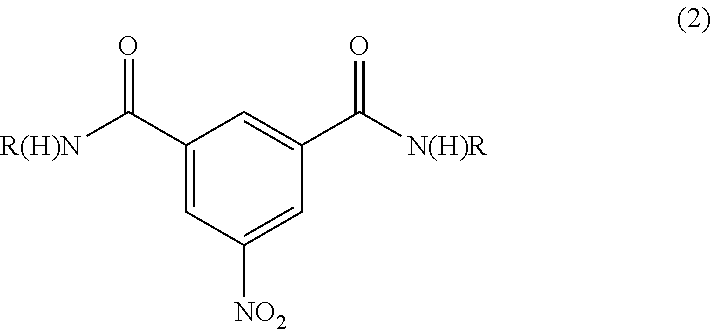
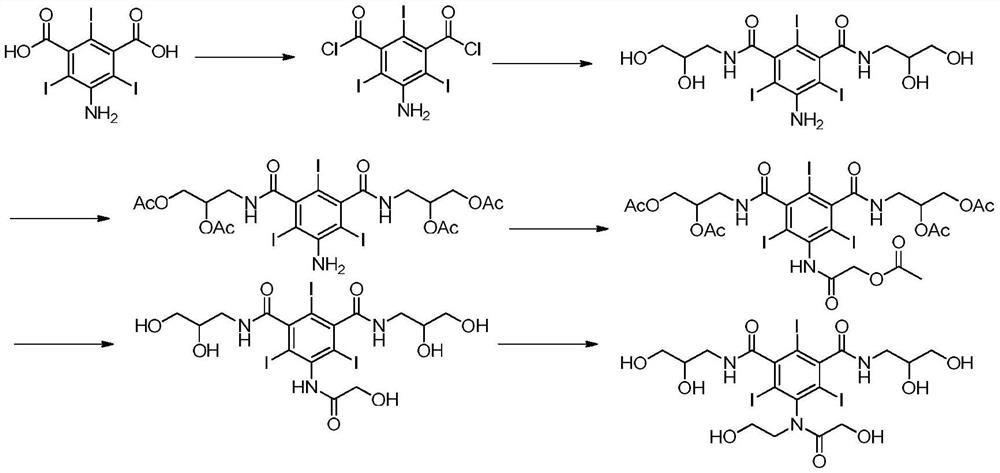
Patents
Literature
37 results about "Ioversol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
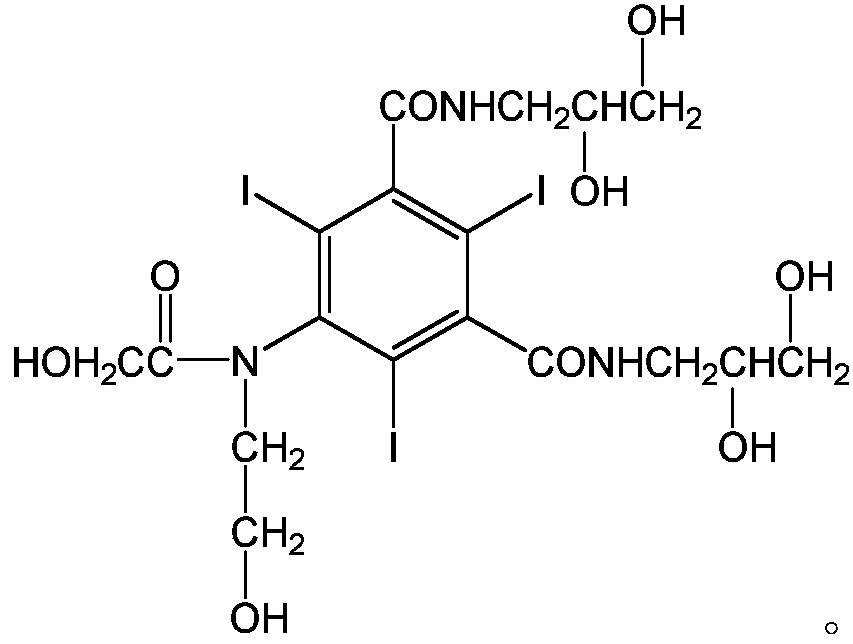
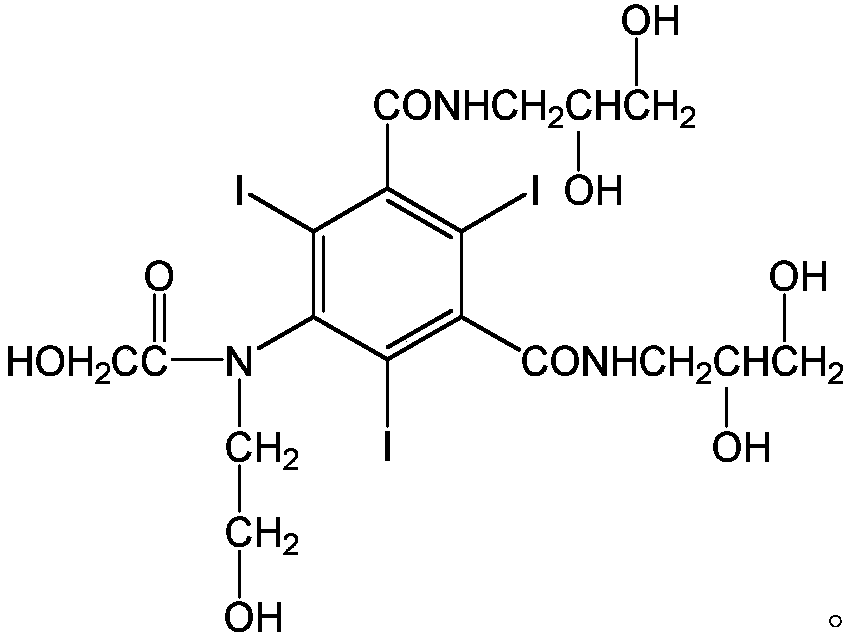
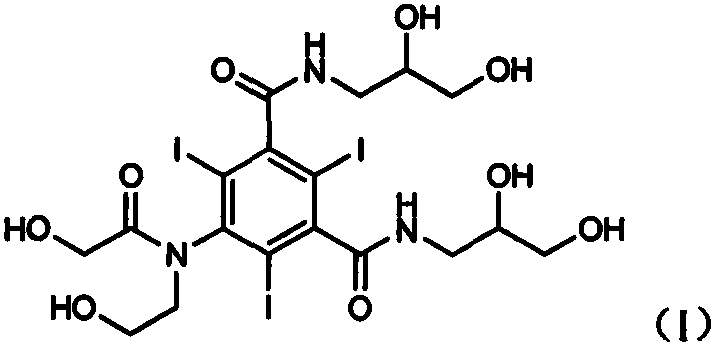
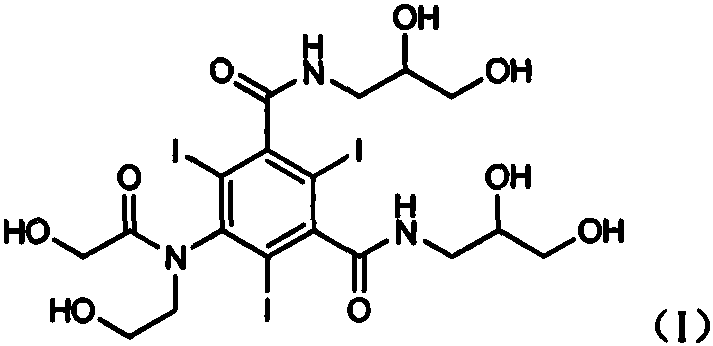
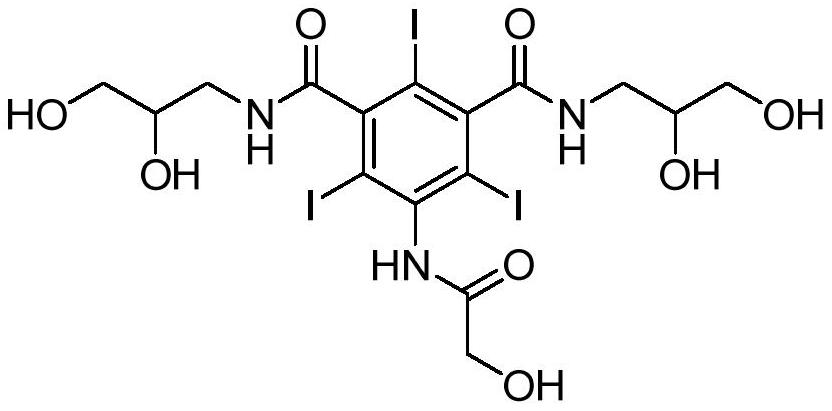
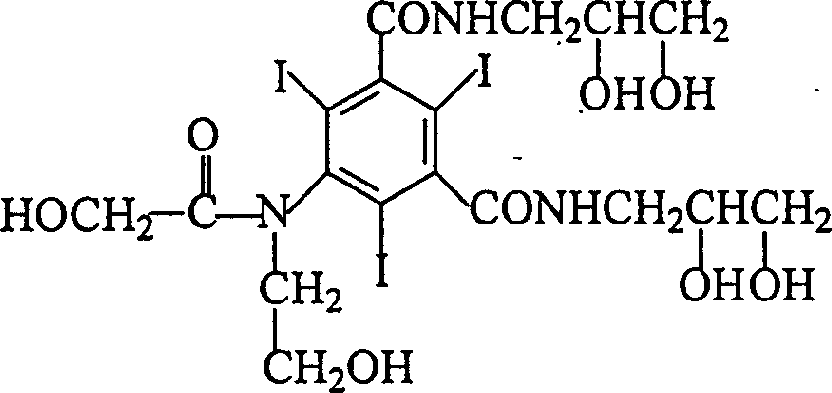
Ioversol (INN) is an organoiodine compound that used as a contrast medium. It features both a high iodine content, as well as several hydrophilic groups.
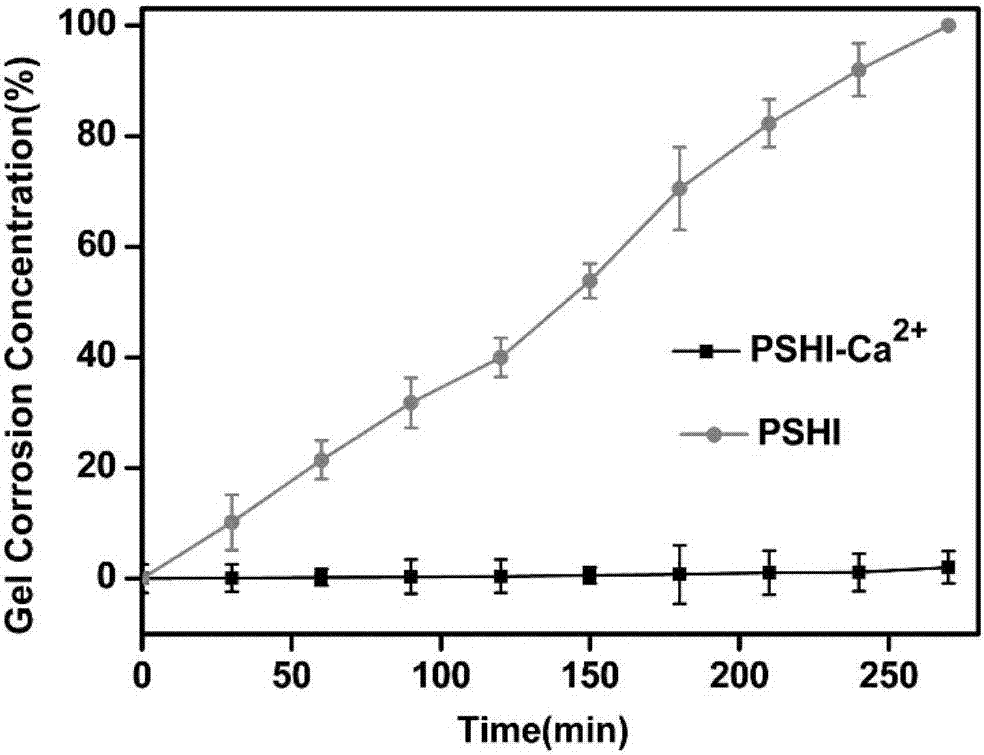
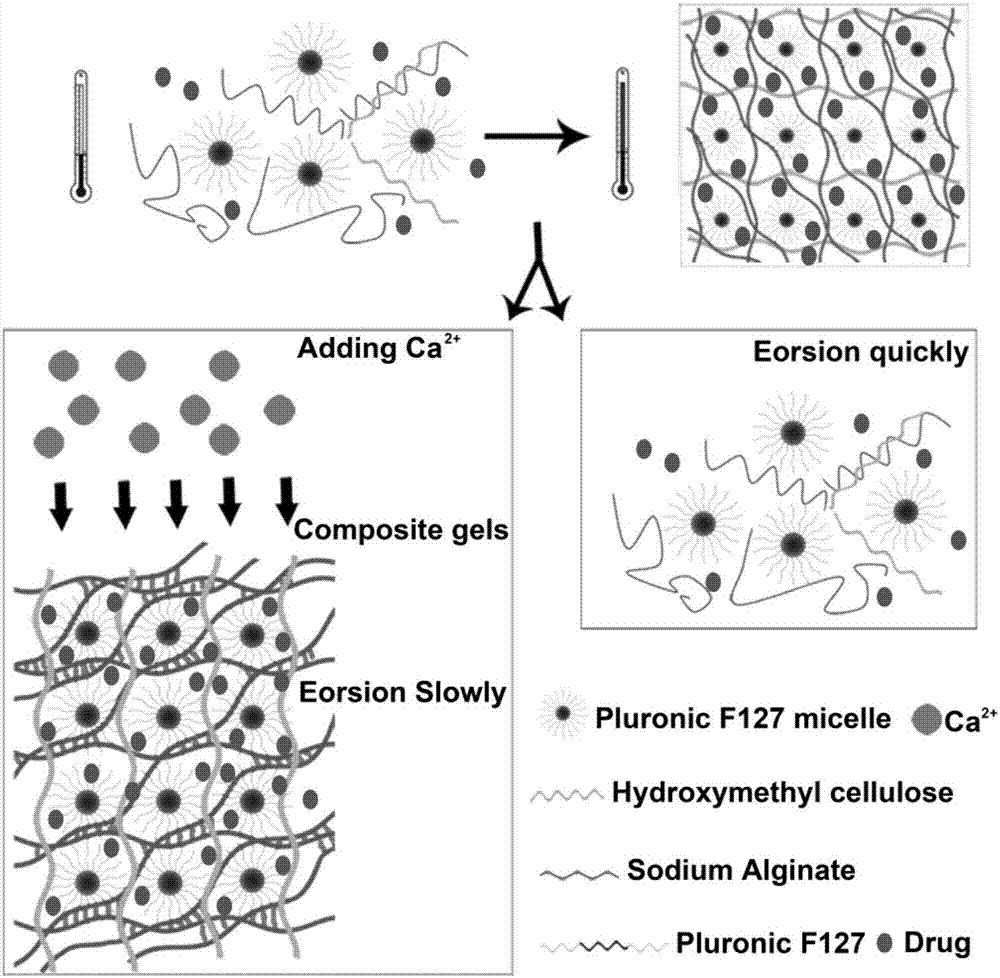
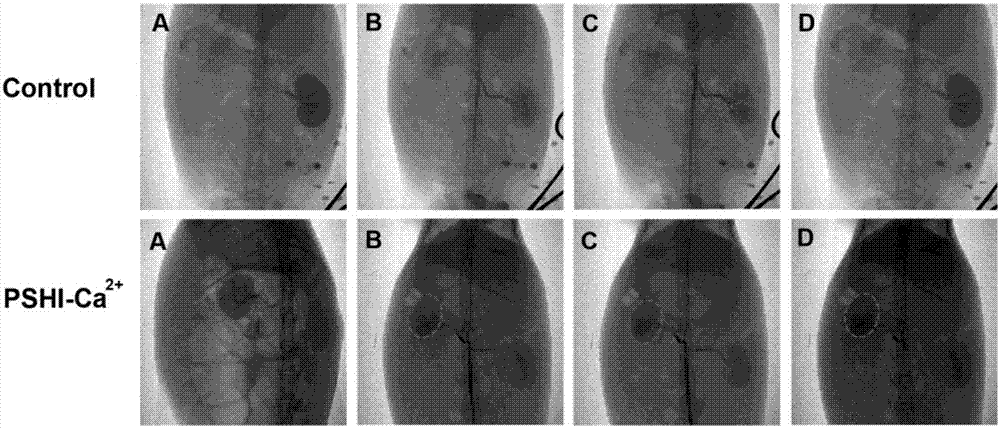
Composite developing thermosensitive gel embolizing agent as well as preparation method and application thereof
ActiveCN107281502AIncrease success rateReduce chanceOrganic active ingredientsHeavy metal active ingredientsCarboplatinMiriplatin
The invention relates to a composite developing thermosensitive gel embolizing agent as well as a preparation method and application thereof. The preparation method comprises the following steps: firstly, preparing a mixed aqueous solution of an anticancer active substance, a thermosensitive material and a developing agent into composite developing thermosensitive gel; secondly, forming a composite developing thermosensitive gel embolizing agent by the composite developing thermosensitive gel and a coagulant on the scene, wherein the thermosensitive material is hydroxyl C1-4 alkyl cellulose, Pluronic, alginate or a mixture of the substances; the anticancer active substance is arsenic trioxide, docetaxel, cisplatin, carboplatin, nedaplatin, oxaliplatin, lobaplatin, miriplatin, siRNA or a mixture of the substances; the developing agent is a water-soluble developing agent of iodixanol, ioversol or iohexol and the like. The preparation method disclosed by the invention is simple and convenient, is suitable for industrial large-scale production, is particularly suitable for preparing an embolizing agent which is biodegradable and good in biocompatibility and is used for hemorrhagic diseases, and is especially suitable for preparing the composite developing thermosensitive gel embolizing agent for treating liver cancer, kidney cancer, lung cancer, prostate cancer, uterine myoma or splenic tumor and the like.
Owner:苏州申润医疗科技有限公司 +1
Multifunctional gold and silver core-shell nanoparticles and preparation method
InactiveCN102908633AUniform sizeUniform particle sizeAntibacterial agentsNMR/MRI constrast preparationsPolyethylene glycolWater chlorination
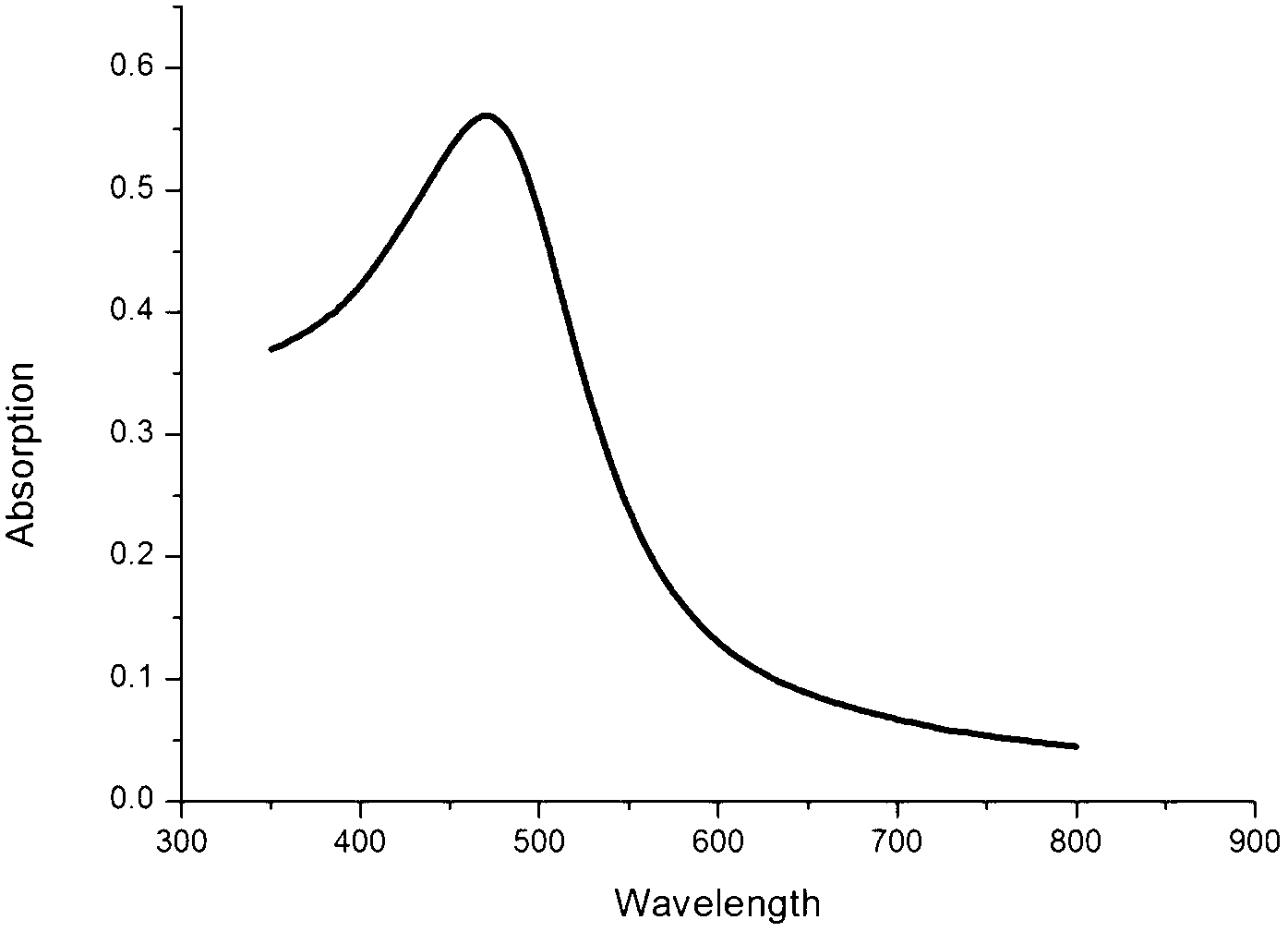
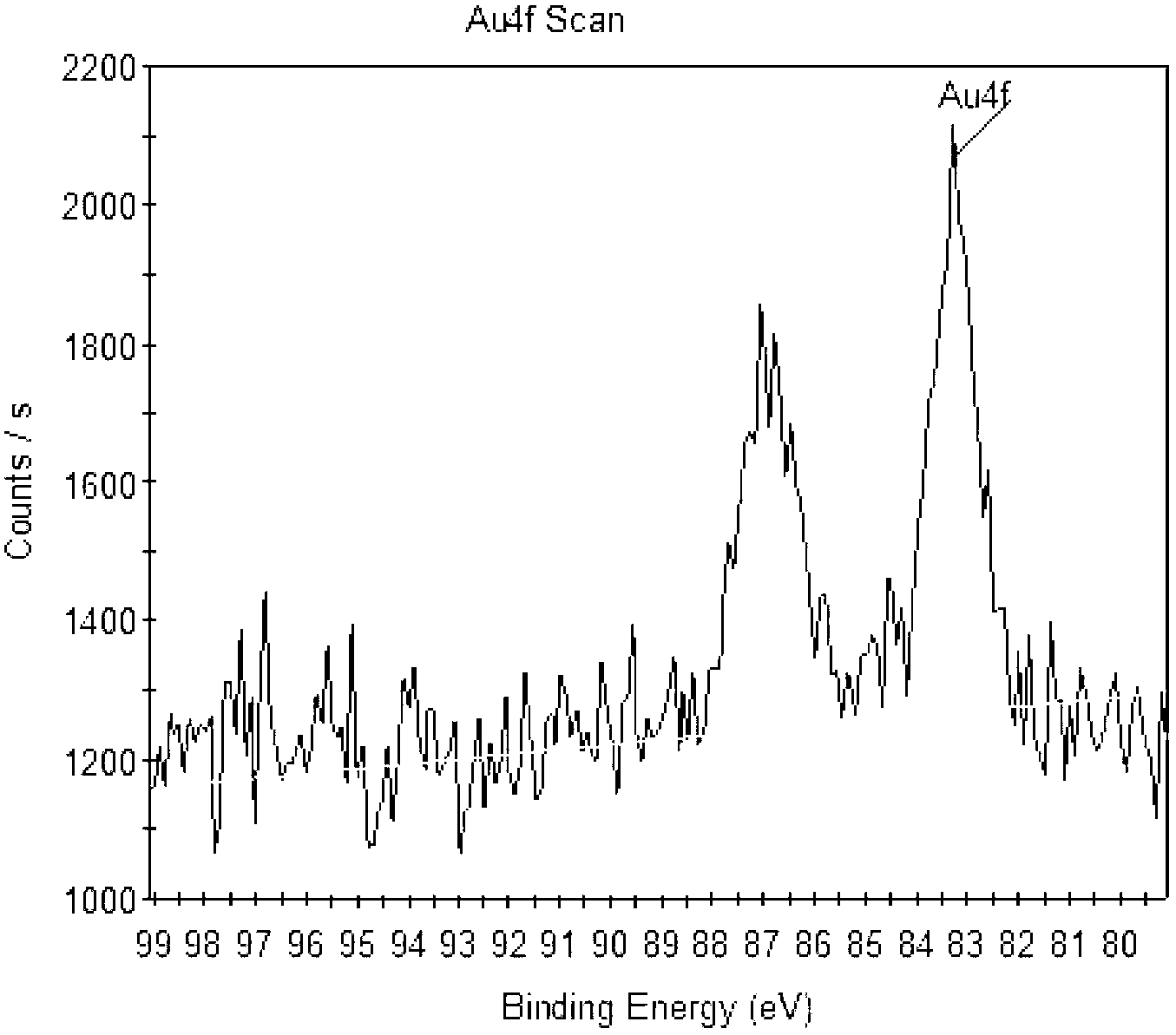
The invention relates to multifunctional gold and silver core-shell nanoparticles. The gold nanopartciles are covered by silver nano shell layers and the nanoparticles with silver core shell structures are conducted for surface modification through sulfydryl polyethylene glycol molecules; and gold nano core particles are 3 to 20 nm in size, and silver nano shells are 2 to 12 nm in size. Especially, the gold and silver core-shell nanoparticles covered by the silver nano core and shells are prepared by reducing chloroauric acid to prepare ultrafine fold nanopartciles through tetrakisphosphonium chloride activated by alkaline liquor as a reducing agent, and reducing a silver nitrate solution by nanoparticles as crystal nucleuses; and a CT (Computed Tomography) contrast medium with long-efficiency blood circulation time and an anti-infective function is obtained by performing surface modification for the gold and silver core-shell nanoparticles through polyethylene glycol modified by tail sulfhydrylation. The multifunctional gold and silver core-shell nanoparticles are uniform in particle size, good in water solubility, and free from obvious agglomeration, and the core-shell nanoparticles have stronger dissipation capacities on X rays, thereby being superior to effective developing time of CT of traditional contrast medium ioversol; and the core-shell nanoparticles effectively inhibit infection of bacteria through a contrast medium injection window.
Owner:NANJING UNIV
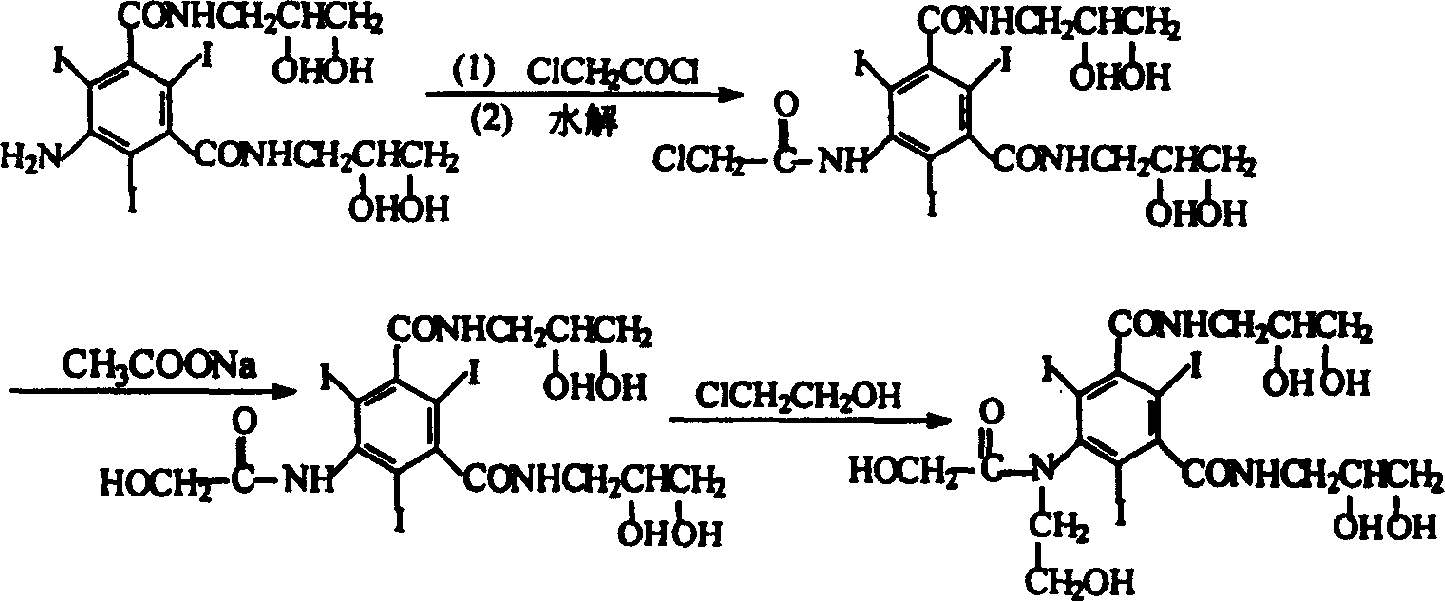
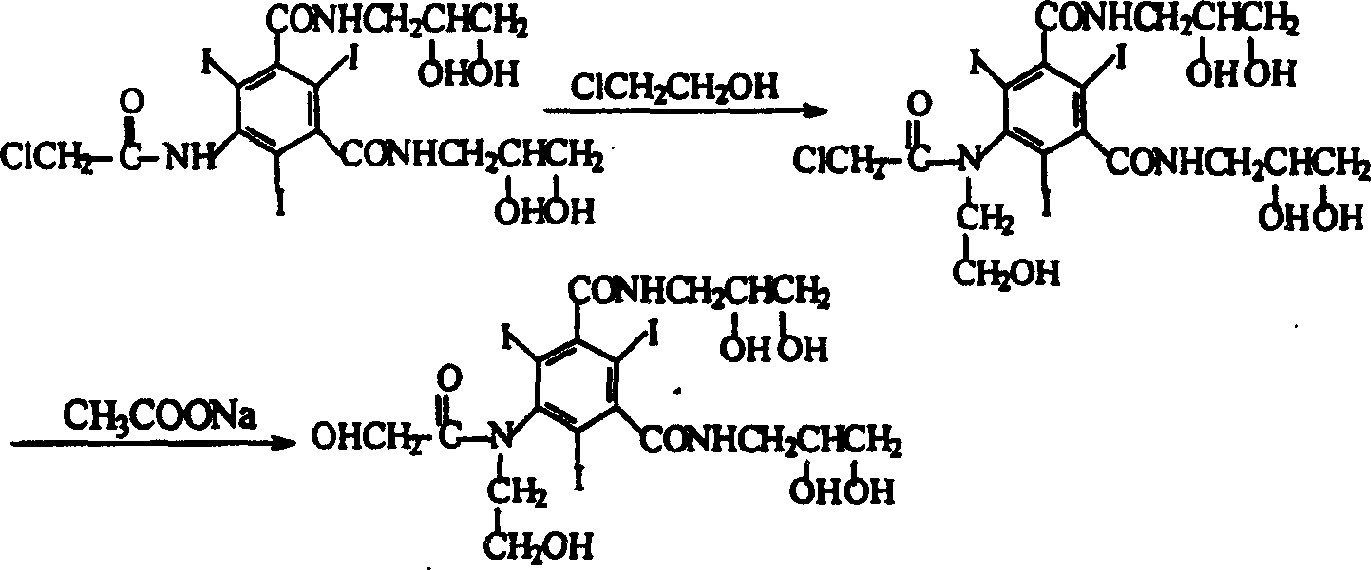
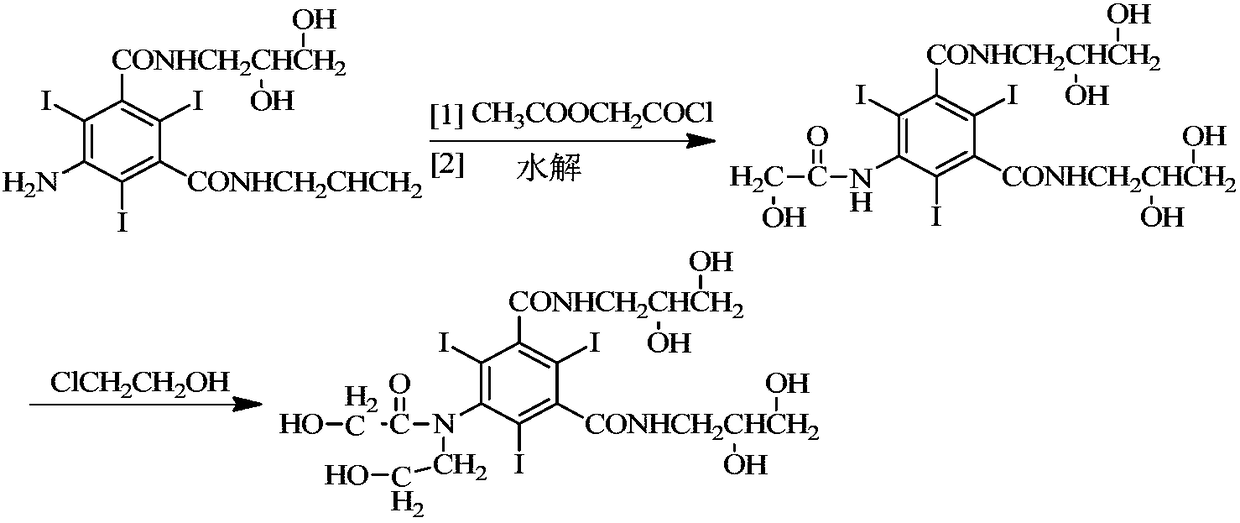
Preparation method of ioversol
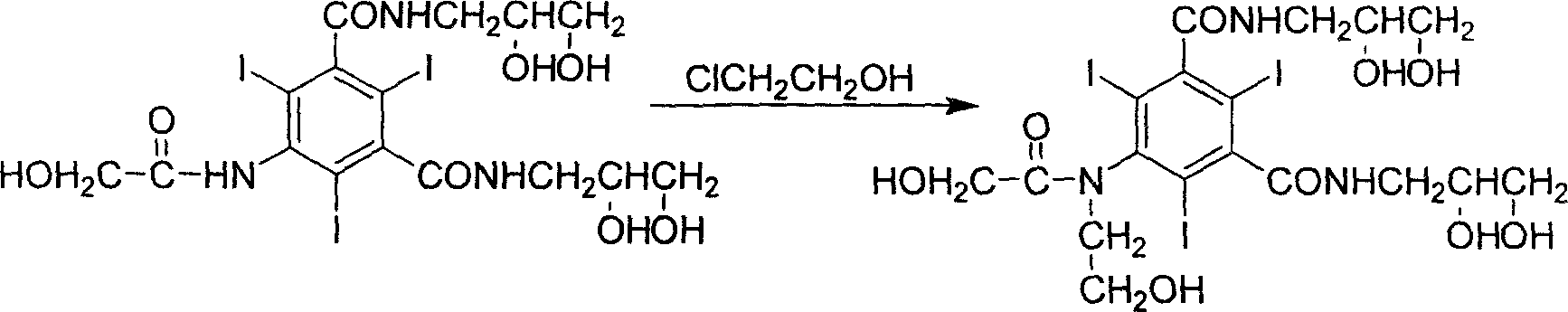
InactiveCN1477093AAvoid decompositionMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationAlkyl transferX-ray
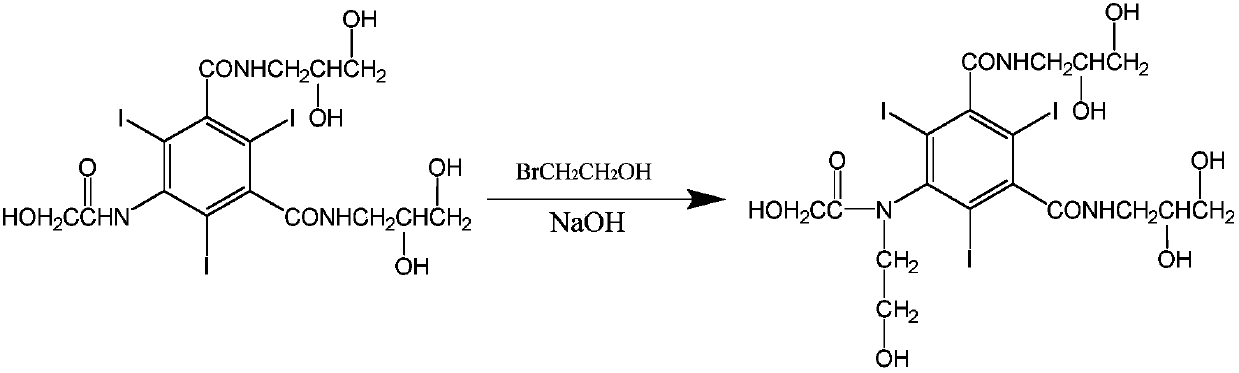
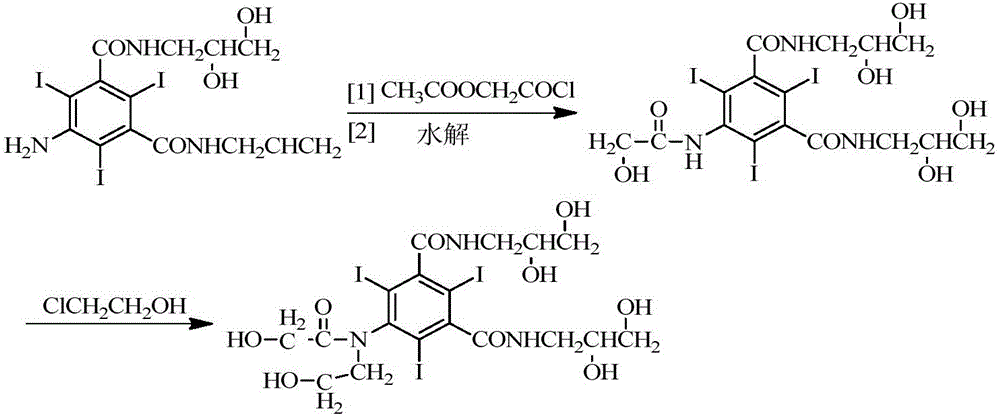
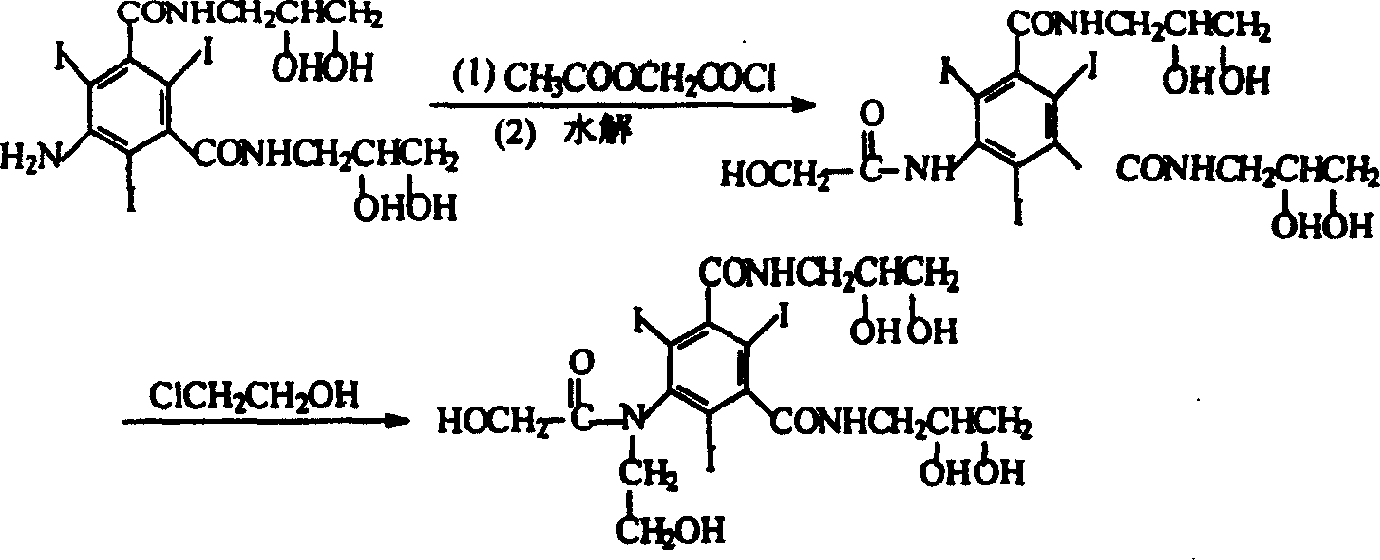
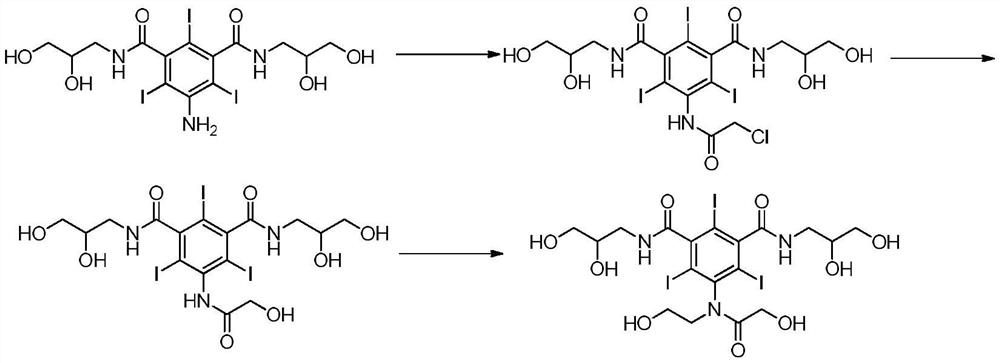
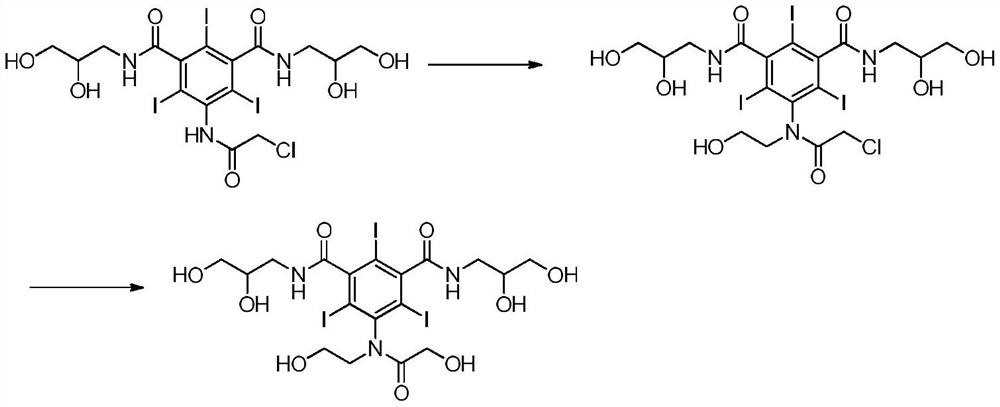
The present invention relates to a preparation method of X-ray contrast agent ioversol. The method adopts 5-chloroacetamino-N,N'-bis(2,3- dihydroxypropyl)-2,4,6-triodo-1,3-phthalamine and chloroethanol to make them implement alkylation reaction in the presence of inorganic alkali to obtain reaction liquor, said reaction liquor has no need of separation, and the anhydrous sodium acetate can be directly added to make reaction.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Improved process for synthesizing ioversol
InactiveCN1884257AGeneration of controlIncrease contentOrganic compound preparationCarboxylic acid amides preparationAlkyl transferTriiodide
The invention provides an improved method for synthesizing Ioversol, belonging to technology preparing non-ionic X contrast agent. The invention employs 5- hydroxyethyl amido- N, N' - (2,3-dihydroxy propyl)- 2, 4, 6- triiodide-1, 3- benezene diformamide and 2- chlorethanol to proceed nitrogen alkylation reaction in mixing solution of caustic soda and acetonitrile, neutralizing, desalting and desalting reacting solution, re-crystallizing with methyl glycol single dimethyl ether, and getting Ioversol. The invention is characterized by soft reacting condition, short reaction time, and stable quality, low cost and suitable for industrialized production.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Completely-biodegradable support capable of developing and preparation method thereof
ActiveCN103169556AGood biocompatibilitySolve the problem of easy falling offStentsHuman bodyAmidotrizoic Acid
The invention relates to the field of medical apparatuses and instruments and provides a completely-biodegradable support capable of developing and a preparation method thereof. A connecting rod of a support main body is provided with holes, markers which can develop in X-rays are filled in the holes, and each marker is composed of an outer protective layer, a developing layer and an inner protective layer. Both the support main body and the protective layers are made of biodegradable materials such as poly L lactic acid (PLLA), polycaprolactone (PCL) and poly lactic-co-glycolic acid (PLGA); the developing layers are made of contrast agents used for human bodies, and one or more than two of preferential amidotrizoic acid, diatrizoate, iohexol, iopromide and ioversol; and crystallization is carried out in advance through purification. The completely-biodegradable support can be clearly seen in the X-rays, the markers can be controlled to be completely degraded in 6 months to 2 years, developing time can be long, accordance can be achieved, and needs for support developing can be fundamentally met.
Owner:SHENZHEN SALUBRIS BIOMEDICAL ENG CO LTD
Contrast medium composition with contrast enhancement effect by comprising highly concentrated agent
InactiveCN103169988AImprove clarityImprove resolutionX-ray constrast preparationsSolution deliveryHigh concentrationIotrolan
The present invention relates to a contrast medium composition (formulation) comprising a highly concentrated contrast agent for use in an imaging method. The contrast medium composition comprises an aqueous contrast agent selected from the group consisting of iopentol, iotrolan, iohexol, ioversol, ioxilan, iodixanol and iobitridol in an amount of 360 mgl / mL to 450 mgI / mL. The contrast medium composition according to the present invention, which comprises iodine working as a contrast agent in a higher concentration than the concentration used before, shows higher contrast enhancement effect in both of arterial phase and portal phase when used for liver CT than a composition comprising the contrast medium in a concentration used before. Accordingly, images having excellent resolution and discrimination can be obtained.
Owner:CENT MEDICAL SERVICE CO LTD +1
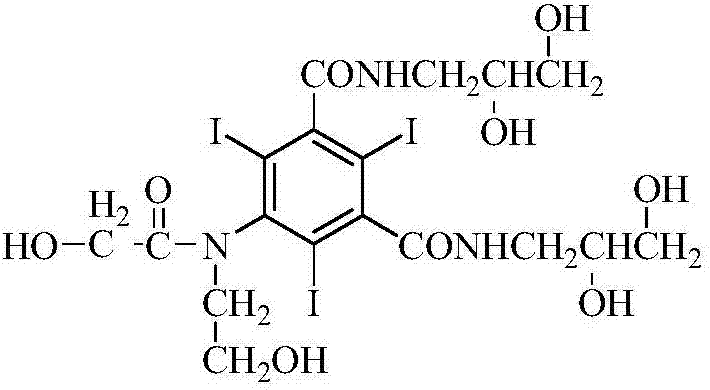
Ioversol and synthesis method thereof
InactiveCN107698456AReduce usageIncrease profitOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsSolvent
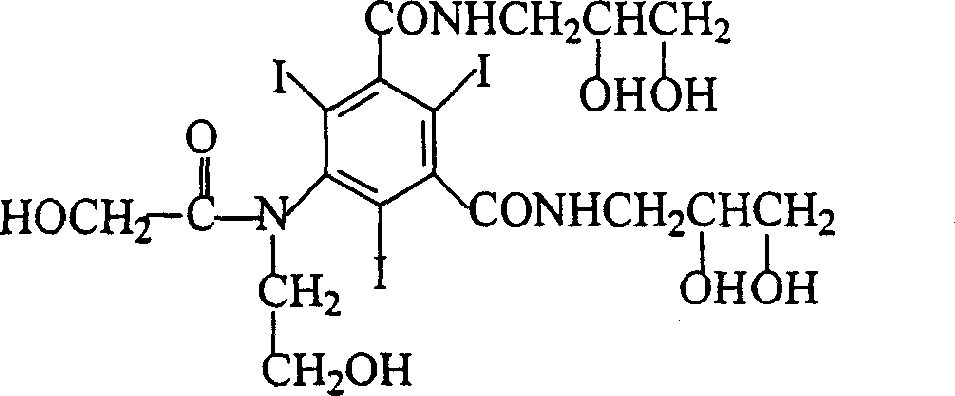
The invention belongs to the technical field of organic compound preparation, and discloses ioversol and a synthesis method thereof. The chemical name of ioversol is N,N'-bis(2,3-dihydroxypropyl)-5-[N-(2-hydroxyethyl)-hydroxyacetamido]-2,4,6-triiodo-1,3-benzenedicarboxamide. 5-hydroxyacetylamino-N,N'-bis-(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide and an alkylation agent carry outalkylation reactions under an alkaline condition to generate ioversol. Bromoethanol and sodium hydroxide are taken as reagents; glycol and anhydrous ethanol are taken as a mixed solvent, in the absence of water, a substrate can be well dissolved; the hydrolysis of the alkylation agent is avoided, the raw material loss is largely reduced, the utilization rate is increased, the reaction yield is increased, and the cost is reduced. At the same time, dangerous and toxic reagents such as chloroethanol, ethylene oxide, and the like are not used, the safety hazard is reduced, the damage to human body is reduced, and the stability of industrial production is guaranteed.
Owner:CHENGDU LAURELSCI TECH
Method for purifying ioversol
InactiveCN1483723AIncrease dosageIncrease contentCarboxylic acid amide separation/purificationPurification methodsAlcohol
The present invention relates to a purification method of X-ray non-ionic contrast agent ioversol. Said invention adopts the solvent recrystallization method to make the ioversol crude product undergo the process of recrystallization at twice, and its used recrystallization solvent includes: normal butyl alcohol, 2-methyl cellosolve and isopropanol mixed solvent and 2-methyl cellosolve and normal butyl alcohol mixed solvent. After having being recrystallized the content of the ioversol can be up to above 99.0%.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Process for purifying ioversol
ActiveCN101337907AHigh yieldImprove efficiencyCarboxylic acid amide separation/purificationX-ray constrast preparationsPurification methodsX-ray
The invention relates to a method for purifying ioversol that is x-ray nonionic contrast agent. The method adopts ethanol as recrystallization solvent to recrystallize ioversol crude-product, and the content of the obtained ioversol pure-product is more than 98.5 percent. The method is simple, easy to operate, economical and practical, and suitable for large-scale industrial production.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
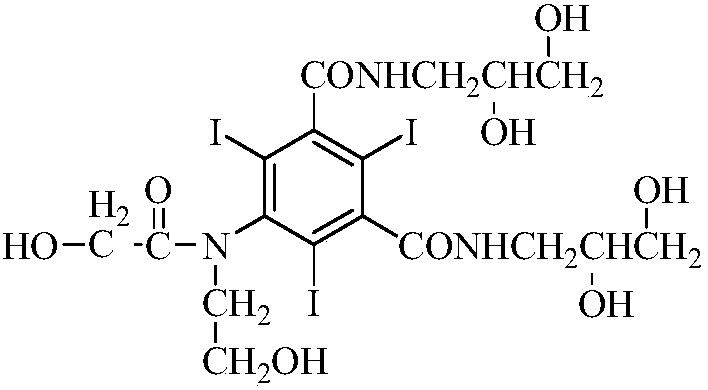
Preparation method of X-ray contrast agent ioversol intermediate
InactiveCN101654417AReduce dosageFew reaction stepsOrganic compound preparationCarboxylic acid amides preparationSimple Organic CompoundsX-ray
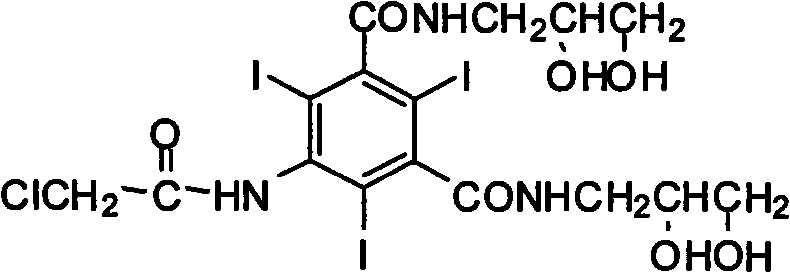
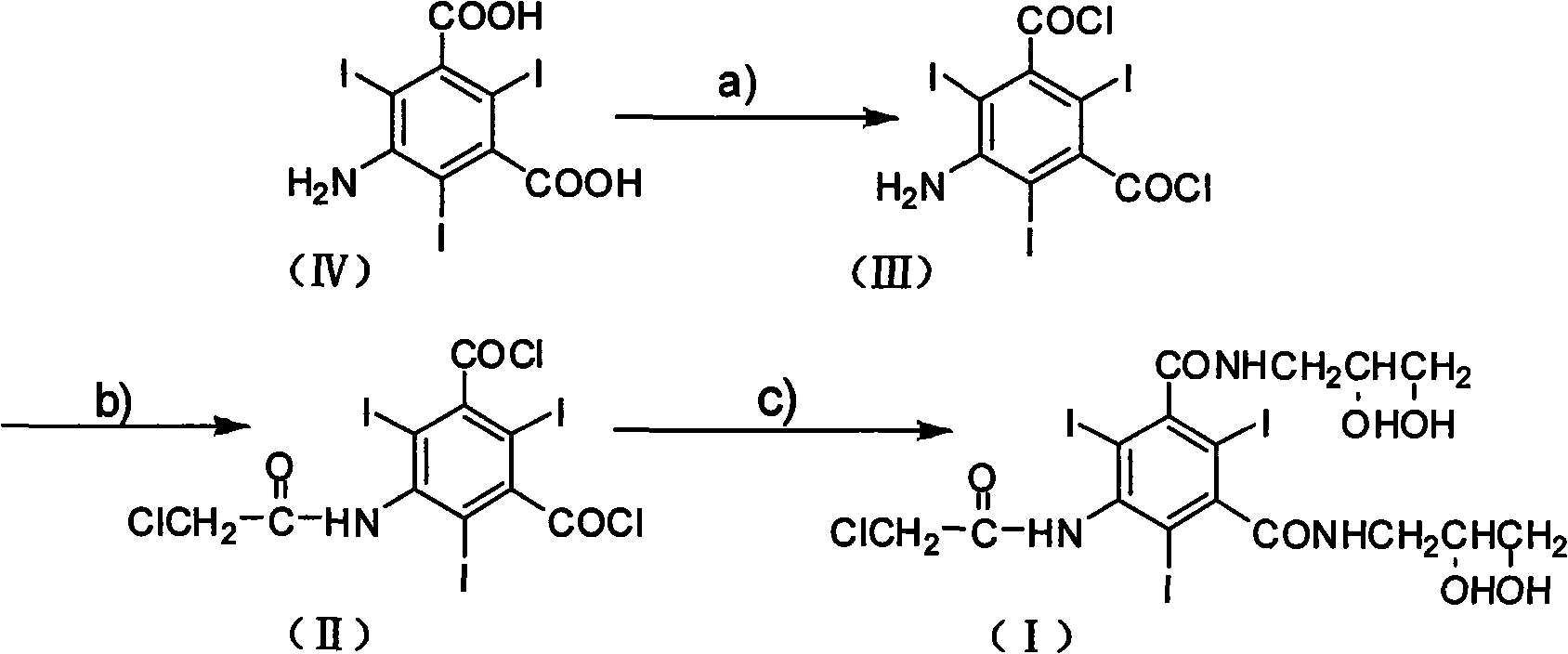
The invention discloses a preparation method of X-ray contrast agent ioversol intermediate, belonging to the technical field of organic compound preparation. The chemical name of the intermediate is 5-chloroacetamide-N,N'-bi(2,3-dyhydroxyl propyl)-2,4,6-triiodo-1,3-benzenedicarboxamide. The method utilizes 5-amino-2,4,6-triiodo-1,3-phthalic acid to react with thionyl chloride, and then reacts withchloroacetic chloride to obtain 5-chloroacetamide-2,4,6-triiodo-1,3-benzenedicarbonyl dichloride, finally reacts with 3- amino-1,2- propylene glycol to obtain the 5-chloroacetamide-N,N'-bi(2,3-dyhydroxyl propyl)-2,4,6-triiodo-1,3-benzenedicarboxamide. The method has simple synthesis process route, shortened reaction steps, mild reaction condition, security and reliability, stable quality, high yield, low cost and less equipment investment, thereby being applicable to large-scale industrial production.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
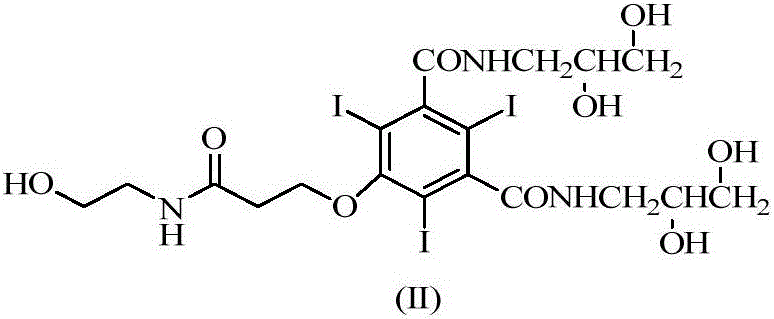
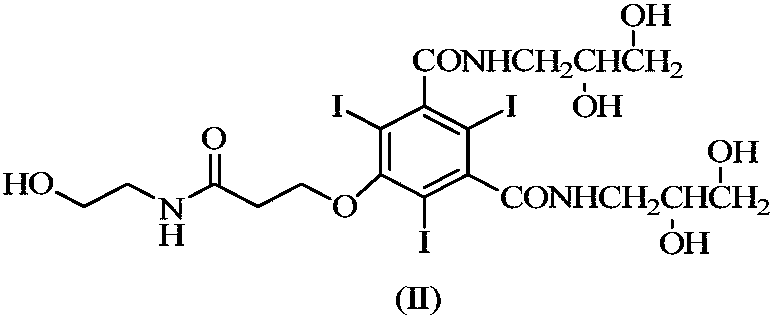
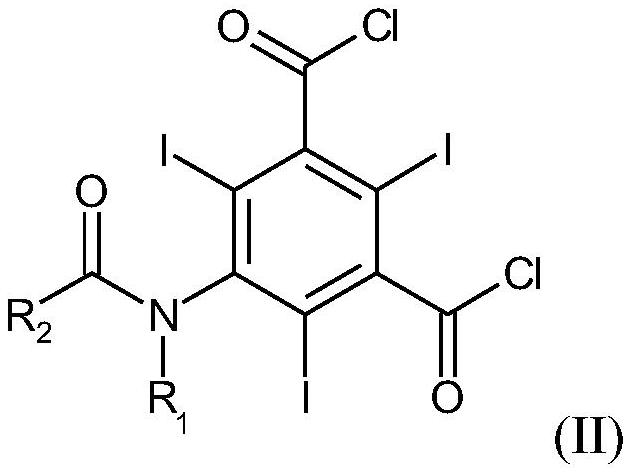
Efficient contrast agent synthesizing method and application thereof
ActiveCN109134289AIn line with the concept of atomic economicsThere is no special requirement for the order of additionOrganic compound preparationCarboxylic acid amide separation/purificationSynthesis methodsIopromide
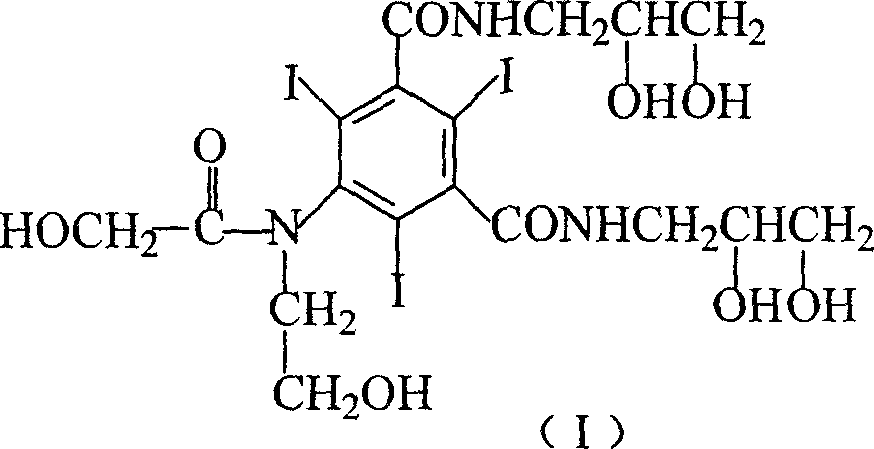
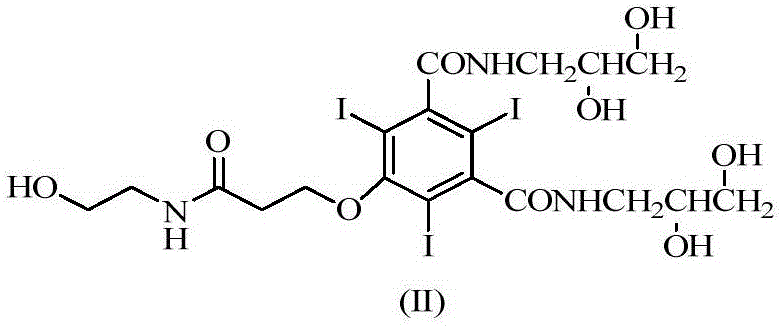
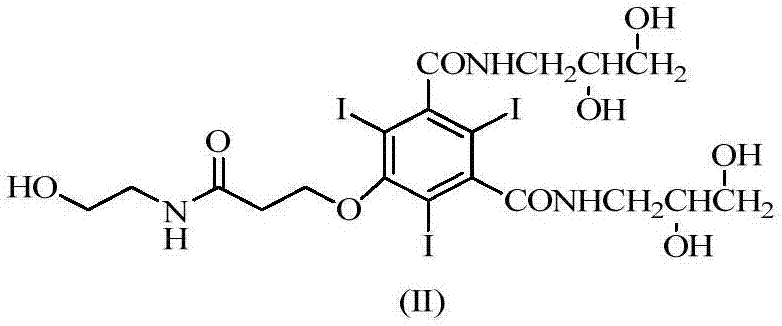
The invention discloses an efficient contrast agent synthesizing method. The method includes the following steps of firstly, preparing an intermediate mixture of a compound shown in the formula (II) and a compound shown in the formula (I) and / or (III); secondly, conducting separating to obtain the compound shown in the formula (II) and the compound shown in the formula (I) and / or (III); thirdly, taking the compound shown in the formula (II) to prepare a contrast agent (iopromide); fourthly, taking the compound shown in the formula (III) to prepare a contrast agent (iobitridol), and / or taking the compound shown in the formula (I) to prepare a contrast agent (iohexol, ioversol, iopentol or iodixanol). In the method, the iodic contrast agent is prepared by synthesizing and separating intermediates in the formula (II) and the formula (I) and / or the formula (III) and using the intermediates as the raw materials, the problem that diacylation byproducts need to be removed in an existing method is effectively solved, all the intermediates are effectively used, efficiency is high, and the actual application prospects are good. The formulas (I), (II) and (III) can be seen in the description.
Owner:XILING LAB CO LTD
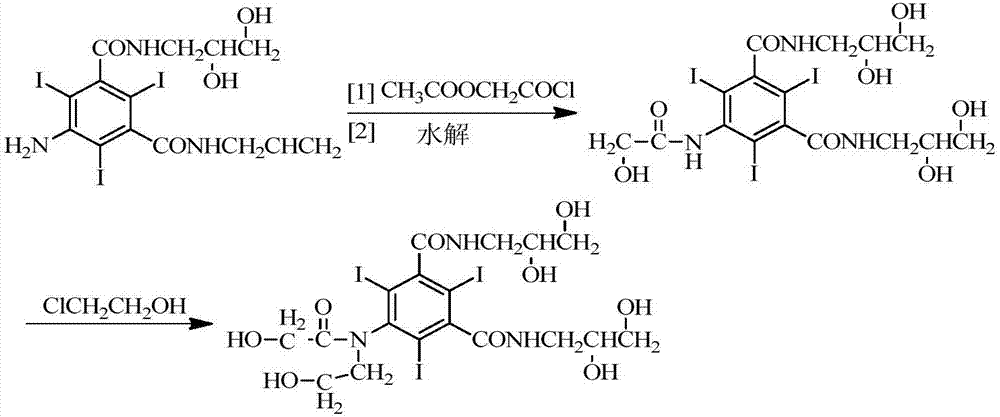
Preparation method of ioversol
PendingCN110028418AMeet medicinal requirementsEasy to operate in industrial productionOrganic compound preparationCarboxylic acid amides preparationAlkyl transferImpurity
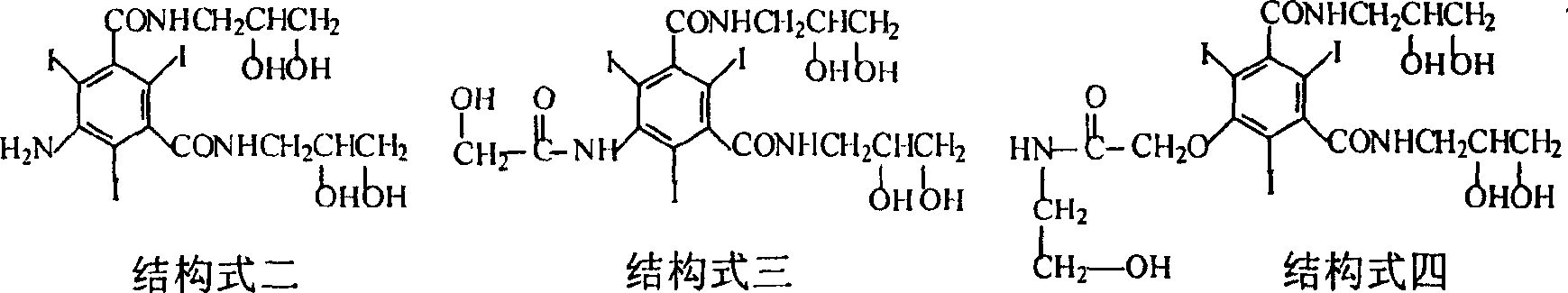
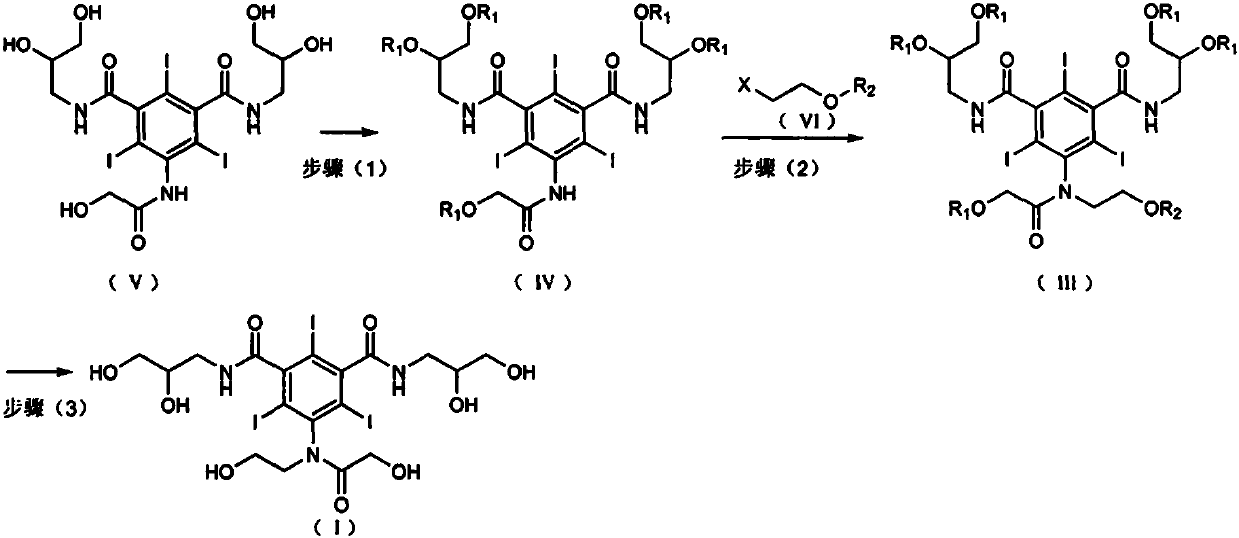
The present invention provides a novel preparation method of ioversol. The method comprises the following steps: adding a protection group to 5-hydroxyacetylamino-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzenedicarboxamide, conducting an alkylation reaction under an alkaline condition, and carrying out deprotection, so as to prepare ioversol. According to the preparation method disclosedby the invention, hydroxyl is protected before alkylation, so that the generation of ioversol rearrangement impurities (impurity II) in the alkylation preparation process is effectively controlled, the refining difficulty of a finished product is reduced, the refining yield is increased, and the preparation method is more suitable for industrialization.
Owner:大道隆达(北京)医药科技发展有限公司
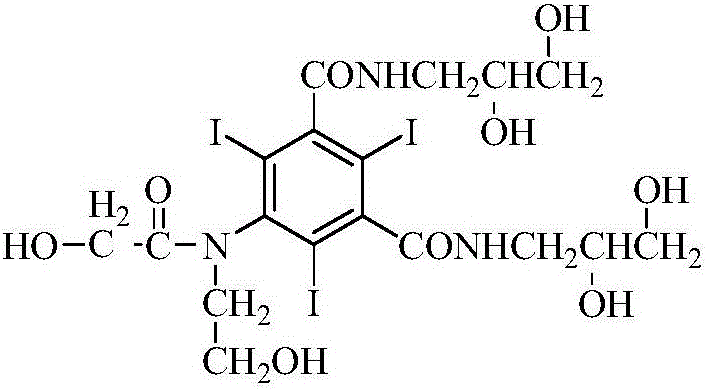
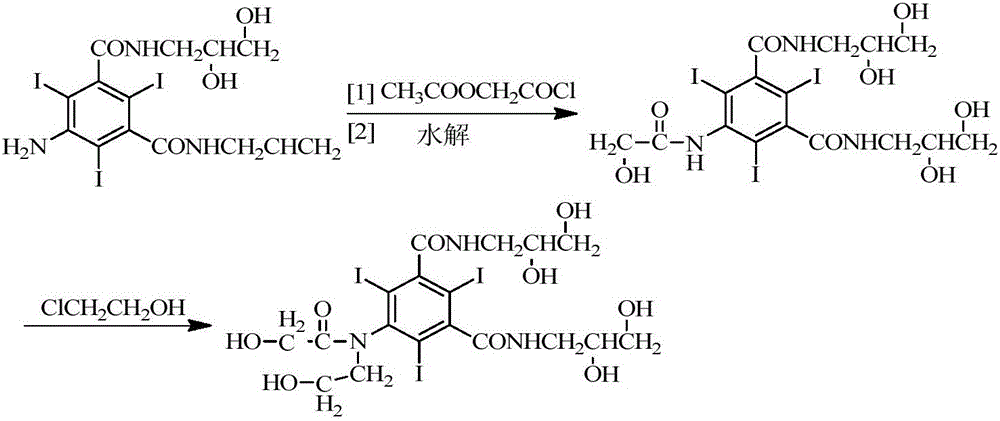
Preparation method for ioversol
ActiveCN106748859AOrganic compound preparationCarboxylic acid amides preparationSodium acetateSodium acetrizoate
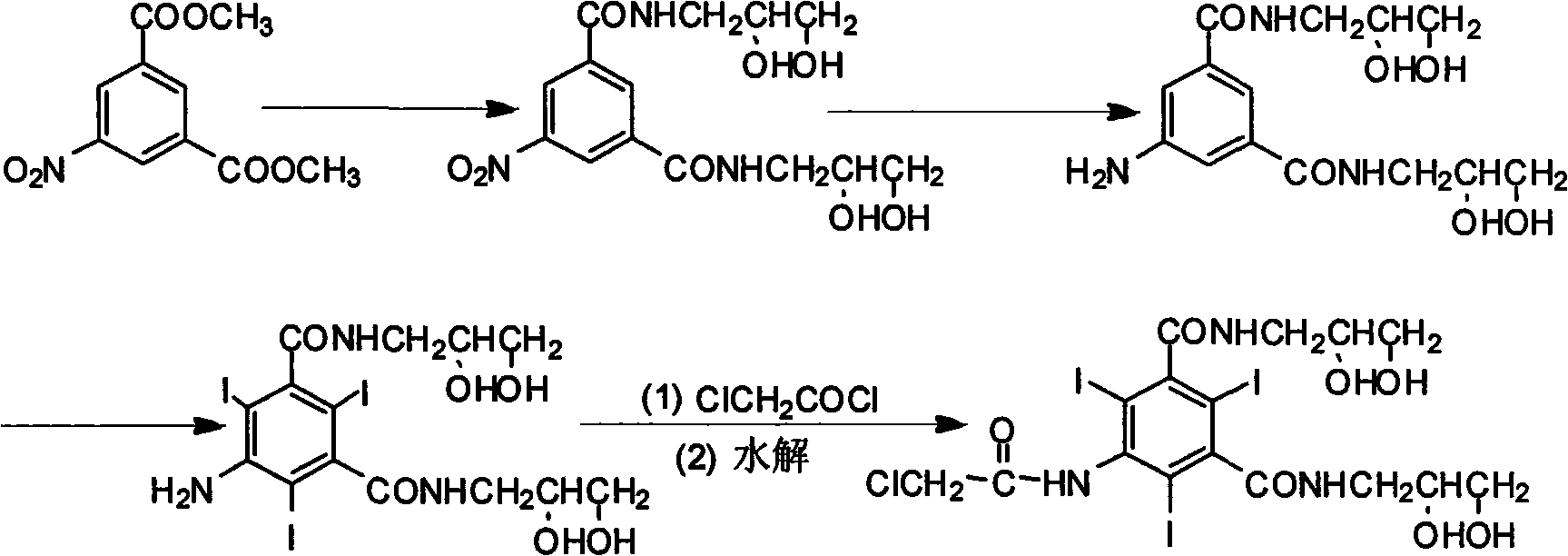
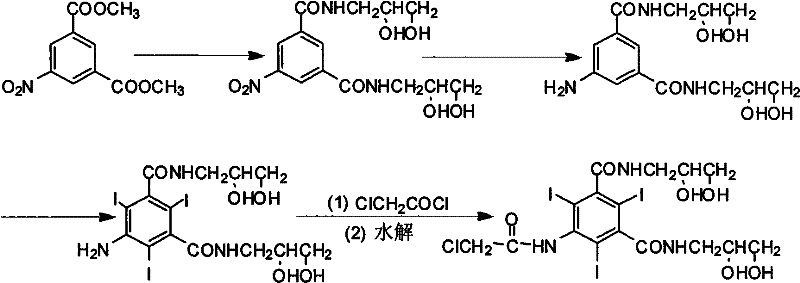
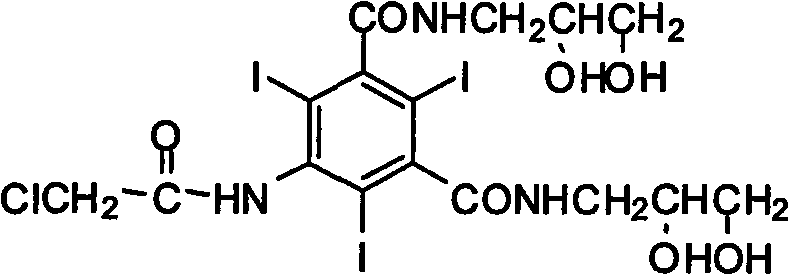
The invention provides a preparation method for ioversol. Specifically speaking, the method comprises the following steps: (1) performing an alkylation reaction on 5-chloracetyl amino-N,N'-bi-(2,3- dyhydroxyl propyl)-2,4,6-triiodo-1,3-phthalic diamide and chlorethyl alcohol under an alkaline condition; (2) hydrolyzing through sodium acetate to obtain the ioversol. A container of which the inner wall is coated with a corrosion-resistant coating is used in the reactions of the former two steps, and the water with the iron content of below 0.05 ppm is used. The method can effectively avoid the production of impurities which are difficult to remove, and is suitable for industrial production.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
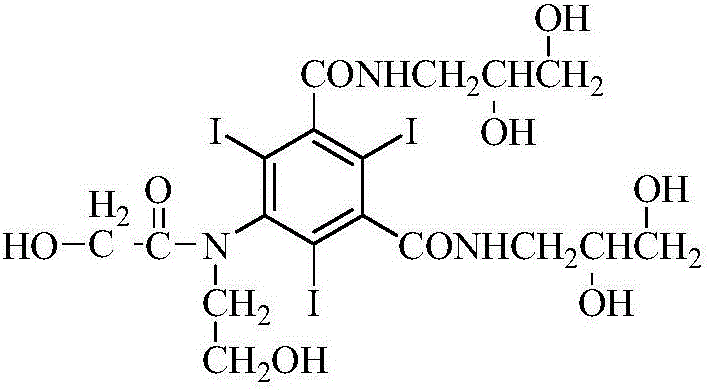
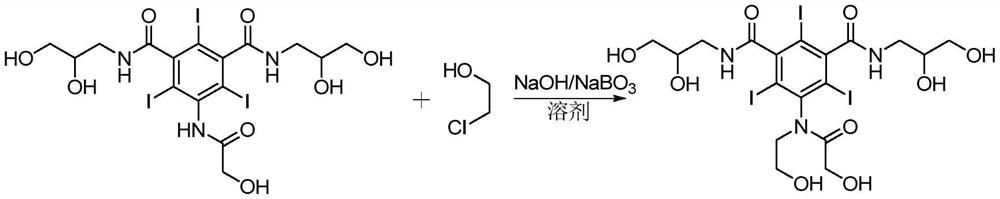
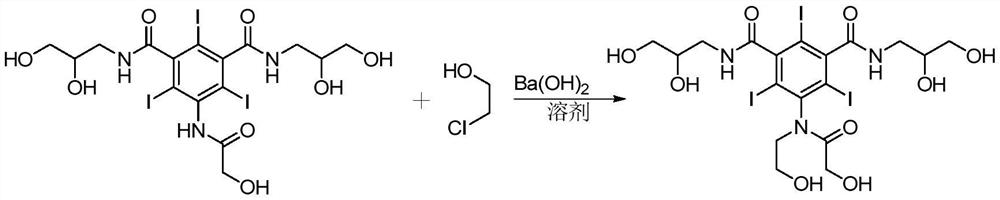
Preparation method of ioversol
ActiveCN106278929AOrganic compound preparationCarboxylic acid amides preparationAlkyl transferLithium chloride
The invention provides a preparation method of ioversol. Particularly, the method provided by the invention comprises the following steps: carrying out alkylation reaction on 5-chloracetylamino-N,N'-bis-(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-phenyldiformamide and chloroethanol by using sodium hydroxide / sodium borate or sodium hydroxide / lithium chloride; and meanwhile, directly hydrolyzing the product by a one-pot multistep process, and carrying out desalting and purification to prepare the ioversol. The method simplifies the production technique process, effectively controls the generation of irremovable impurities, and is suitable for industrial production.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
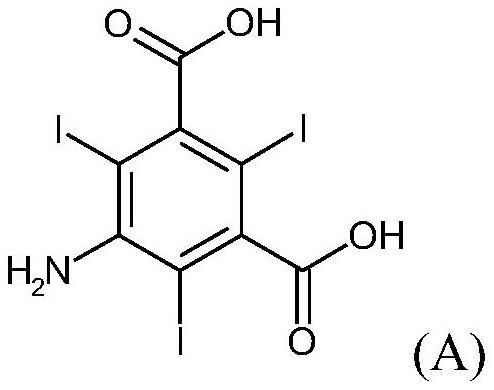
Method for preparing 5-amino-N,N'-di(2,3-dihydroxypropyl)-2,4,6-triiodoisophthal amide
InactiveCN102399167AHigh yieldReduce manufacturing costOrganic compound preparationCarboxylic acid amides preparationX-rayIoversol
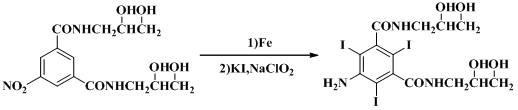
The invention discloses a method for preparing 5-amino-N,N'-di(2,3-dihydroxypropyl)-2,4,6-triiodoisophthal amide, and belongs to the technical field of pharmaceutical intermediates. The compound is an important intermediate of non-ionic x-ray contrast agents of iohexol and ioversol. The invention provides process reaction conditions for preparing the 5-amino-N,N'-di(2,3-dihydroxypropyl)-2,4,6-triiodoisophthal amide; and the process reaction conditions are mild, the operation is simple and convenient, corrosion to equipment is low, the reaction yield is high, the production cost is obviously reduced, and the industrial production of the product is easy to implement.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Preparation method of ioversol
InactiveCN1187317CAvoid decompositionMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationAlkyl transferX-ray
The present invention relates to a preparation method of X-ray contrast agent ioversol. The method adopts 5-chloroacetamino-N,N'-bis(2,3- dihydroxypropyl)-2,4,6-triodo-1,3-phthalamine and chloroethanol to make them implement alkylation reaction in the presence of inorganic alkali to obtain reaction liquor, said reaction liquor has no need of separation, and the anhydrous sodium acetate can be directly added to make reaction.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
A kind of method for preparing ioversol
ActiveCN110156623BMild reaction conditionsOvercome the disadvantage of unstable pHOrganic compound preparationCarboxylic acid amide separation/purificationDipotassium hydrogen phosphateAlkoxy group
The invention discloses a method for preparing ioversol. Compound I: [5-hydroxyacetamido]-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo ‑1,3‑phthalamide, chloroethanol or its analogues are used as raw materials, and disodium hydrogen phosphate or dipotassium hydrogen phosphate is used as an acid application agent, reacted in a solvent, and after the reaction is completed, ioversol is obtained through post-treatment. By buffering alkali disodium hydrogen phosphate or dipotassium hydrogen phosphate as an acid-binding agent, the reaction conditions are relatively mild, which overcomes the shortcoming of unstable pH in the reaction process, so the present invention prepares ioversol with few and small impurities, especially alkoxy Basic impurities can be controlled below 0.5%, the yield is high, up to 90-98%, green and environmentally friendly, and the operation is simple.
Owner:ZHEJIANG HAIZHOU PHARMA CO LTD
A kind of preparation method of ioversol
ActiveCN106748859BOrganic compound preparationCarboxylic acid amides preparationSodium acetateEthyl Chloride
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Process for the monotopic preparation of intermediate organo-iodinated compounds for the synthesis of ioversol
PendingCN112543751AReduce or avoid the use ofAvoid separabilityOrganic compound preparationCarboxylic acid amides preparationCombinatorial chemistryOrganic chemistry
The invention relates to a process for the preparation of organo-iodinated compounds as well as the preparation intermediates thereof. More specifically, the invention relates to a process for the preparation of organo-iodinated compounds useful as preparation intermediates in the synthesis of the contrast product ioversol.
Owner:GUERBET SA
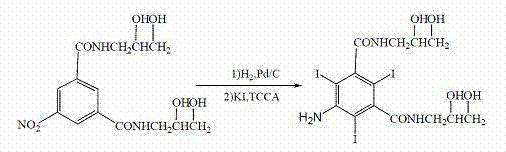
Improved preparation process of 5- amino-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzene dicarboxamide
ActiveCN102702018AMild reaction conditionsSimple and efficient operationOrganic compound preparationCarboxylic acid amides preparationChemistryIohexol
The invention discloses an improved preparation process of 5- amino-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzene dicarboxamide. The improved preparation process is characterized in that potassium iodide is taken as propiodal of 5- amino-N,N'-bis(2,3-dihydroxypropyl)-1,3-benzene dicarboxamide, and trichloroisocyanuric acid is taken as an oxidant thereof, so that the 5- amino-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzene dicarboxamide is obtained by iodination reaction. Further, the 5- amino-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-benzene dicarboxamide is a key intermediate for a non-ionic x-ray contrast medium such as iohexol, ioversol and iodixanol. The improved preparation process requires mild reaction conditions, and is convenient to operate, free of toxicity or pollution, high in iodine utilization rate and good in reaction selectivity; and production cost is lowered remarkably.
Owner:浙江海昌药业股份有限公司
Method for purifying contrast agent ioversol
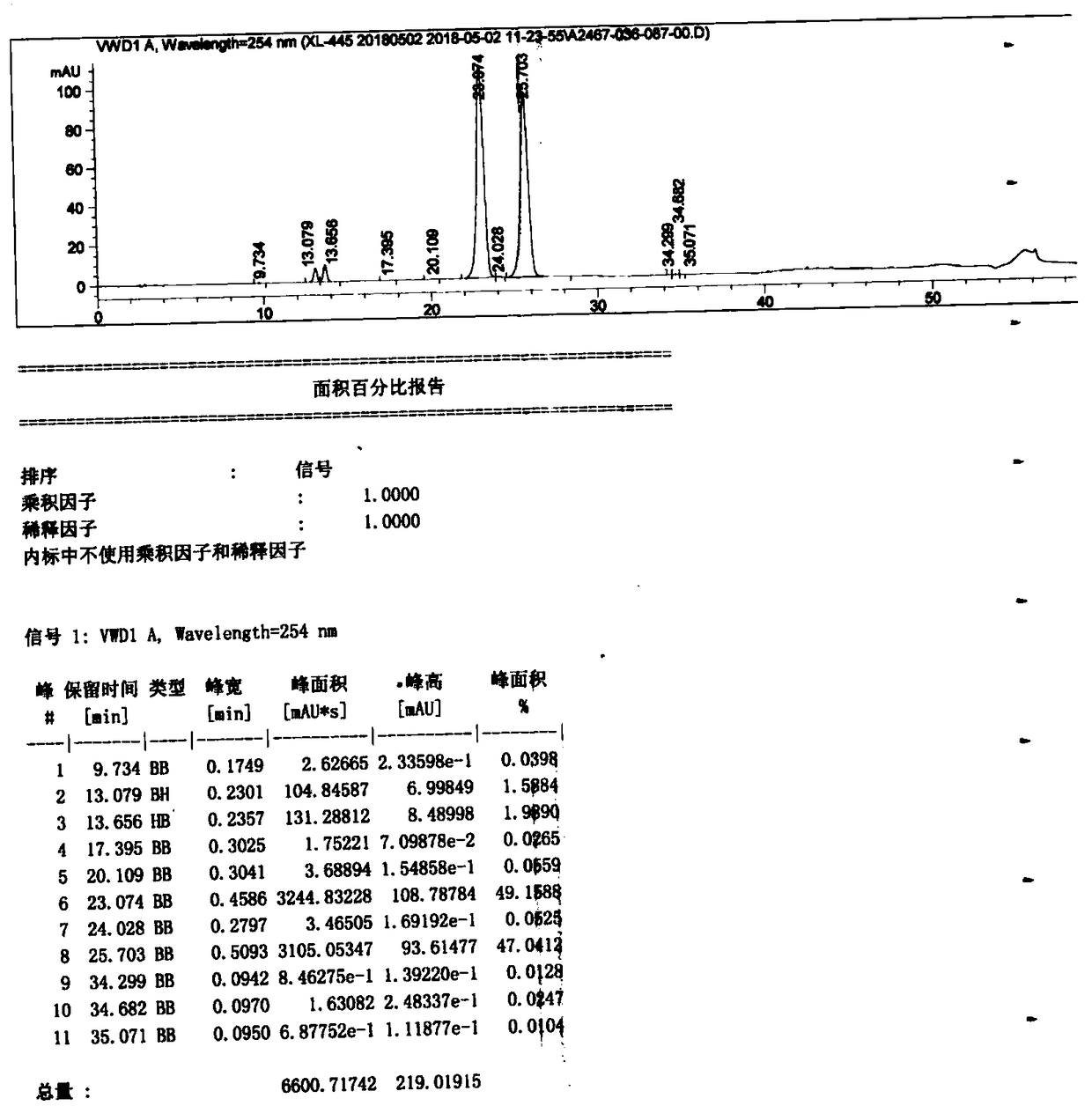
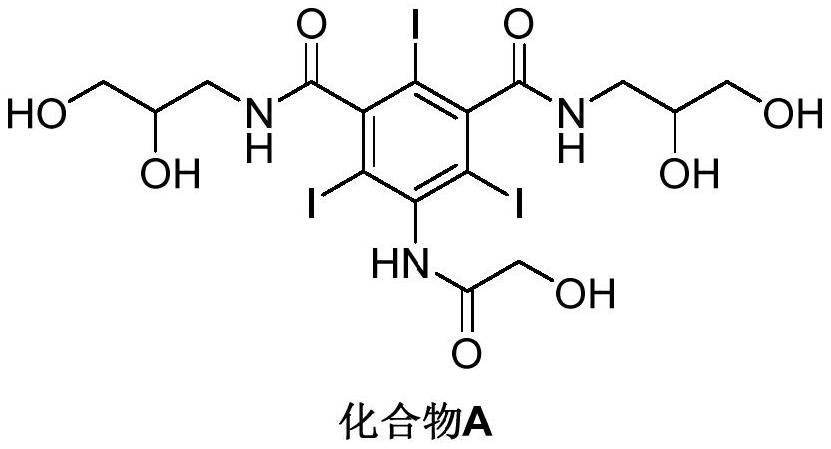
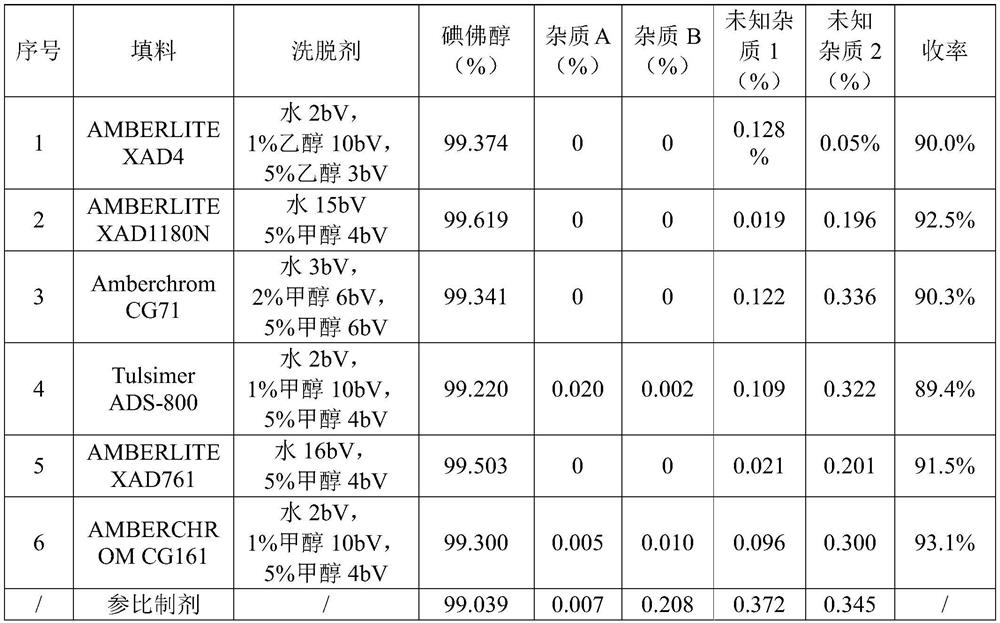
PendingCN114478298AHighly selective adsorptionIncrease productivitySolid sorbent liquid separationCarboxylic acid amide separation/purificationPurification methodsBiochemical engineering
The invention discloses a method for purifying a contrast agent ioversol, which comprises the following steps: purifying an ioversol crude product solution through a macroporous adsorption resin packed column to obtain an ioversol pure product. The high-quality pure ioversol product is obtained by using the purification method, and the purity of the high-quality pure ioversol product is as high as 99.220%-99.619% and even higher than that of a reference preparation. In addition, the purification method disclosed by the invention is carried out at room temperature, and is low in cost, high in yield, easy in solvent recovery, large in batch, short in period, highly automatic in process, environment-friendly and pollution-free, easy in production process amplification and capable of realizing large-scale industrial production.
Owner:XILING LAB CO LTD +1
A kind of preparation method of ioversol
ActiveCN106278929BOrganic compound preparationCarboxylic acid amides preparationAlkyl transferLithium chloride
The invention provides a preparation method of ioversol. Particularly, the method provided by the invention comprises the following steps: carrying out alkylation reaction on 5-chloracetylamino-N,N'-bis-(2,3-dihydroxypropyl)-2,4,6-triiodo-1,3-phenyldiformamide and chloroethanol by using sodium hydroxide / sodium borate or sodium hydroxide / lithium chloride; and meanwhile, directly hydrolyzing the product by a one-pot multistep process, and carrying out desalting and purification to prepare the ioversol. The method simplifies the production technique process, effectively controls the generation of irremovable impurities, and is suitable for industrial production.
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Traceable polylactic acid/rifampicin drug-loaded microspheres and preparation method thereof
ActiveCN114042044ASimple processHigh drug loadingAntibacterial agentsOrganic active ingredientsUltrasonic emulsificationPolyvinyl alcohol
The invention discloses a preparation method of traceable polylactic acid / rifampicin drug-loaded microspheres, which comprises the following steps: dissolving polylactic acid in dichloromethane, then adding span-80 and rifampicin, and carrying out ultrasonic emulsification to obtain an emulsion; pouring the emulsion into a PVA (Polyvinyl Alcohol) aqueous solution, and shearing and dispersing to obtain an oil-in-water emulsion; stirring the oil-in-water emulsion , adding an ioversol contrast agent solution dropwise, then adding sodium tripolyphosphate dropwise , and curing and forming microspheres to obtain polylactic acid two-layer composite drug loading ; putting the microspheres into a dichloromethane solution dissolved with polylactic acid to obtain three-layer composite drug-loaded microspheres, adding the three-layer composite drug-loaded microspheres into an ioversol contrast agent solution, and dropwise adding TPP to obtain a solution; and centrifuging the obtained solution, and taking out the microspheres at the bottom. The preparation process of the drug-loaded microspheres is simple and rapid, the product has a tracing function, high drug loading rate and encapsulation efficiency and long slow release time, polylactic acid with good biodegradability is used in the preparation process, and the drug-loaded microspheres have wide application prospects.
Owner:SHANGHAI INST OF TECH
Preparation method of X-ray contrast agent ioversol intermediate
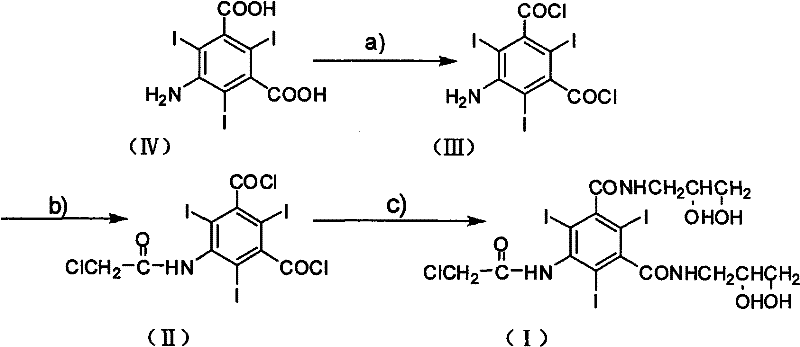
InactiveCN101654417BReduce dosageFew reaction stepsOrganic compound preparationCarboxylic acid amides preparationX-rayChloroacetyl chloride
The invention discloses a preparation method of X-ray contrast agent ioversol intermediate, belonging to the technical field of organic compound preparation. The chemical name of the intermediate is 5-chloroacetamide-N,N'-bi(2,3-dyhydroxyl propyl)-2,4,6-triiodo-1,3-benzenedicarboxamide. The method utilizes 5-amino-2,4,6-triiodo-1,3-phthalic acid to react with thionyl chloride, and then reacts with chloroacetic chloride to obtain 5-chloroacetamide-2,4,6-triiodo-1,3-benzenedicarbonyl dichloride, finally reacts with 3- amino-1,2- propylene glycol to obtain the 5-chloroacetamide-N,N'-bi(2,3-dyhydroxyl propyl)-2,4,6-triiodo-1,3-benzenedicarboxamide. The method has simple synthesis process route, shortened reaction steps, mild reaction condition, security and reliability, stable quality, high yield, low cost and less equipment investment, thereby being applicable to large-scale industrial production.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Synthesis of N1,N3-bis(2,3-dihydroxypropyl)-5-nitroisophthalamide
ActiveUS9006486B2Organic compound preparationCarboxylic acid amides preparationImaging agentCombinatorial chemistry
The present disclosure generally relates to a new process for the preparation of high purity 5-nitro-isophthalamide compounds, which are useful as intermediates for the preparation of imaging agents, such as iodinated x-ray contrast imaging agents like ioversol, iohexyl and iopamidol.
Owner:LIEBEL FLARSHEIM CO
Process for purifying ioversol
ActiveCN101337907BHigh yieldImprove efficiencyCarboxylic acid amide separation/purificationX-ray constrast preparationsPurification methodsX-ray
Owner:JIANGSU HENGRUI MEDICINE CO LTD +1
Method for preparing ioversol
PendingCN113717074AAvoid generatingImprove qualityOrganic compound preparationCarboxylic acid amides preparationBenzenePharmaceutical drug
The invention discloses a preparation method of a medicine namely ioversol, and belongs to the technical field of medical intermediates. The preparation method comprises the following steps: carrying out acetonylidene protection on (5-hydroxyacetamido)-N,N'-bis(2,3-dihydroxypropyl)-2,4,6-triiodine-1,3-benzene dicarboxamide (1) to obtain an intermediate (2), carrying out substitution reaction on the intermediate (2) and chlorohydrin to obtain an intermediate (3), and finally, carrying out acetonylidene removal protection reaction on the intermediate (3) to obtain ioversol. Through protection of different levels, no rearrangement isomer is generated even under a strong alkaline condition, the generation of an impurity II is avoided, the process reproducibility is good, and the method can be smoothly amplified to a kilogram-level reaction scale.
Owner:SHANGHAI ZAIQI BIO TECH
Gastrointestinal tract oral CT (Computed Tomography) contrast agent
PendingCN114288427AProlong CT Imaging TimeLong imaging timeX-ray constrast preparationsCelluloseFood additive
The invention discloses an oral CT (Computed Tomography) contrast agent for gastrointestinal tracts. The oral CT contrast agent comprises the following components in percentage by mass: 0.5-1.5% of edible liquid paraffin, 0.5-1.5% of soybean salad oil, 0.5-1% of sodium carboxymethyl cellulose, 0.1-0.3% of a food additive, 0.1-0.2% of a preservative, 0.5-1% of ioversol or iohexol and the balance of purified water. The gastrointestinal tract contrast performance can be remarkably improved, the contrast agent CT imaging time can be prolonged, the diagnosis efficiency of doctors is effectively improved, the problem that a large amount of water and contrast agents are taken again is reduced, the economic burden of patients is reduced, and the situation that the bodies of the patients are uncomfortable is prevented.
Owner:福建宸润生物科技有限公司
Method for purifying ioversol
InactiveCN1197842CSuitable for large industrial productionCarboxylic acid amide separation/purificationPurification methodsX-ray
A method for purifying ioversol, the invention relates to a method for purifying ioversol as an x-ray non-ionic contrast agent. The method adopts the solvent recrystallization method to carry out two recrystallizations to the ioversol crude product, and the recrystallization solvent used includes: n-butanol, 2-methoxyethanol and isopropanol mixed solvent, 2-methoxyethanol and n-butanol Solvents are mixed, and the content of ioversol reaches more than 99.0% after recrystallization. The method is economical and practical, easy to operate and suitable for industrial production.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
Method for subfissure teeth vacuum and contrast agent crack infiltrating
PendingCN111557686AImprove the detection rateImprove diagnostic capabilitiesRadiation diagnostics for dentistryMaterial analysis by transmitting radiationVisual field lossHigh density
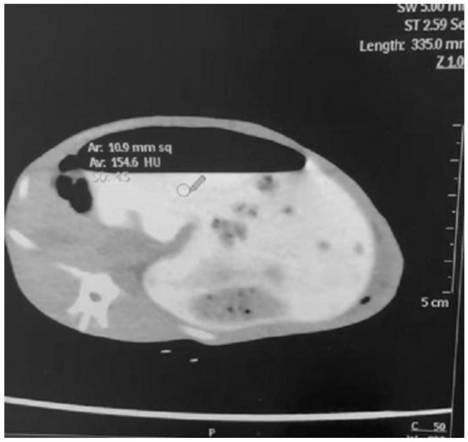
The invention relates to the technical field of dentistry, in particular to a method for enabling a contrast agent to permeate into cracks of in-vitro subfissure teeth under vacuum assistance so as toachieve better development on CBCT. The method comprises the following steps: carrying out a subfissure tooth vacuum contrast agent adding experiment; shooting an enhanced small visual field CBCT; and collecting image data. According to the method, the liquidity and the X-ray radiation resistance of ioversol are utilized; the method comprises steps: placing ioversol liquid at the crown part of atooth crack; vacuumizing in a closed environment; after the ioversol contrast agent permeates into the structural cracks of the teeth, shooting CBCT as enhanced CBCT, comparing with a conventional CBCT before the crack permeation experiment, so that the detection rate of the CBCT on the fine crack of the tooth is increased, the invasion depth of the CBCT can be determined by measuring the high-density linear crack shadow, and the diagnosis capability of the CBCT on the subfissure of the tooth is improved.
Owner:STOMATOLOGICAL HOSPITAL TIANJIN MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com