Preparation method of ioversol
A technology of ioversol and intermediate, applied in the field of preparation of ioversol, can solve problems such as affecting the quality and yield of the final product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the synthesis of ioversol
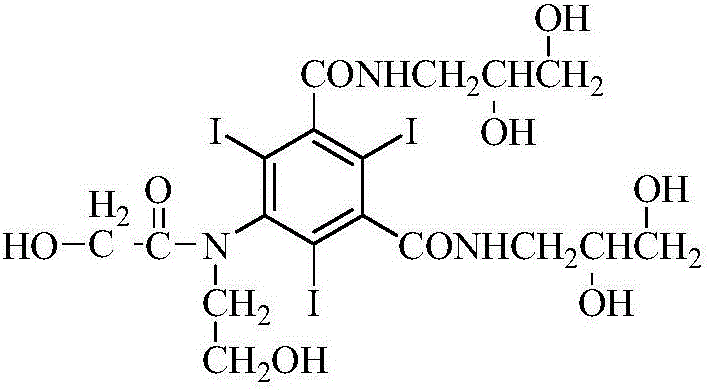
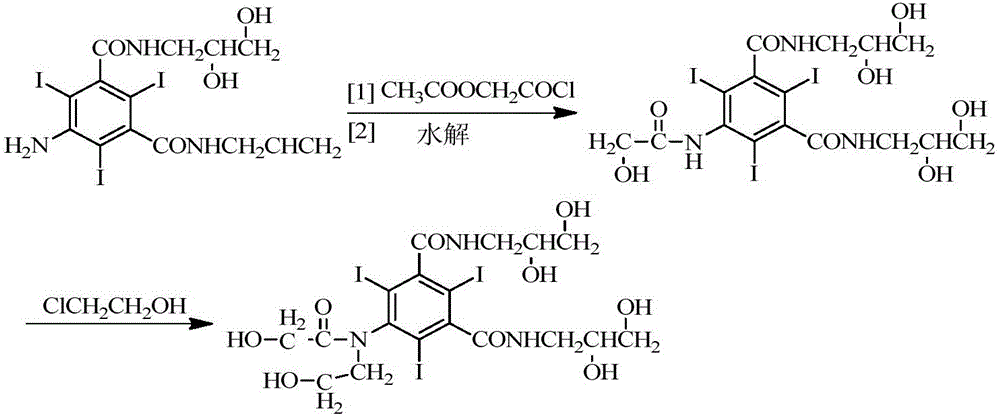
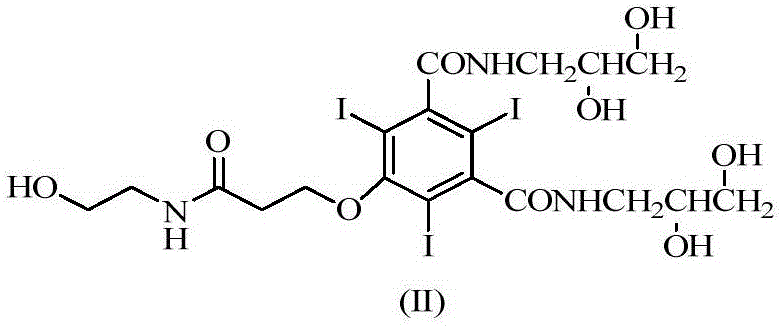
[0041]In a three-necked flask equipped with a stirrer and a reflux condenser, add 50g (0.06mol) of 5-chloroacetamido-N,N'-bis-(2,3-dihydroxypropyl)-2,4,6- Triiodo-1,3-phthalamide, sodium hydroxide-sodium borate buffer solution (sodium hydroxide 4.8g, water 100g, add sodium borate dodecahydrate to adjust the pH to 12.5), tetrahydrofuran 30ml, stir to dissolve, add 14.5 g (0.18mol) chloroethanol, warmed up to 50°C, and stirred for 11.5h. Use 5 mol / L hydrochloric acid solution to adjust the pH of the reaction solution to 6.0, add 26 g (0.317 mol) of anhydrous sodium acetate, and add concentrated hydrochloric acid dropwise under stirring to adjust the pH to 6.5. Heat to reflux for 13 hours, during which time 5mol / L hydrochloric acid is added to adjust the pH and maintain it at 5.5-6.5. The HPLC detection purity was 96.4%, wherein the impurity B was 1.32%, and the impurity D was 0.12% (direct sampling for detection).
[0042] Afte...
Embodiment 16
[0052] Embodiment 16: the synthesis of ioversol
[0053] In a three-necked flask equipped with a stirrer and a reflux condenser, add 50g (0.06mol) of 5-chloroacetamido-N,N'-bis-(2,3-dihydroxypropyl)-2,4,6- Triiodo-1,3-phthalamide, sodium hydroxide-sodium borate buffer (sodium hydroxide 4.8g, water 100g, add sodium borate 12 water to adjust the pH to 11.5), ethylene glycol 20ml, stir to dissolve, add 15.4g (0.19mol) of chloroethanol was heated up to 50°C and stirred for 15h. Use 5 mol / L hydrochloric acid solution to adjust the pH of the reaction solution to 6.0, add 26 g (0.317 mol) of anhydrous sodium acetate, and add concentrated hydrochloric acid dropwise under stirring to adjust the pH to 6.5. Heat to reflux for 15 hours, during which 5mol / L hydrochloric acid is added to adjust the pH and maintain it at 5.5-6.5.
[0054] The reaction was completed, and the refining method was referred to Example 1 to obtain 36.7 g of a white solid with a yield of 75.9%. The HPLC detectio...
Embodiment 17
[0055] Embodiment 17: the synthesis of ioversol
[0056] In a three-necked flask equipped with a stirrer and a reflux condenser, add 50g (0.06mol) of 5-chloroacetamido-N,N'-bis-(2,3-dihydroxypropyl)-2,4,6- Triiodo-1,3-phthalamide, sodium hydroxide-sodium borate buffer solution (sodium hydroxide 4.8g, water 100g, add sodium borate 12 water to adjust the pH to 13), tetrahydrofuran 50ml, stir to dissolve, add 14.5g (0.18mol) chloroethanol, the temperature was raised to 50°C, and the reaction was stirred for 10h. Use 5 mol / L hydrochloric acid solution to adjust the pH of the reaction solution to 6.0, add 26 g (0.317 mol) of anhydrous sodium acetate, and add concentrated hydrochloric acid dropwise under stirring to adjust the pH to 6.5. Heat to reflux for 11 hours, during which time 5 mol / L hydrochloric acid is added to adjust the pH and maintain it at 5.5-6.5. The purity detected by HPLC was 96.4%, the impurity B was 1.32%, and the impurity D was 0.02%.
[0057] The reaction wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com