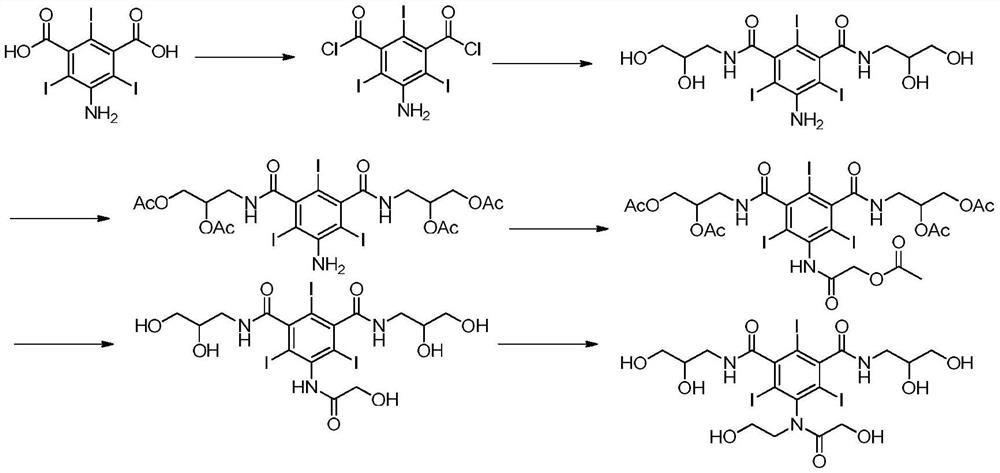
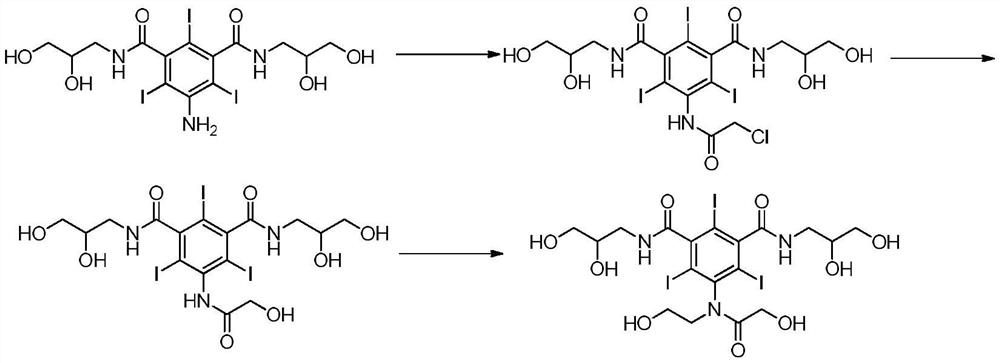
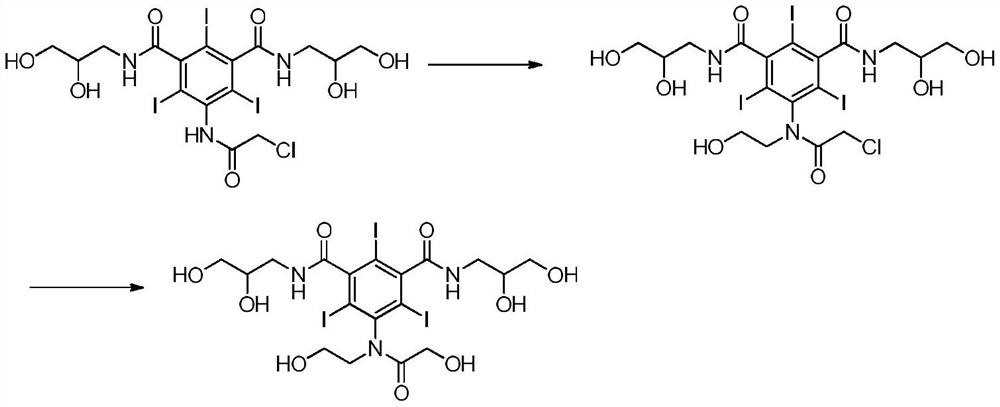
Method for preparing ioversol
A technology of ioversol and chloroethanol, applied in the field of medicine, can solve problems such as the generation of impurity II that is difficult to avoid, and achieve the effects of improving quality, good process reproducibility and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] first step:
[0041] (5-hydroxyacetamide) -N, N'-bis (2,3-dihydroxypropyl) -2,4,6-triiodine-1,3-phenylene terephthalamide (1) (1.0 g 1.31 mmol) and acetone (2.26 mg, 0.013 mmol) were added to acetone (163.78 mg, 1.57 mmol) / acetone (18 mL) solution, and the reaction reflux was heated to 0-5 ° C after cooling to 0-5 ° C. The solution of vinylmethyl ether (83 mg, 1.44 mmol) / acetone (2 mL) was slowly added to the reaction system, and then stirred at room temperature for 18 hours, aqueous sodium hydrogencarbonate solution was washed, dichloromethane extraction, and coated to obtain intermediate 2 ( 0.98g, yield: 84%).
[0042] Step 2:
[0043] Intermediate 2 (0.71 g, 0.79 mmol), chlorocanol (0.126 g, 1.58 mmol) was added with potassium carbonate (0.327 g, 2.37 mmol), and stirred at room temperature for 4 hours, saturated aqueous ammonium chloride solution, Extraction of dichloromethane. The organic layer is coated with intermediate 3 (0.74 g), which is directly used in the n...
Embodiment 2
[0047] first step:
[0048] (5-hydroxyacetamide) -N, N'-bis (2,3-dihydroxypropyl) -2,4,6-triiodine-1,3-phenylenediamide (1) (100g, 131.05 mmol) and ammonium chloride (83.9 mg, 1.57 mmol) were added to a solution of acetone (20.79 g, 157.26 mmol) and tetrahydrofuran (1.8L), and the reaction reflux was heated to 0-5 ° C after cooling to 0-5 ° C. Ethylene ether (11.34 g, 157.26 mmol) / tetrahydrofuran (0.2 L) solution was slowly added to the reaction system, then stirred at room temperature for 18 hours, add aqueous sodium hydrogencarbonate solution, dichloromethane extraction, and dry to dry the intermediate 2 (103.2g, yield: 86%).
[0049] Step 2:
[0050] Intermediate 2 (74.37 g, 81.25 mmol), chlorocyaxethanol (9.81 g, 121.88 mol) were added to N, N-dimethylformamide (1.5 L), and stirred at room temperature 4 at room temperature 4 Hour, saturated aqueous solution of ammonium chloride, extract of dichloromethane. The organic layer is coated with intermediate 3 (78g), which is direc...
Embodiment 3
[0054] first step:
[0055](5-hydroxyacetamide) -N, N'-bis (2,3-dihydroxypropyl) -2,4,6-triiodine-1,3-phenylenediamide (1) (1 kg, 1.31 mol) and acetone (2.71 g, 15.73 mmol) were added to the solution of acetone (252.02 g, 1.57 mol) / dichloromethane (18 L) solution, and the reaction was refluxed for 5 hours and then cooled to 0-5 EtOAc EtOAc EtOAc m. Dry from 0.95 kg of intermediate 2.
[0056] Step 2:
[0057] Intermediate 2 (0.71 kg, 787.78 mmol), chlorocol (63.43 g, 787.78 mmol) was added to potassium t-butoxide (88.9 mmol, 787.78 mmol), and stirred at room temperature for 4 hours, saturated ammonium chloride Aqueous solution, dichloromethane extraction. The organic layer is coated with intermediate 3 (0.74 kg), which is used directly in the next step;
[0058] third step:
[0059] The glacial acetic acid (70.51 g, 1.17 mol) was added to the intermediate 3 (0.74 kg, 0.78 mol) / tetrahydrofuran (3.7 L) solution at 0-5 ° C, stirred at room temperature for 2 hours, the solvent was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com