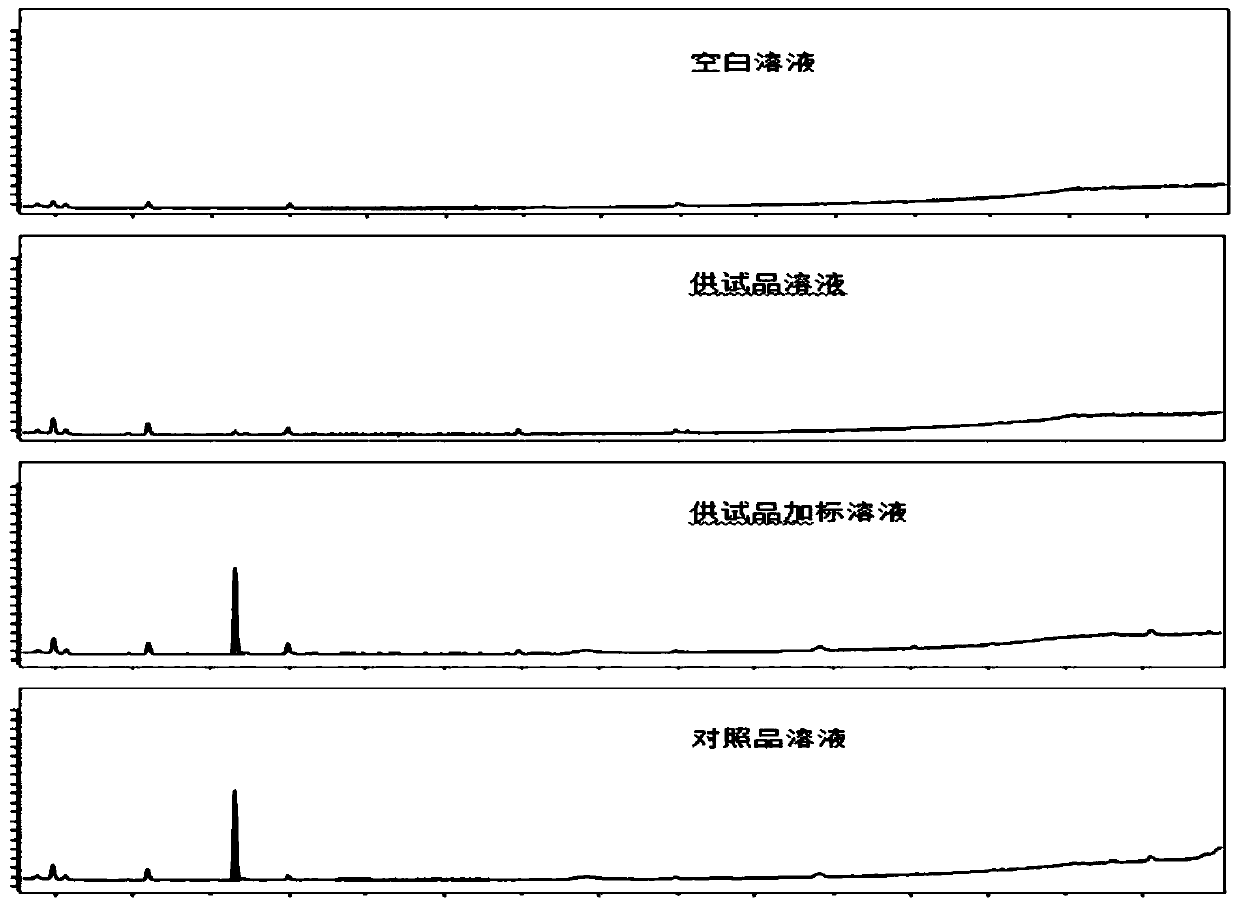
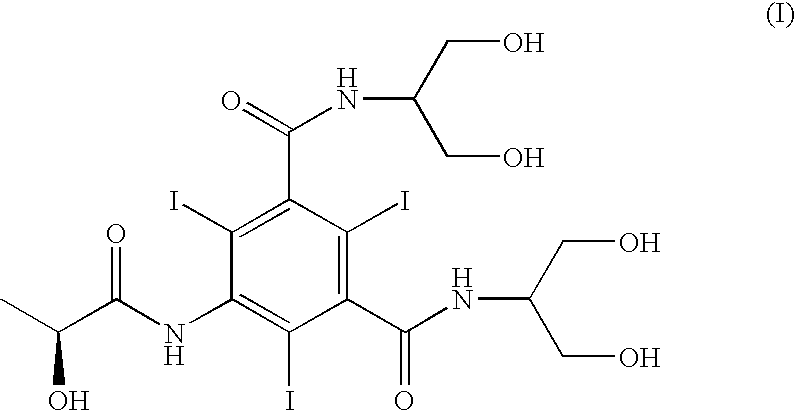
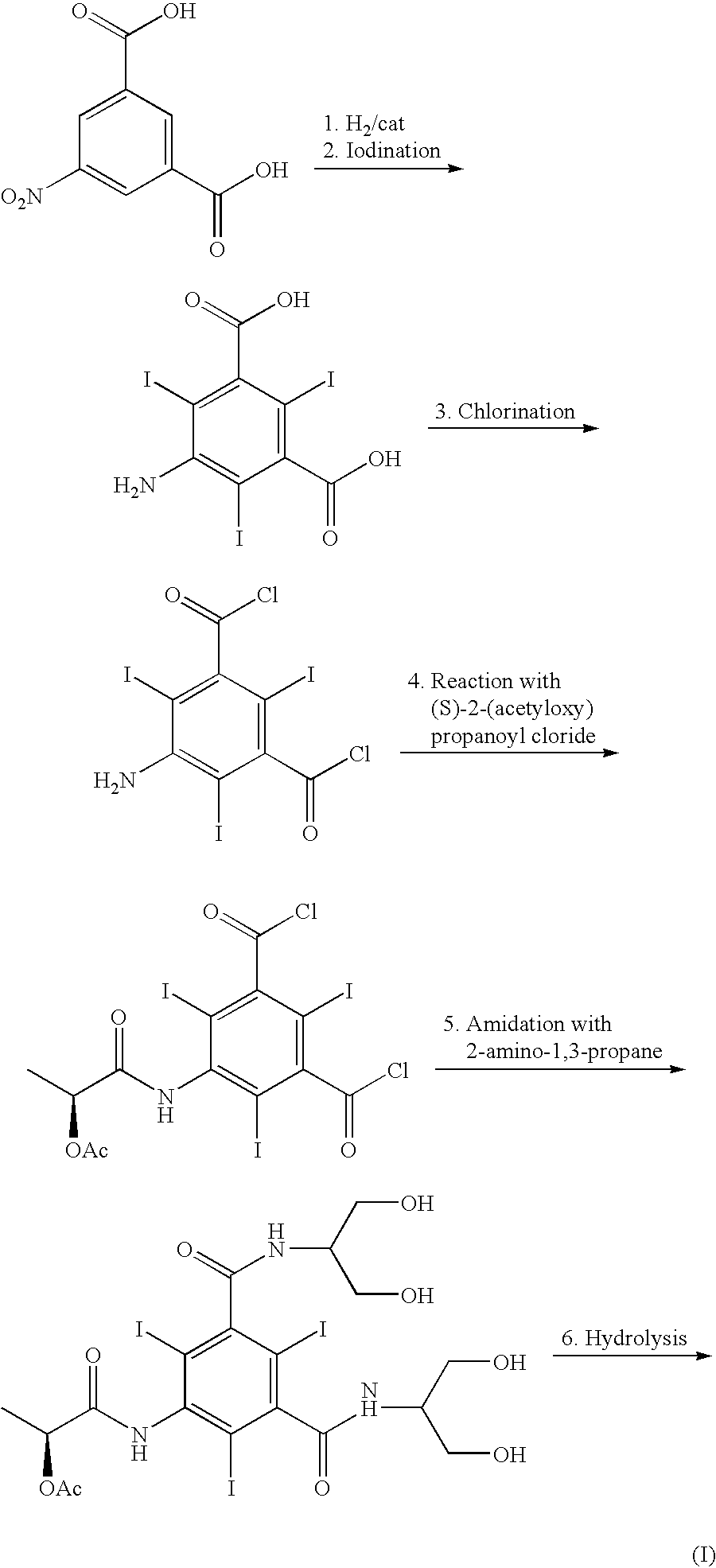
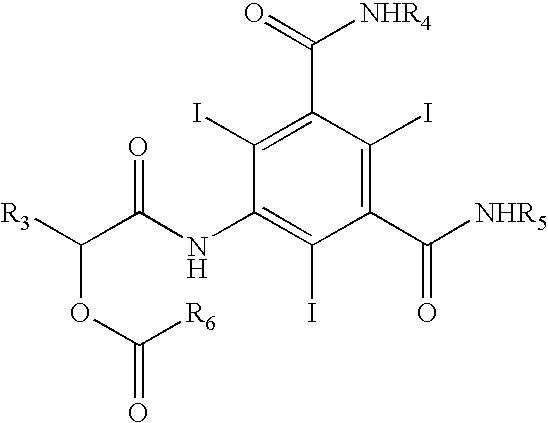
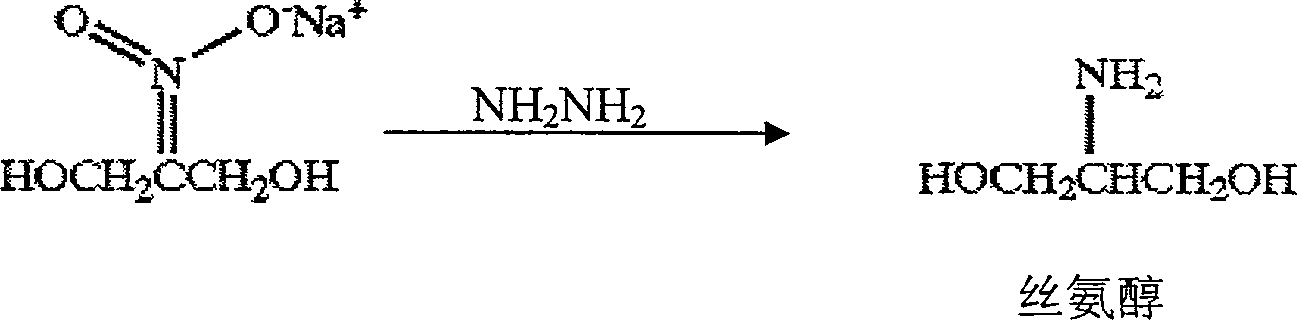Patents
Literature
36 results about "Iopamidol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
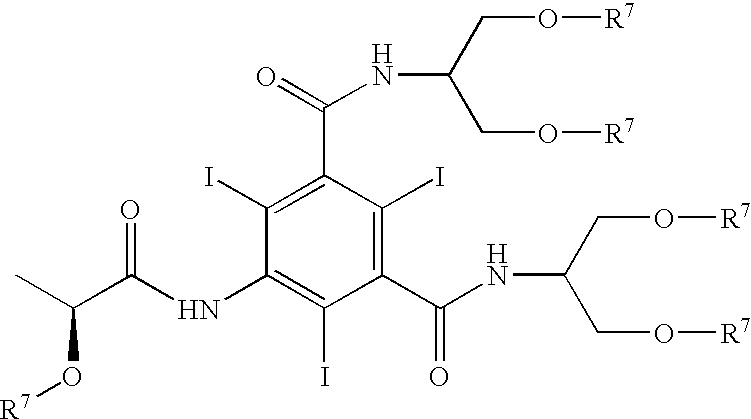
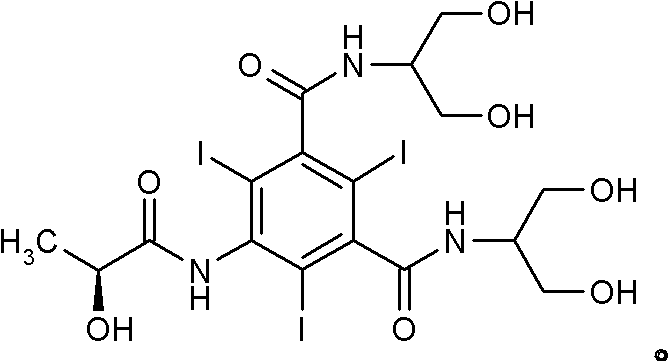
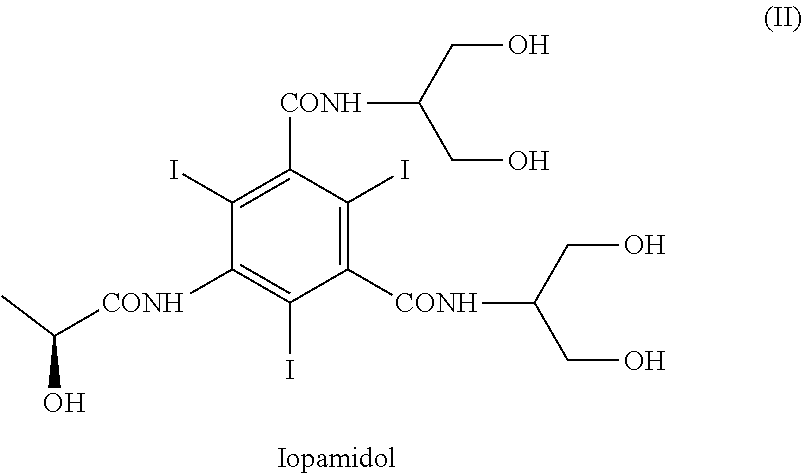
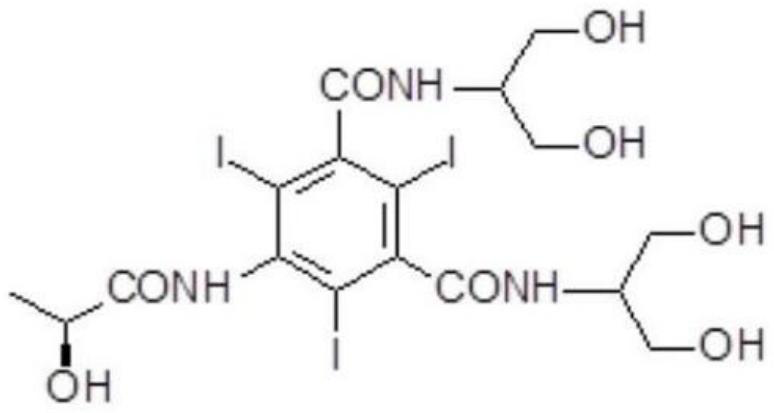
Iopamidol (INN, trade names Iopamiro, Isovue, Iopamiron, and Niopam) is a nonionic, low-osmolar iodinated contrast agent, developed by Bracco. It is available in various concentrations, from 200 to 370 mgI/mL.
Bismuth selenide nanometer material, preparation method and applications thereof
InactiveCN103288061AEasy to makeLow toxicityMaterial nanotechnologyX-ray constrast preparationsDispersityX-ray
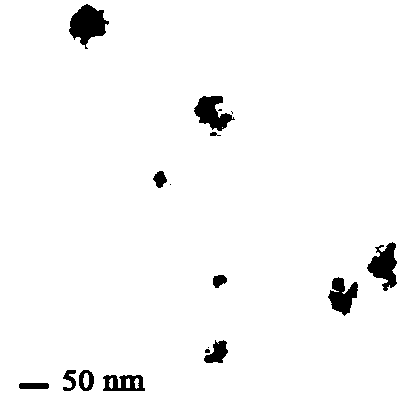
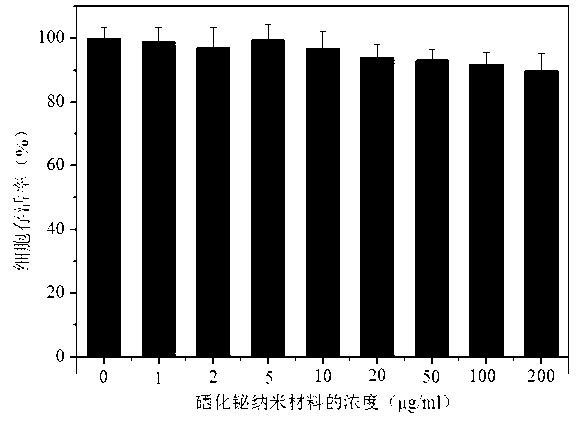
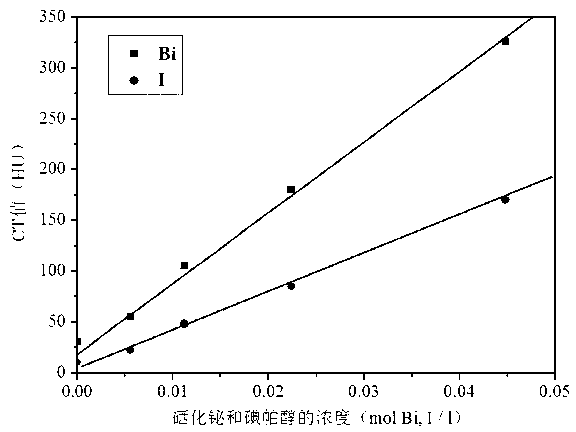
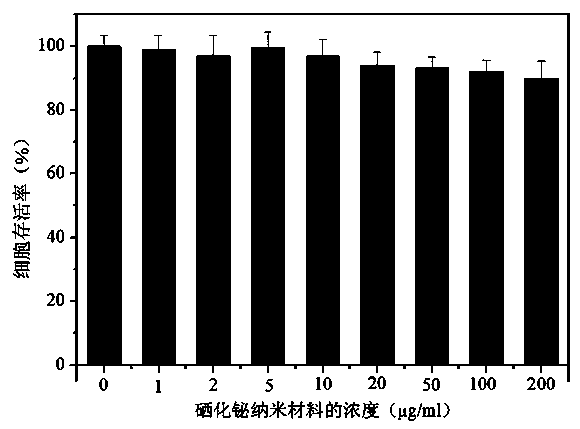
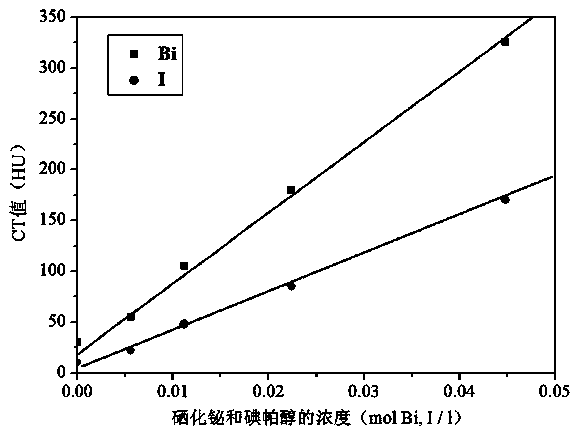
The present invention discloses a bismuth selenide nanometer material, a preparation method and applications thereof. The preparation method comprises: dissolving a bismuth salt, a selenium-containing compound and a stabilizer in a solvent, carrying out an ultrasonic treatment, uniformly mixing, heating to a temperature of 80-200 DEG C, adding a reducing agent, cooling to a room temperature after completing a reaction, and carrying out centrifugation separation washing three times to obtain the bismuth selenide nanometer material with a diameter of 20-200 nm. The preparation method has characteristics of simple operation, less time consuming, low energy consumption and easy scalization. The prepared particles have a uniform particle size and good dispersity. With application of the bismuth selenide nanometer material as a living body X-ray CT imaging contrast agent, a significant imaging effect is provided compared with imaging effects of the traditional contrast agents such as an iodine reagent iopamidol and the like so as to provide important application prospects in the future tumor imaging field.
Owner:FUZHOU UNIV
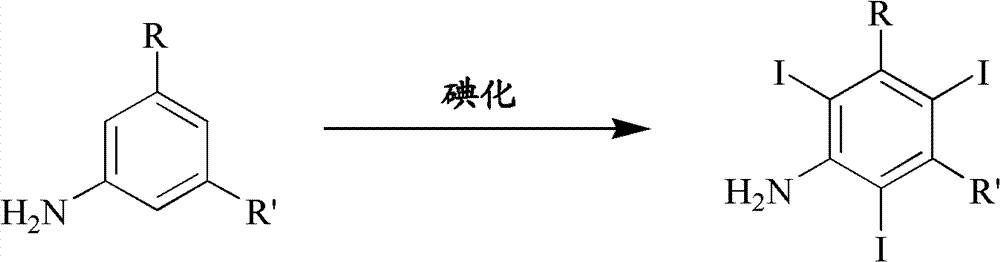
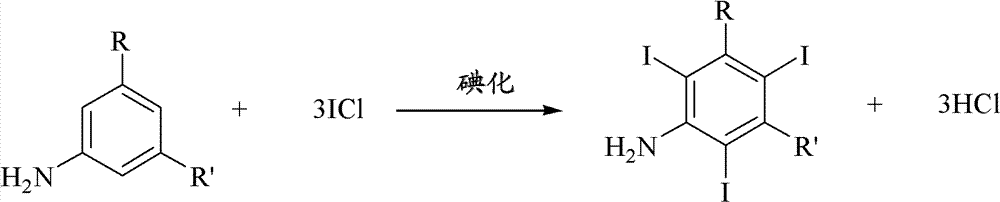
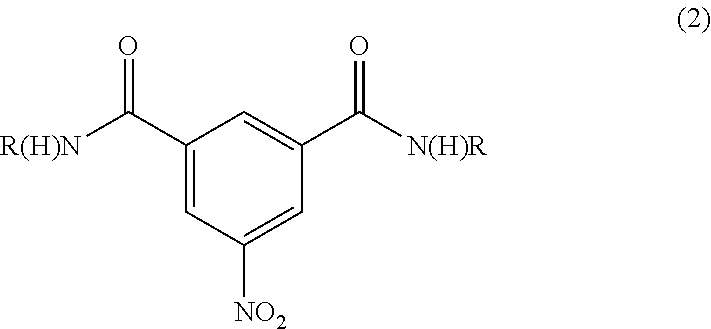
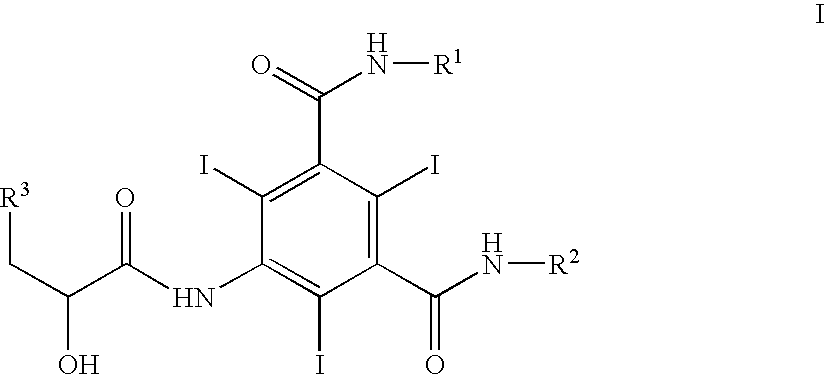
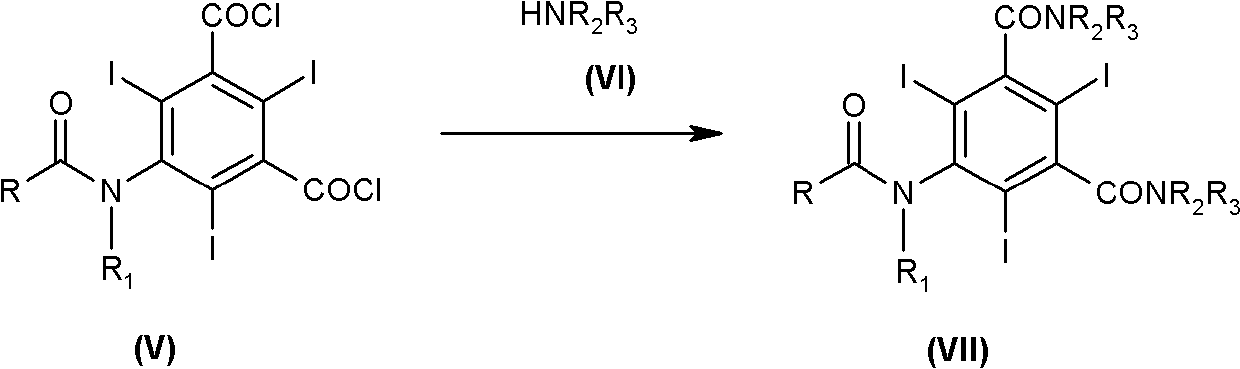
Iodination method for preparing 3,5-disubstituted-2,4,6-triiodo aromatic amine compound
InactiveCN103086915AOrganic compound preparationCarboxylic acid amides preparationIodixanolIodination reaction
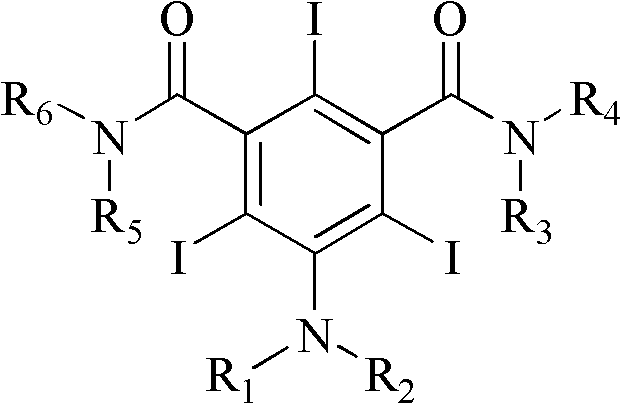
The invention discloses an improved method for preparing 3,5-disubstituted-2,4,6-triiodo aromatic amine represented by a formula (II), wherein R1 and R2 are defined in the instruction, and the compound represented by the formula (II) is a key intermediate for synthesizing iopamidol, iohexol, iodixanol and a series of non-ionic contrast agents. The method comprises: adopting a chlorine-free iodination reagent and a 3,5-disubstituted aromatic amine compound to carry out an iodination reaction to obtain the 3,5-disubstituted-2,4,6-triiodo aromatic amine represented by the formula (II), wherein a mole yield of the iodination reaction can be 89%.
Owner:上海亿脉利医药科技有限公司
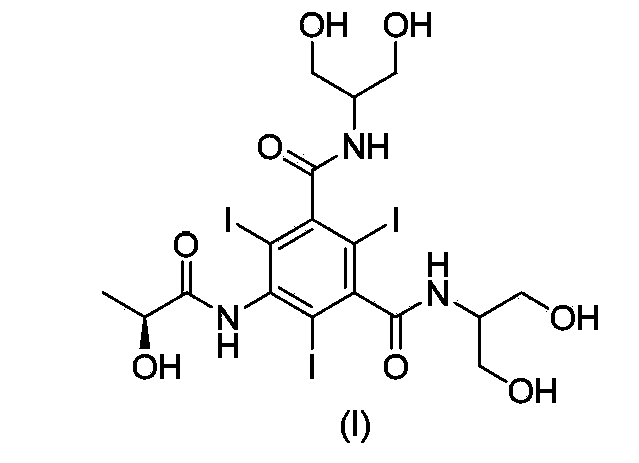
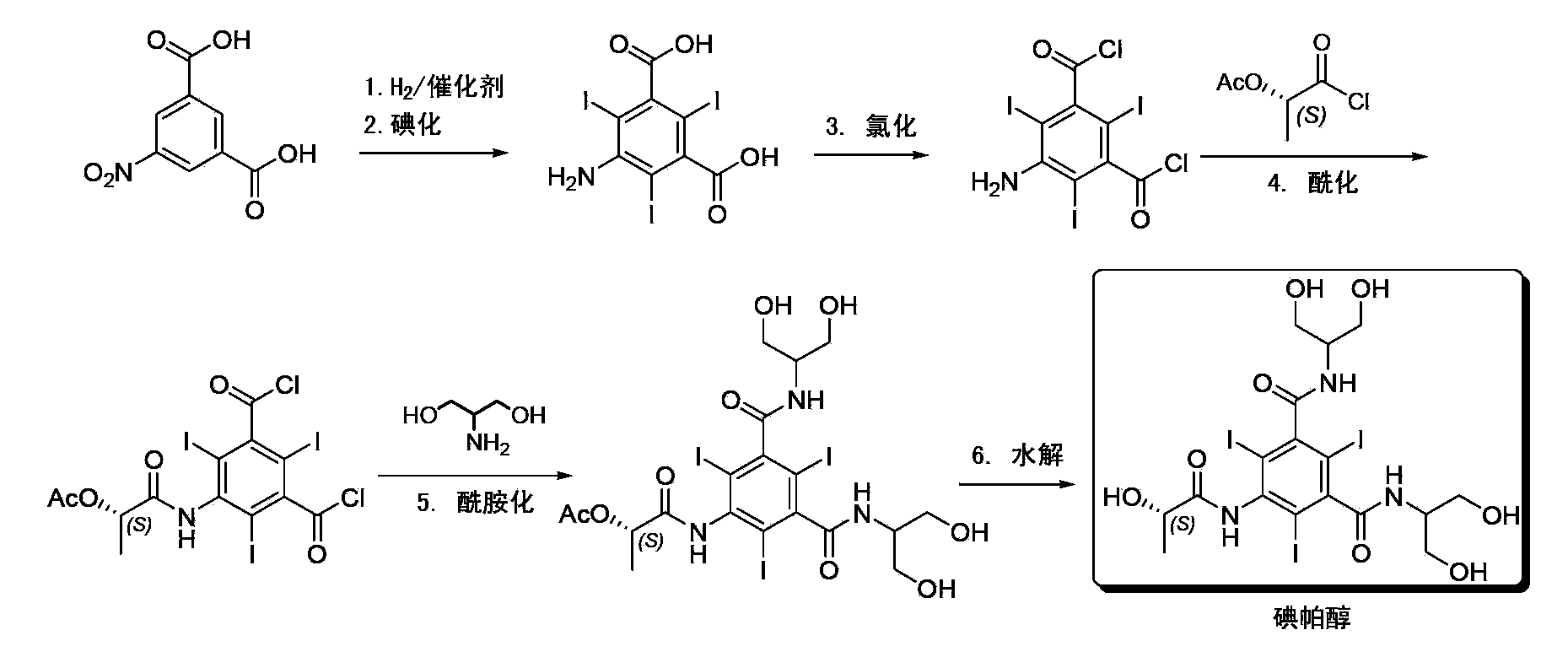
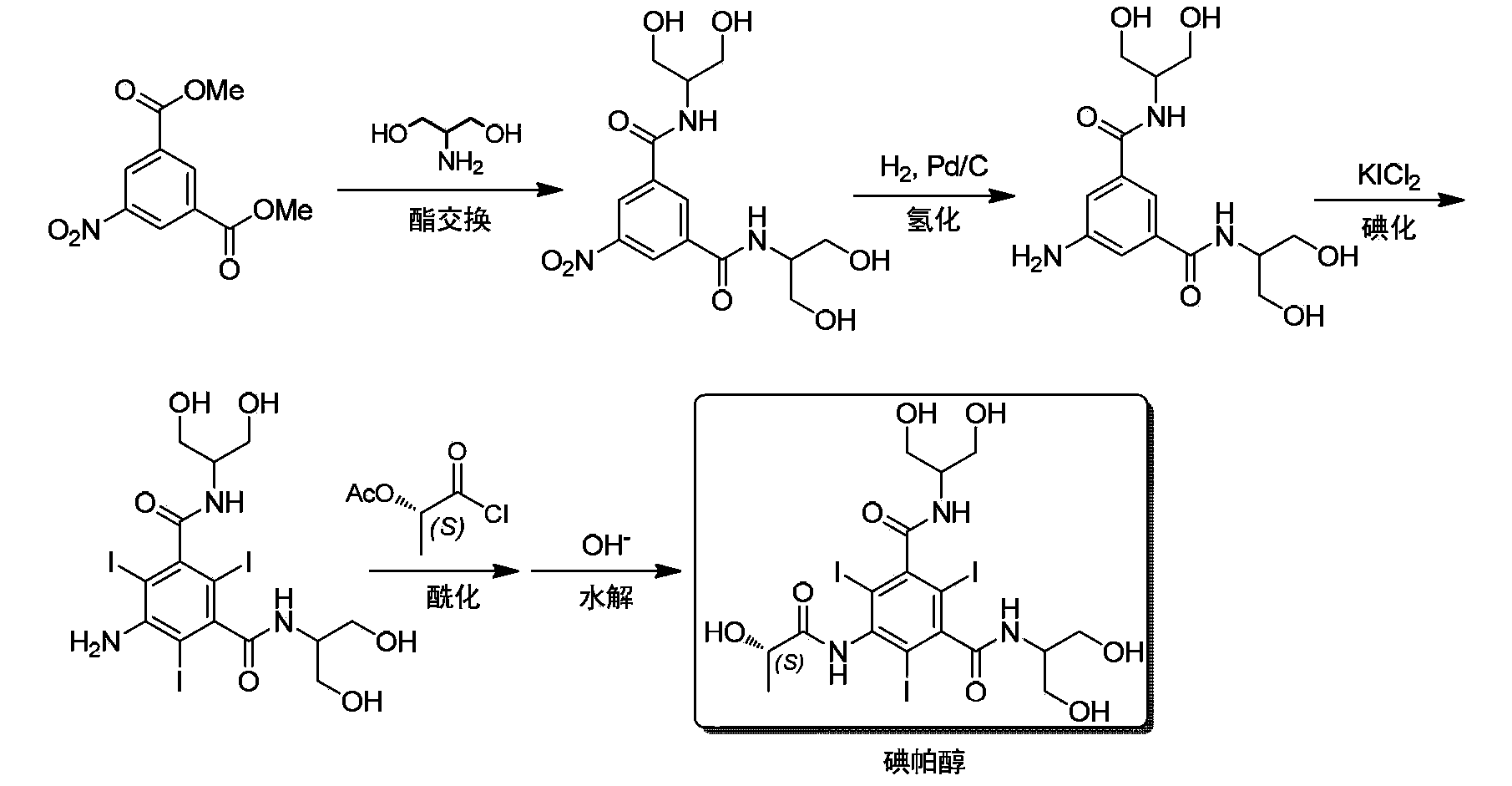
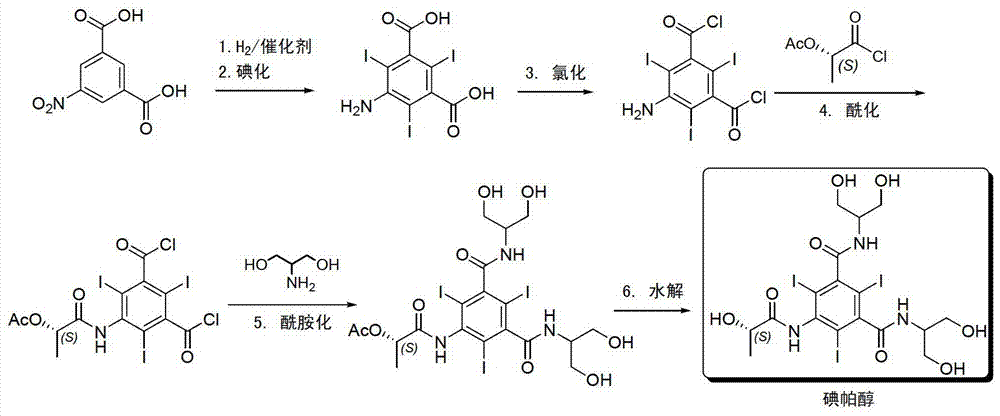
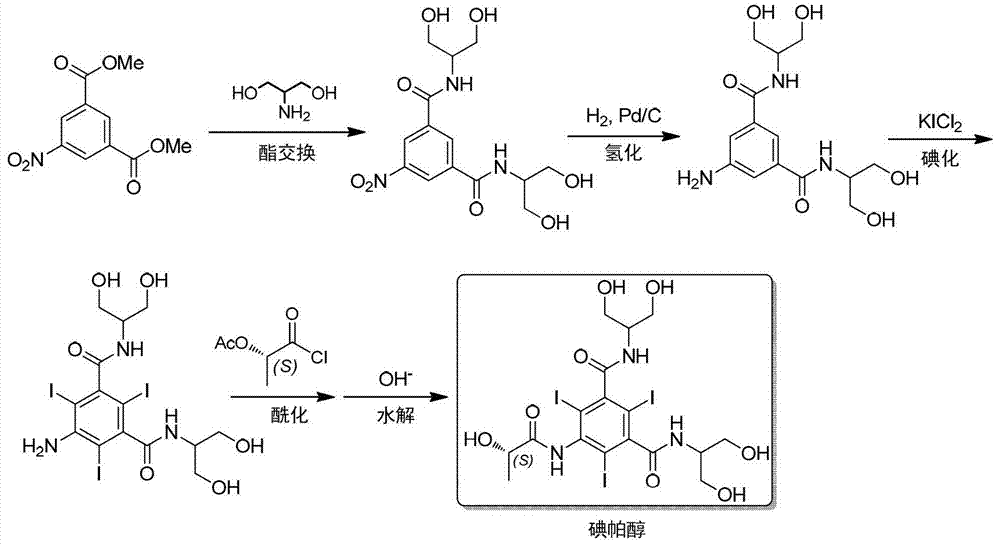
Synthesis of iopamidol and preparation of iopamidol synthesis intermediate
ActiveCN103382160AEasy to separateReduce difficultyOrganic compound preparationCarboxylic acid amides preparationPropionyl chlorideIodine
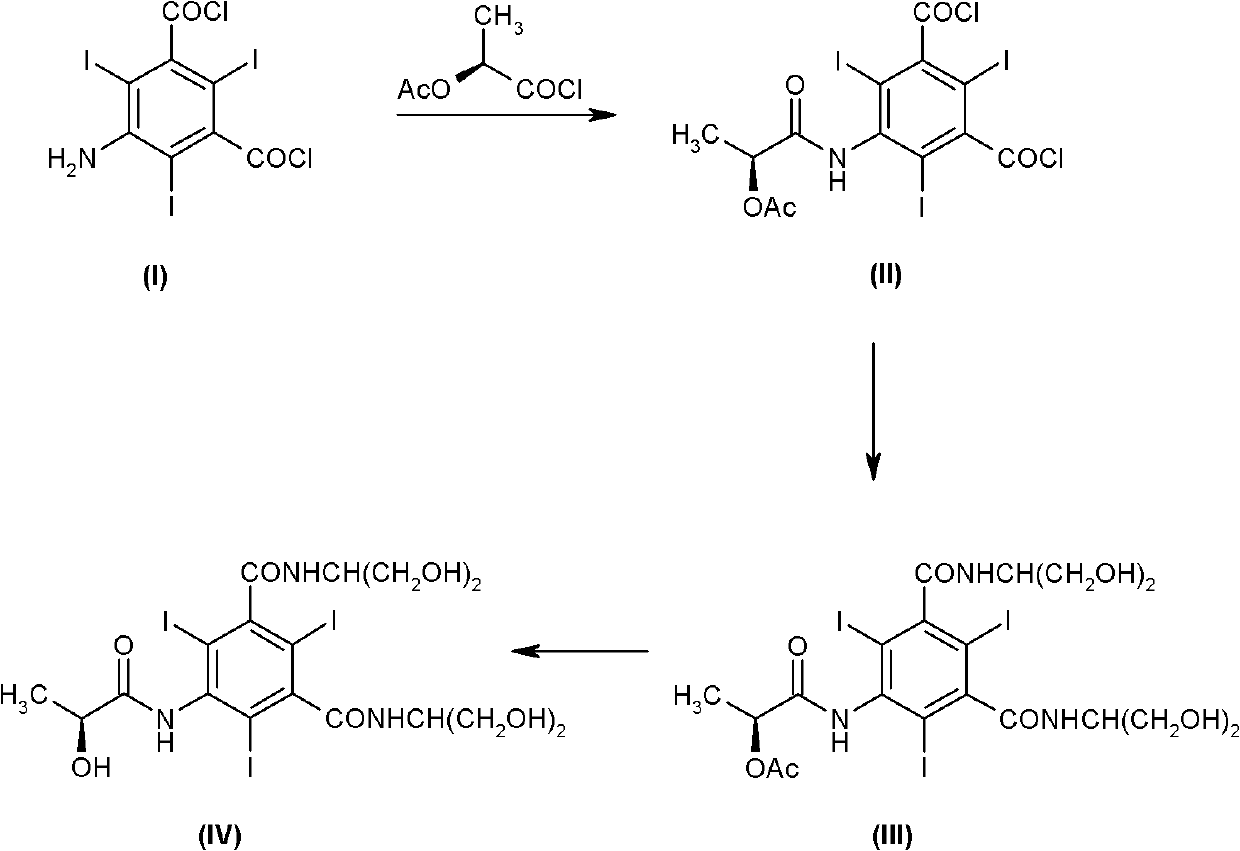
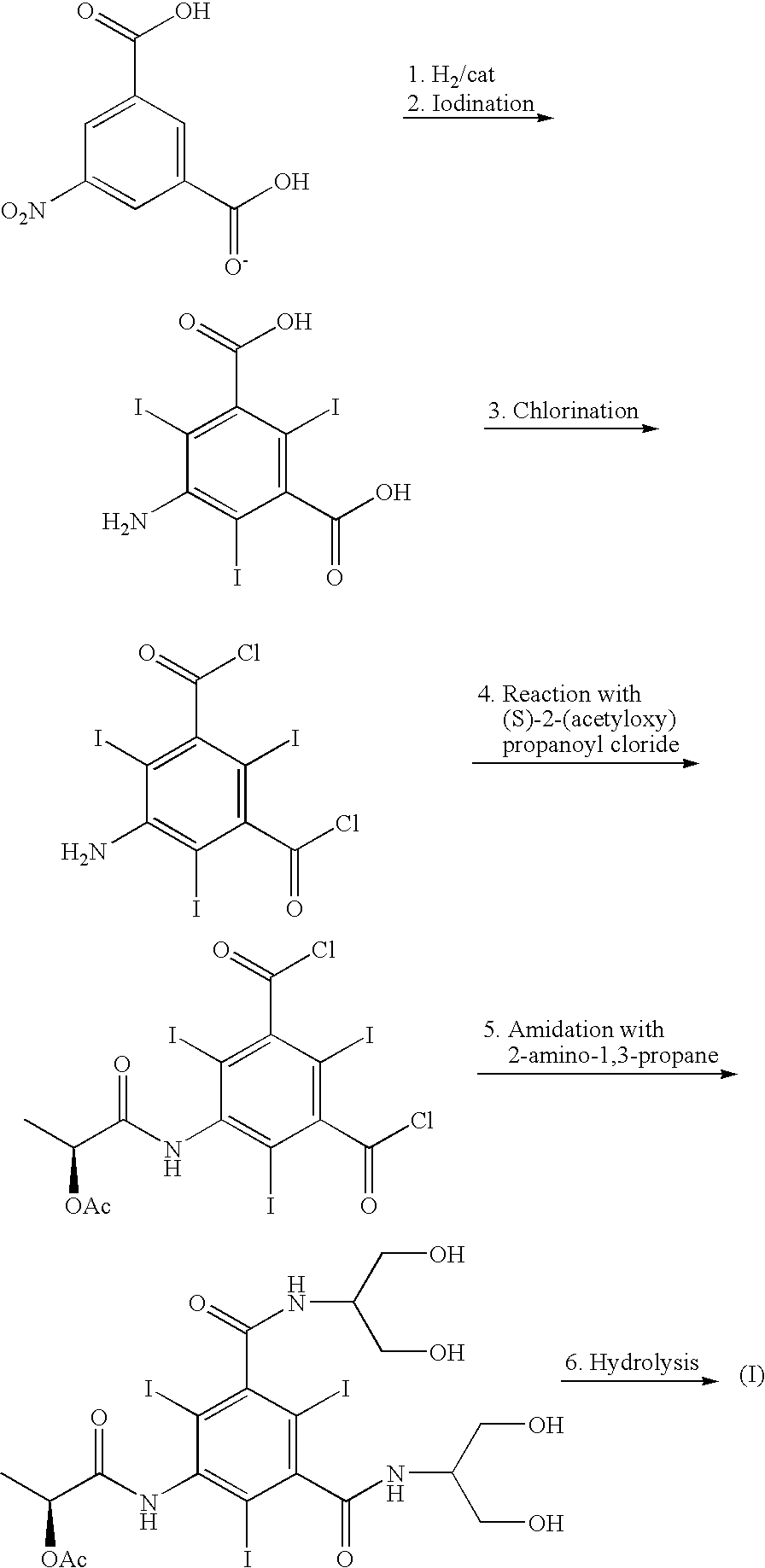
The invention discloses a method for preparation of (S)-N,N'-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-5-[(2-hydroxy-1-oxopropyl)amino]-2,4,6-triiodo-1,3-benzenedicarboxamide (iopamidol) shown in the formula I from 5-amino-N,N'-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-2,4,6-triiodo-1,3-benzenedicarboxamide shown in the formula II. The method comprises the following steps that a, the compound shown in the formula II and a mixed anhydride as an appropriate protective agent undergo a reaction to produce a mixed ester shown in the formula III; b, the mixed ester shown in the formula III and (S)-2-(acetoxy)propionyl chloride undergo a reaction so that an amino group at the 5th site is acylated and a compound shown in the formula IV is obtained; and c, the compound shown in the formula IV undergoes a hydrolysis reaction under acidic or alkaline conditions or undergoes an alcoholysis reaction so that all acyl groups of the compound shown in the formula IV are removed and the iopamidol shown in the formula III is obtained. The invention relates to a synthesis intermediate of the iopamidol shown in the formula III.
Owner:ZHEJIANG HISYN PHARMA
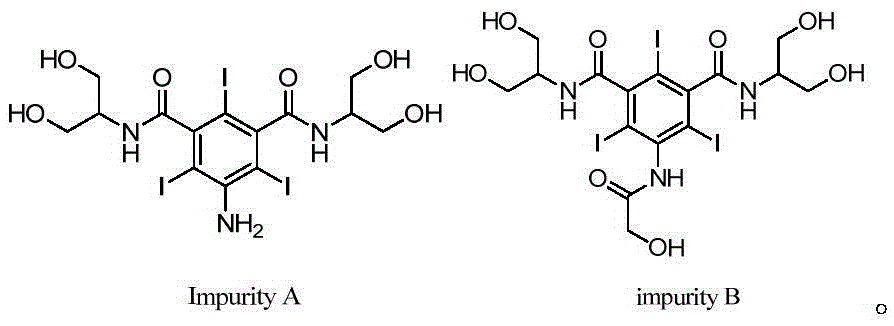
Synthetic methods of an impurity A and an impurity B of iopamidol
InactiveCN105461581ASimple categoryHigh purityOrganic compound preparationCarboxylic acid amides preparationRadiographic contrast mediaCombinatorial chemistry
The invention relates to synthetic methods of an impurity A and an impurity B of iopamidol that is a nonionic X-radiographic contrast medium. 5-amino-1,3-phthaloyl dichloride is adopted as an initial raw material. The impurity A and the impurity B of the iopamidol can provide qualified contrasts for quality control of the iopamidol.
Owner:ZHEJIANG HAIZHOU PHARMA CO LTD
Synthesis of N1,N3-BIS(2,3-DIHYDROXYPROPYL)-5-NITROISOPHTHALAMIDE
ActiveUS20140200367A1High purityOrganic compound preparationCarboxylic acid amides preparationIoversolCombinatorial chemistry
The present disclosure generally relates to a new process for the preparation of high purity 5-nitro-isophthalamide compounds, which are useful as intermediates for the preparation of imaging agents, such as iodinated x-ray contrast imaging agents like ioversol, iohexyl and iopamidol.
Owner:LIEBEL FLARSHEIM CO
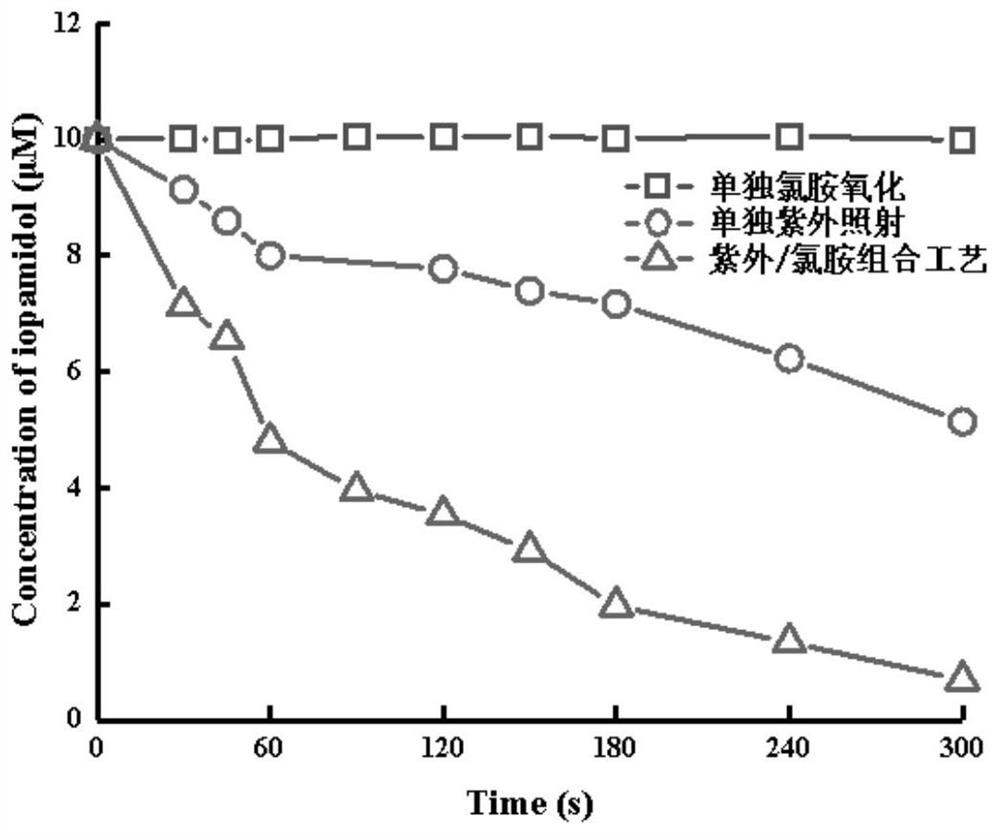
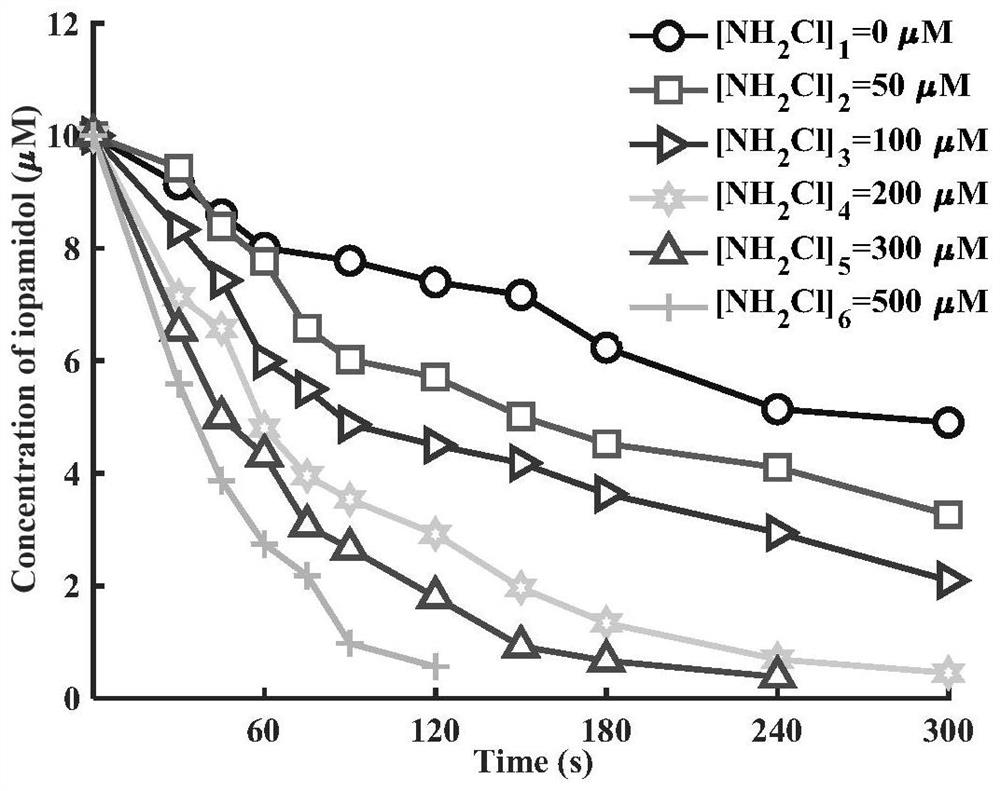
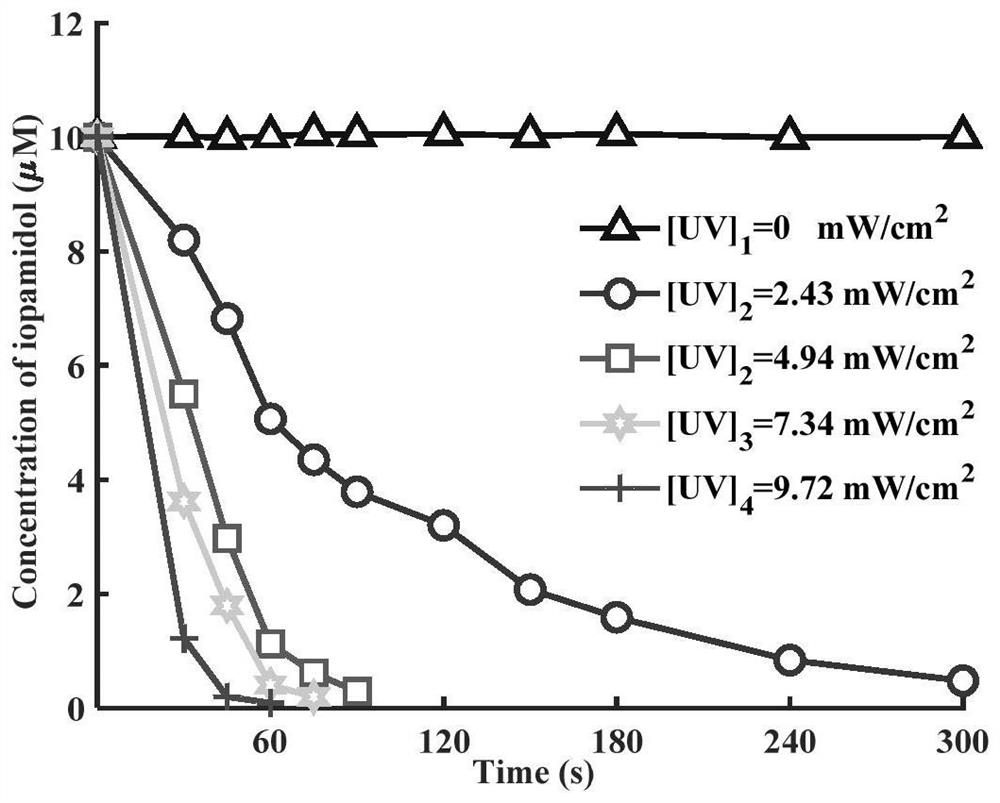
Method for removing iopamidol in water by UV/NH2Cl combined process
PendingCN111762840AEfficient removalSimple methodWater/sewage treatment by irradiationWater treatment compoundsHazardous substanceOrganopónicos
The invention relates to a method for removing iopamidol in water by a UV / NH2Cl combined process. The method comprises the following specific steps of: (1) pretreating a water sample to be treated; and (2) adding an NH2Cl solution into the water sample pretreated in the step (1), adjusting the pH value, and carrying out a photocatalytic oxidation reaction by ultraviolet irradiation to remove iopamidol in the water. According to the method of the invention, the pH value of the reaction water body is adjusted, so that the content of the iopamidol in the water body can be quickly reduced with theremoval effect reaching 99 percent or higher; degradation is quite complete; organic pollutant iopamidol existing in drinking water can be rapidly and effectively reduced; the potential risk of generating high-toxicity iodinated disinfection byproducts can be greatly reduced; the method is simple to operate; reaction conditions of the method are easy to control; used chemical reagents and materials are common products for water treatment, other toxic and harmful substances are not introduced, so that the safety is particularly outstanding; and the reaction conditions are easy to realize.
Owner:SHANGHAI INSTITUTE OF TECHNOLOGY
Process for the preparation of iopamidol
InactiveUS20050192465A1Organic compound preparationCarboxylic acid amides preparationPhotochemistryIopamidol
Owner:BRACCO IMAGINIG SPA
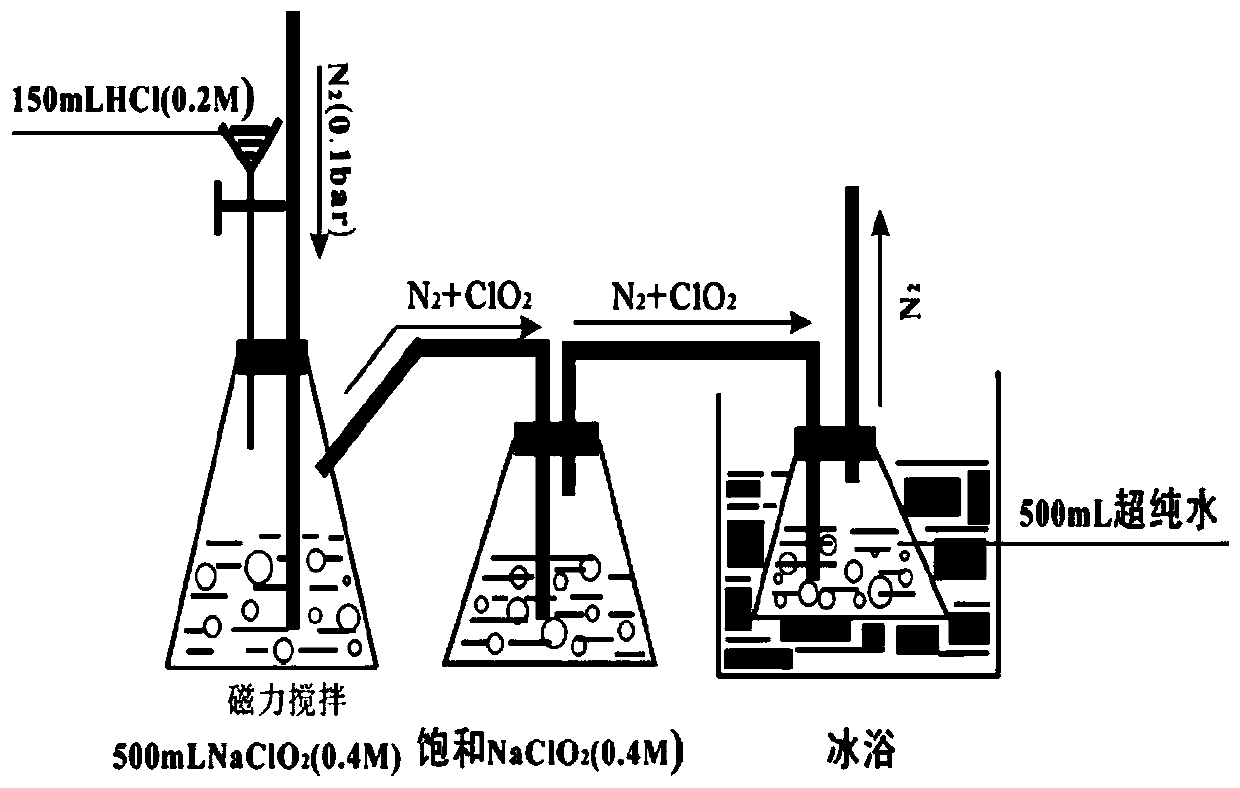
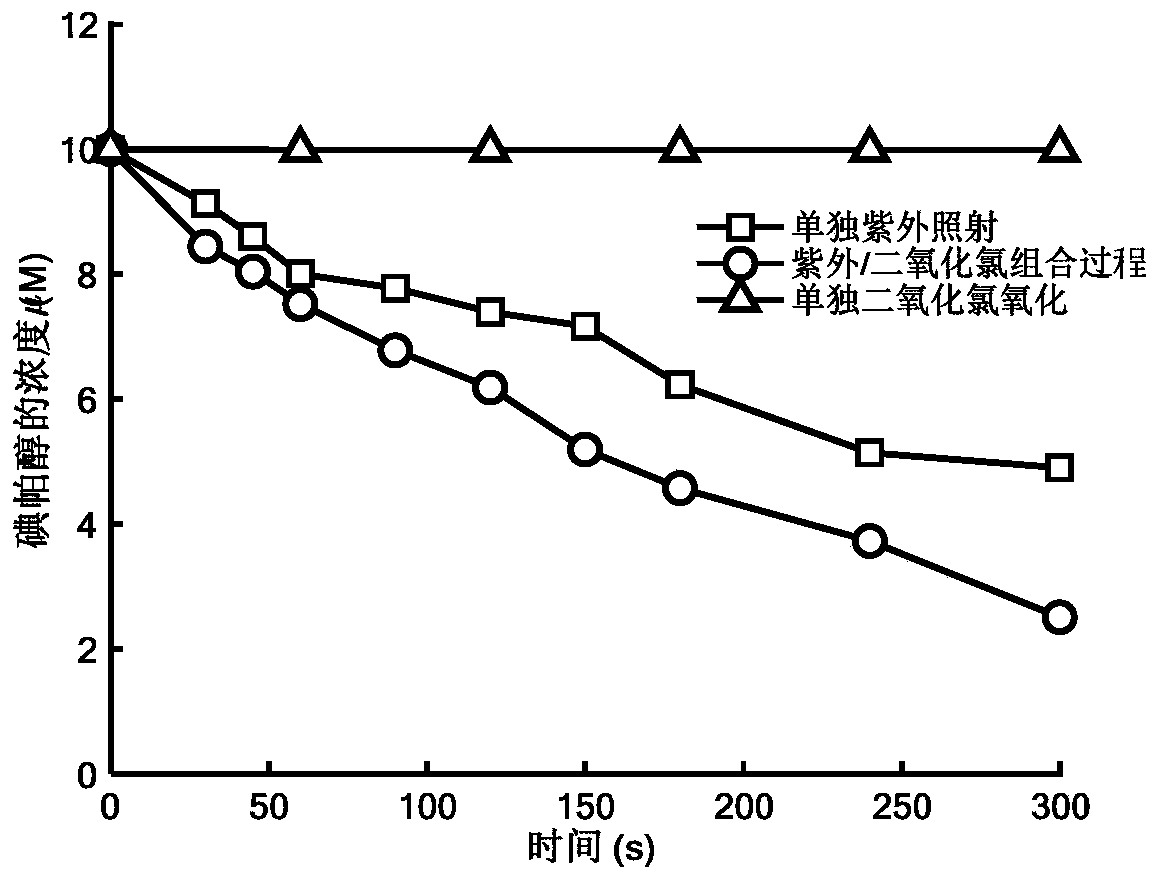
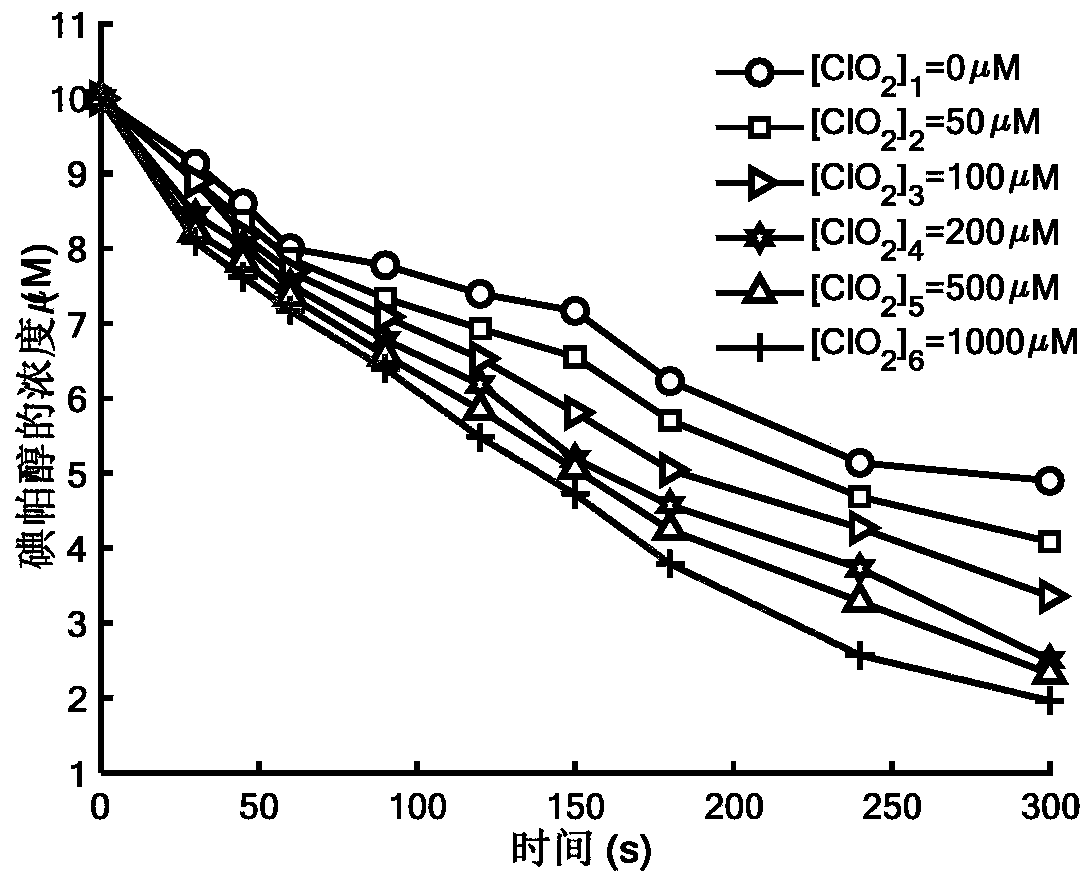
Method for removing iopamidol in water through ultraviolet/chlorine dioxide combined process
InactiveCN110272157AEfficient removalSimple methodWater treatment parameter controlWater/sewage treatment by irradiationChlorine dioxideHazardous substance
The invention relates to a method for removing iopamiol in water through the ultraviolet / chlorine dioxide combined process. The method specifically comprises the following steps that (1), a to-be-treated water sample is pre-treated; (2), a ClO2 solution is added in the water sample obtained after pretreatment in step (1), the pH value is adjusted, a photocatalytic oxidation reaction is conducted through ultraviolet irradiation, iopamidol in the water is removed. Compared with the prior art, the method is combined with the UV / ClO2 combination process, by adjusting the pH of a reaction water body, the content of iopamidol in the water body can be rapidly reduced, the removal effect can reach 99% or above, degradation is thorough, and the degree of mineralization is high, the potential risk that iopamidol existing in drinking water generates I-DBPs in the drinking water can be rapidly lowered, operation is easy, the reaction conditions are easy to control, applied chemical reagents and materials are conventional products for water treatment, other toxic and harmful substances are not introduced, the safety is particularly prominent, and the reaction environment is easy to achieve.
Owner:SHANGHAI INST OF TECH
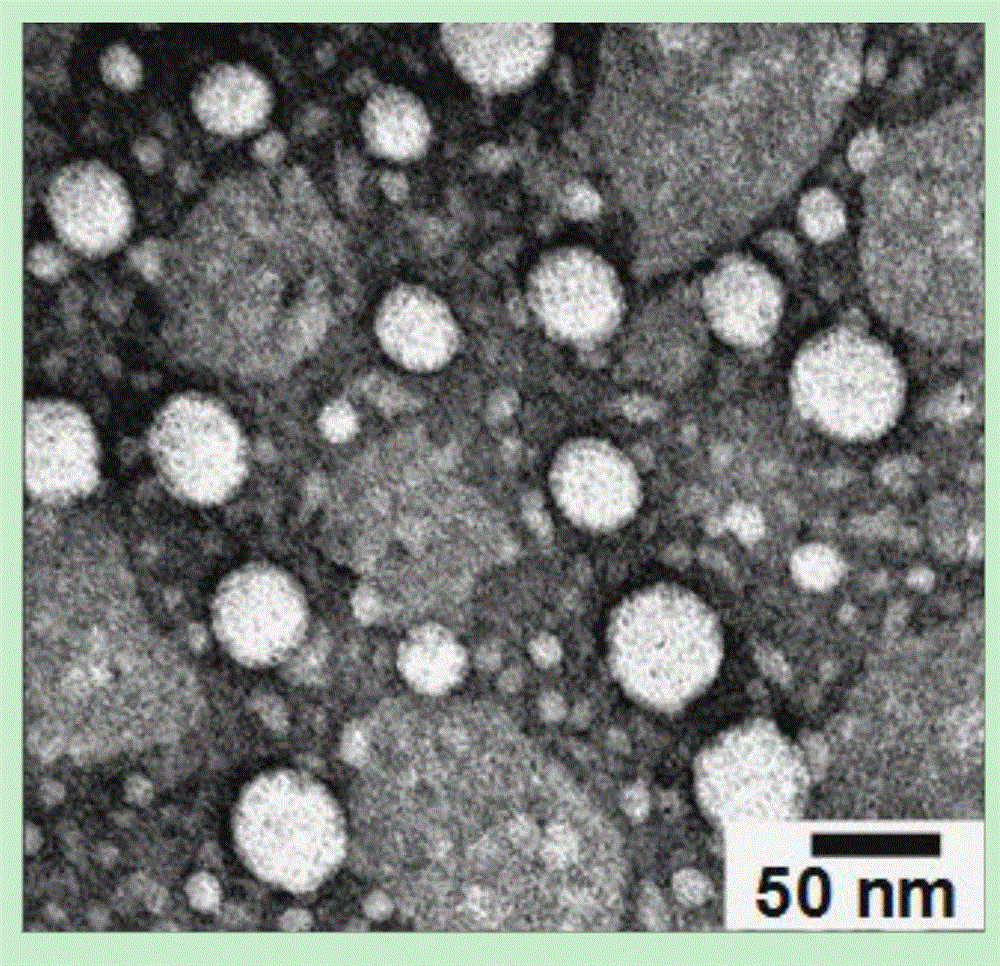
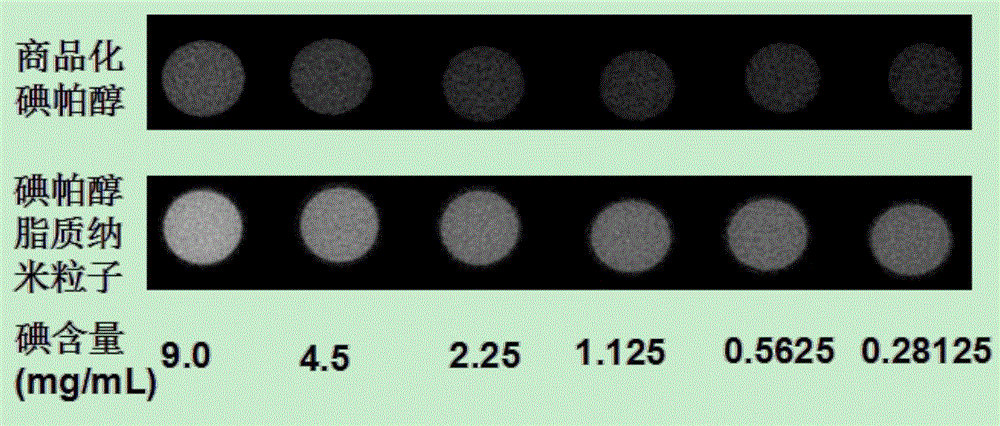
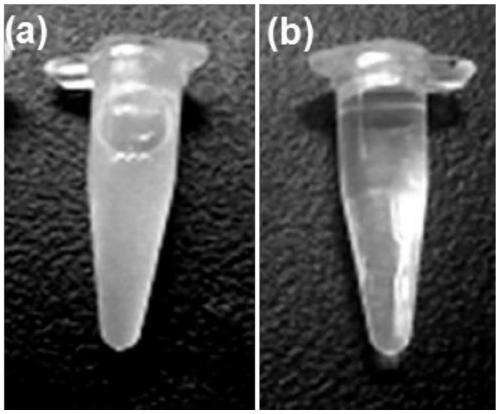
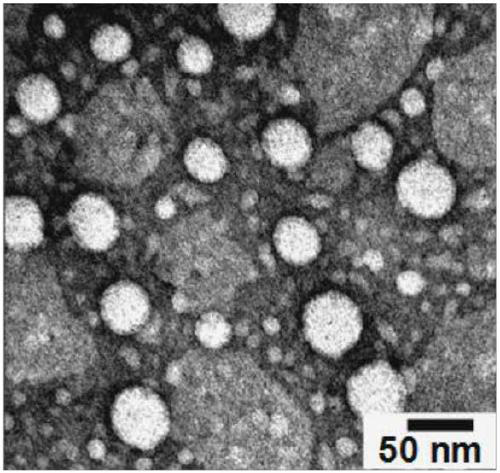
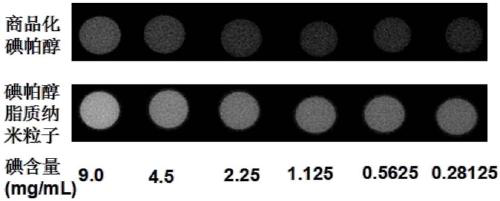
Contrast medium based on iopamidol lipid derivative as well as preparation method and application of contrast medium
ActiveCN106831480ASave raw materialsMild reaction conditionsOrganic compound preparationX-ray constrast preparationsNanoparticleBiocompatibility Testing
The invention discloses a contrast medium based on an iopamidol lipid derivative, as well as a preparation method and application of the contrast medium. The lipid molecule structure comprises both hydrophobic aliphatic chains and hydrophilic iopamidol groups which can be combined to form nanoparticle structures by selves in a water solution, and in addition, as the aliphatic chains and the iopamidol can be connected through degradable ester bonds, the biocompatibility of the contrast medium can be further improved; moreover, as covalent bonds in the molecule structures of such substances are connected with iopamidol functional groups, the content of iopamidol in nanoparticles can be increased, and meanwhile the problems that the contrast medium is released and leaked too early in the particle circulation process can be effectively avoided. The nanoparticles formed by lipid of the contrast medium can be used as a functional material which is used as a contrast medium and a carrier of various medicines and other contrast mediums, thereby having wide clinical application prospects.
Owner:PEKING UNIV
Process for the preparation of iodinated contrast agent
The present invention relies on a process for the preparation of non ionic iodinated contrast agents and, in more details, it relates to a process for the preparation of Iopamidol in high yields and with a high degree of purity. In more details, the invention discloses a process for the preparation of a compound of formula (III) comprising the 5 condensation reaction a compound of formula (II) with 2-amino-1,3-propandiol, being said reaction carried out in an aprotic dipolar solvent and in the presence of an alkaline or alkaline rare earth metal oxide or hydroxide.
Owner:BRACCO IMAGINIG SPA
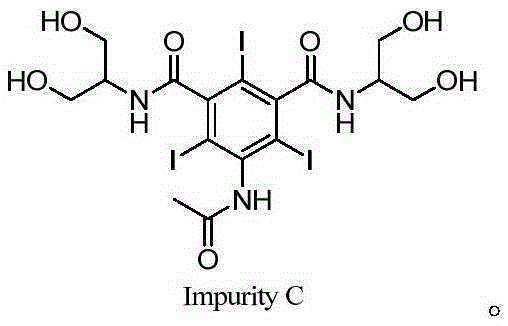
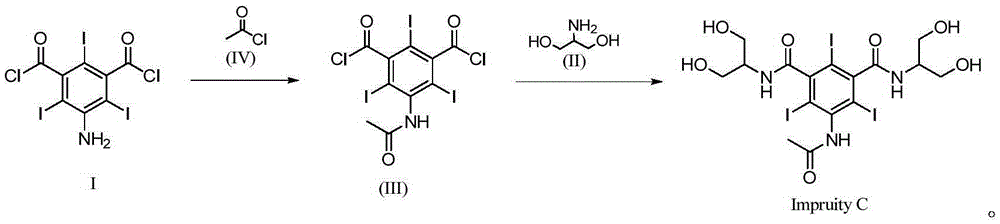
Synthetic method of iopamidol impurity C
InactiveCN105294472ASimple categoryHigh purityOrganic compound preparationCarboxylic acid amides preparationRadiographic Contrast AgentCombinatorial chemistry
The invention relates to a synthetic method of a non-ionic X-radiographic contrast agent iopamidol impurity C. according to the synthetic method, 5-amino-1,3-dibenzoyl dichloride is used as a starting material. The iopamidol impurity C can provides a qualified reference substance for quality control of iopamidol.
Owner:ZHEJIANG HAIZHOU PHARMA CO LTD
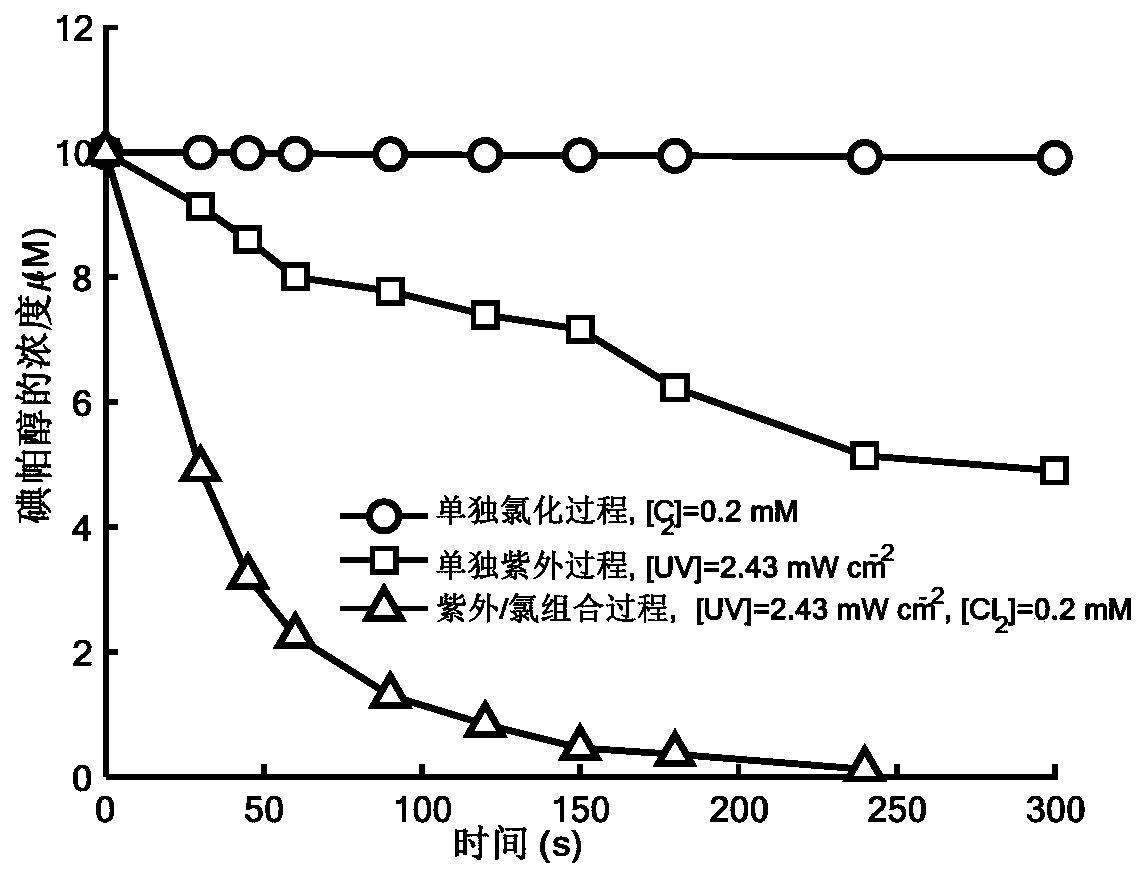
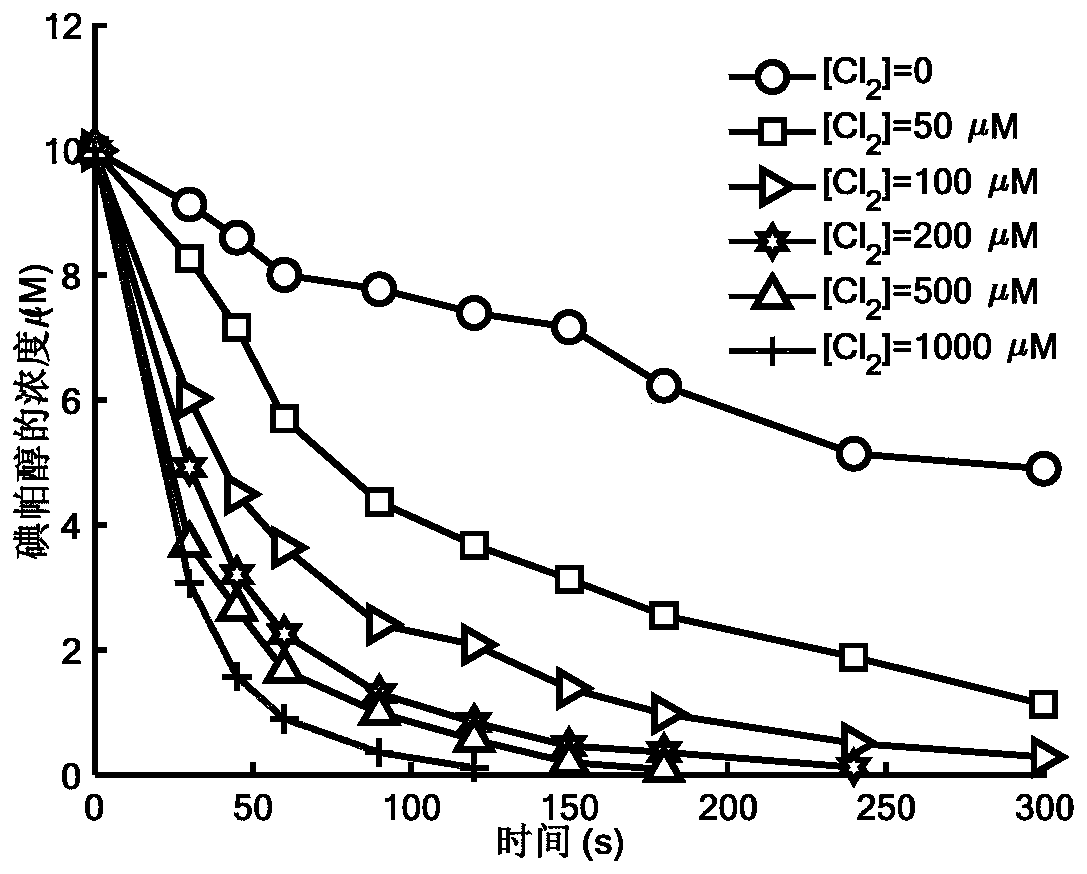
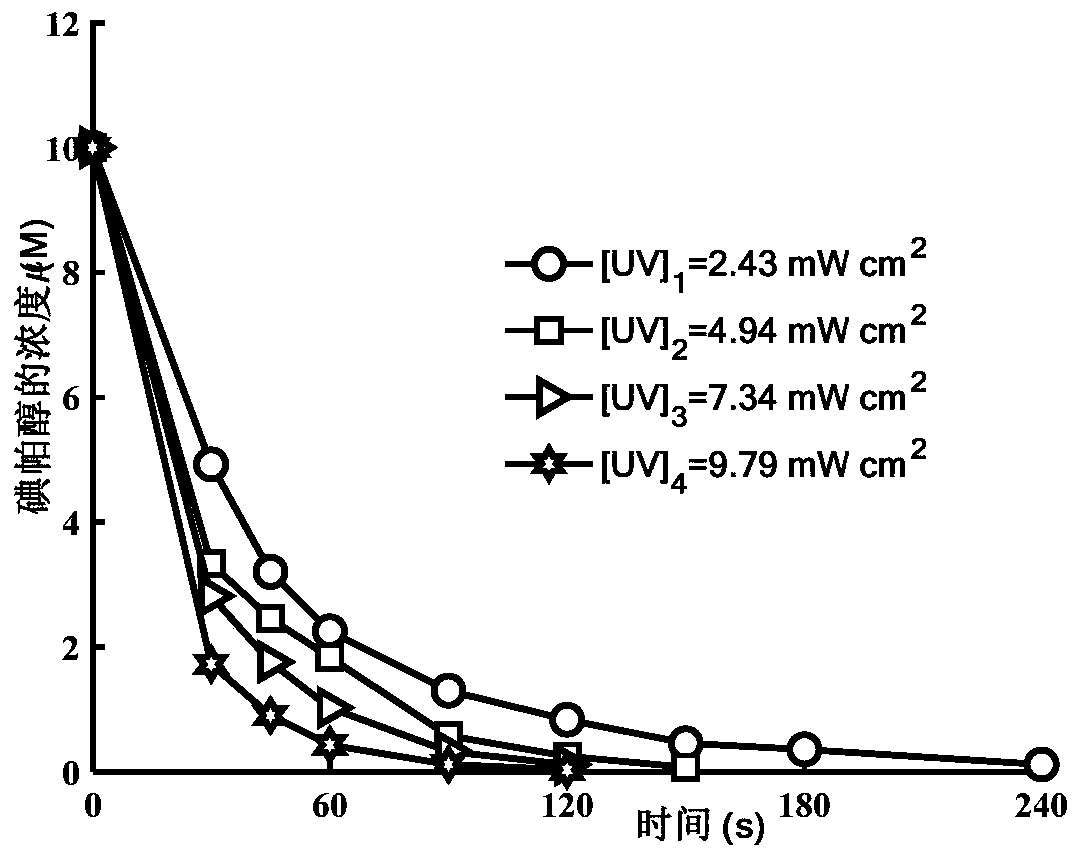
Method for removing iopamidol in water by ultraviolet/chlorine combined process
InactiveCN110282693AReduce contentEasy to operateWater treatment parameter controlWater/sewage treatment by irradiationHazardous substanceTreated water sample
The invention relates to a method for removing iopamidol in water by an ultraviolet / chlorine combined process. The method comprises the following specific steps: (1) pretreating a to-be-treated water sample; (2) adding a solution which contains or can produce free chlorine to the pretreated water sample in step (1), adjusting the pH value, and carrying out an ultraviolet catalytic oxidation reaction to remove the iopamido in the water. Compared with the prior art, according to the method, the pH value of a reaction water body is appropriately adjusted by an ultraviolet / chlorine combined technological process, so that the content of the iopamidol in a water body can be rapidly reduced, and the removal effect can reach 99% or above; the operation is simple; the reaction conditions are easy to control; engineering application is easy to realize; in addition, chemical reagents and materials used are both conventional products for water treatment; no other toxic and harmful substances are introduced; the safety is particularly prominent; the reaction environment is easy to realize; treatment can be carried out under room temperature conditions; the feasibility and operability of the method are effectively improved.
Owner:SHANGHAI INST OF TECH
Refining method of iopamidol intermediate
PendingCN114656370AAvoid generatingSimple production processOrganic compound preparationComponent separationCombinatorial chemistryCatalytic effect
The invention belongs to the field of medicine synthesis, and particularly provides a refining method of an iopamidol intermediate, which is characterized in that a compound shown as a formula II is used for preparing a compound shown as a formula III of the iopamidol intermediate under the catalytic action of Lewis soft acid. According to the technical scheme, the high-purity and high-yield intermediate compound shown in the formula III can be obtained, the yield can reach 99.9%, the reaction is thorough, no raw material is left, the purity can reach 96% or above, and generation of a diiodo-substituted impurity 1 compound is thoroughly avoided. In addition, high-purity and high-yield iopamidol can be prepared by utilizing the intermediate compound as shown in the formula III, so that the technical scheme is more suitable for expanding industrial production.
Owner:HAINAN PULIN PHARMA +2
Preparation method of serinol
InactiveCN100408547CMeet the requirements of the preparationHigh purityOrganic compound preparationAmino-hyroxy compound preparationHydrazine compoundX-ray
The present invention relates to a preparation method of serinol, belonging to the field of serinol preparation technology by using reduction reaction. Said serinol is an important intermediate body for preparing nonionic X-ray contrast agent iopamidol. Said invention uses 2-nitro-1,3-propanediol sodium salt as raw material, uses hydrazine hydrate as reducing agent and uses palladium / carbon as catalyst to prepare serinol at normal pressure.
Owner:JIANGSU INST OF NUCLEAR MEDICINE
A kind of bismuth selenide nanomaterial and its preparation method and application
InactiveCN103288061BEasy to makeLow toxicityMaterial nanotechnologyX-ray constrast preparationsDispersityX-ray
The present invention discloses a bismuth selenide nanometer material, a preparation method and applications thereof. The preparation method comprises: dissolving a bismuth salt, a selenium-containing compound and a stabilizer in a solvent, carrying out an ultrasonic treatment, uniformly mixing, heating to a temperature of 80-200 DEG C, adding a reducing agent, cooling to a room temperature after completing a reaction, and carrying out centrifugation separation washing three times to obtain the bismuth selenide nanometer material with a diameter of 20-200 nm. The preparation method has characteristics of simple operation, less time consuming, low energy consumption and easy scalization. The prepared particles have a uniform particle size and good dispersity. With application of the bismuth selenide nanometer material as a living body X-ray CT imaging contrast agent, a significant imaging effect is provided compared with imaging effects of the traditional contrast agents such as an iodine reagent iopamidol and the like so as to provide important application prospects in the future tumor imaging field.
Owner:FUZHOU UNIV
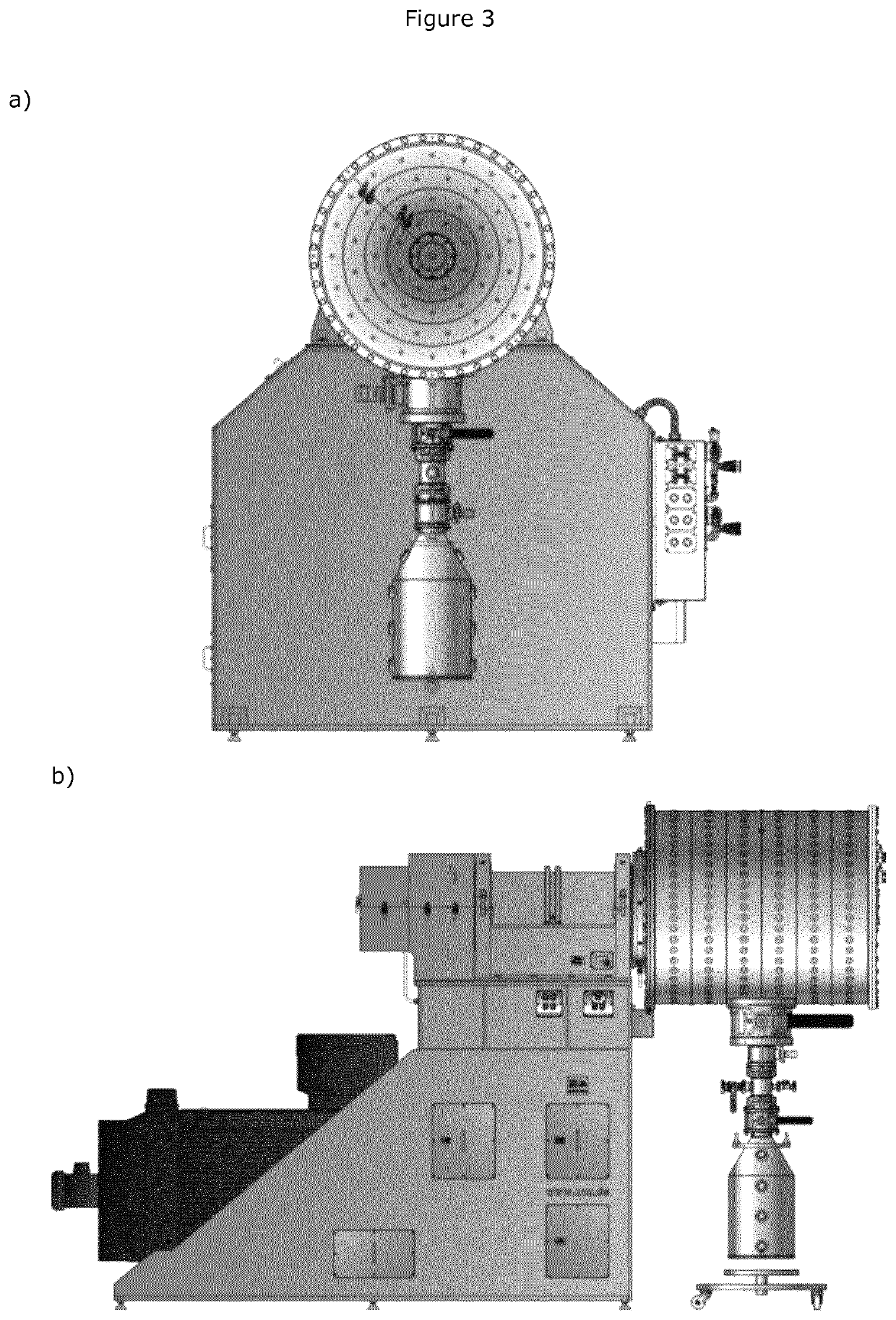
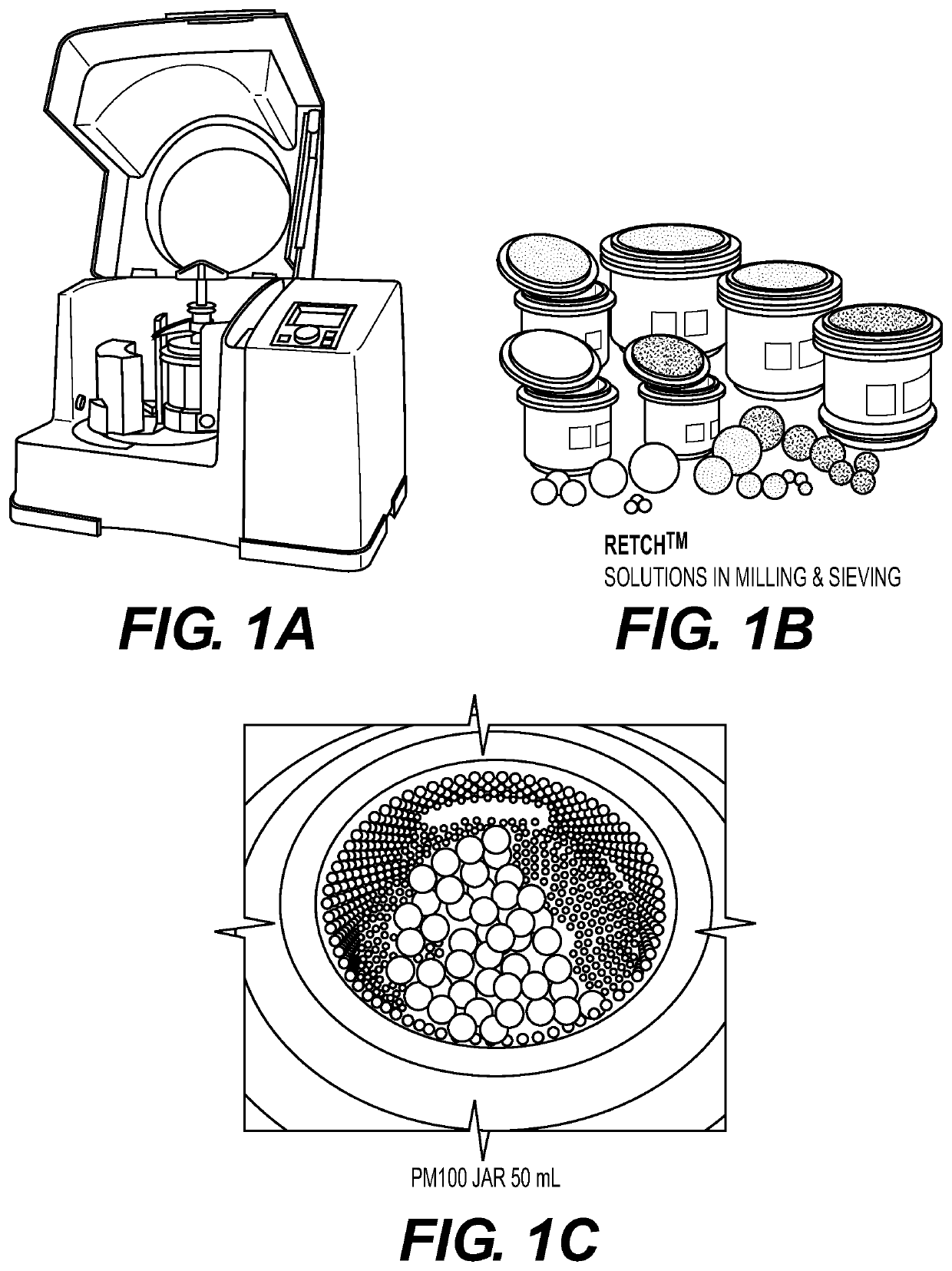
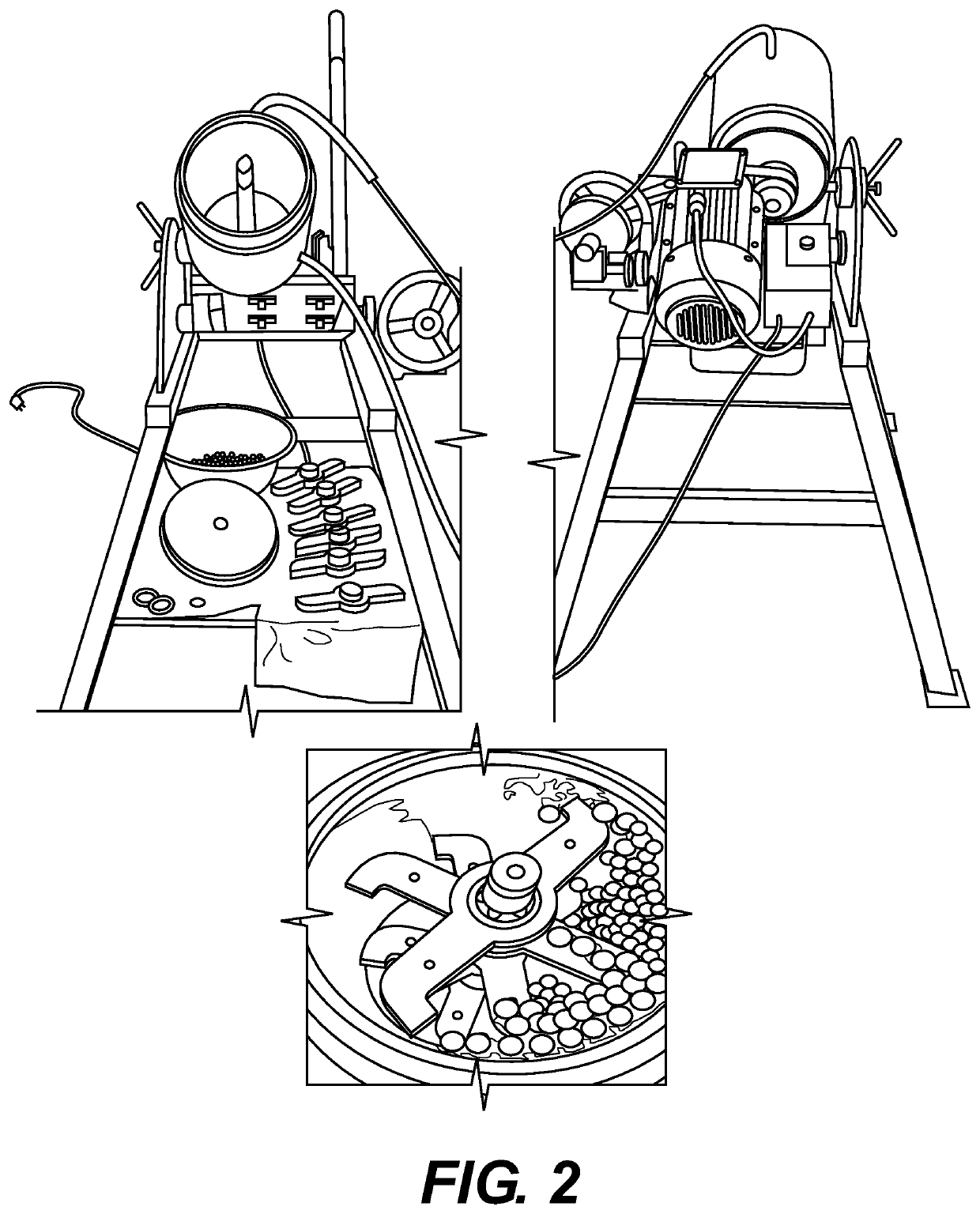
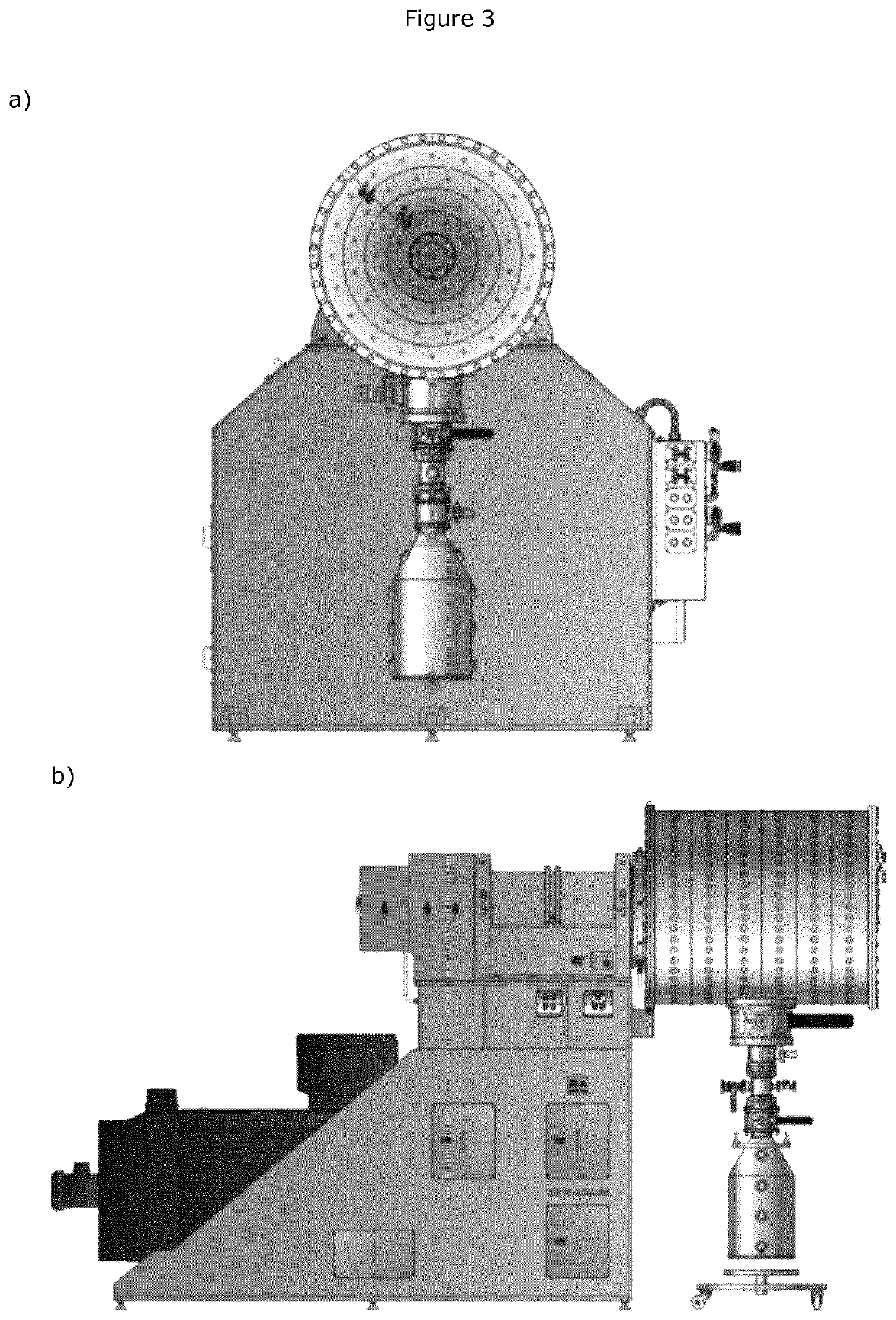
Mechanochemical synthesis of radiographic agents intermediates
ActiveUS20200079728A1Organic compound preparationCarboxylic acid amides preparationChemical synthesisMechanical milling
The present invention generally relates to a process using a mechanochemical approach exploiting the mechanical milling of reactants for the manufacturing of acetyl Iopamidol and, more generally, of key intermediates of radiographic contrast agents, and of the contrast agents themselves.
Owner:BRACCO IMAGINIG SPA
Mechanochemical synthesis of radiographic agents intermediates
ActiveUS10836710B2Organic compound preparationCarboxylic acid amides preparationChemical synthesisMechanical milling
The present invention generally relates to a process using a mechanochemical approach exploiting the mechanical milling of reactants for the manufacturing of acetyl Iopamidol and, more generally, of key intermediates of radiographic contrast agents, and of the contrast agents themselves.
Owner:BRACCO IMAGINIG SPA
Contrast agent based on iopamidol lipid derivative and its preparation method and use
ActiveCN106831480BSave raw materialsMild reaction conditionsOrganic compound preparationX-ray constrast preparationsNanoparticleBiocompatibility Testing
The invention discloses a contrast medium based on an iopamidol lipid derivative, as well as a preparation method and application of the contrast medium. The lipid molecule structure comprises both hydrophobic aliphatic chains and hydrophilic iopamidol groups which can be combined to form nanoparticle structures by selves in a water solution, and in addition, as the aliphatic chains and the iopamidol can be connected through degradable ester bonds, the biocompatibility of the contrast medium can be further improved; moreover, as covalent bonds in the molecule structures of such substances are connected with iopamidol functional groups, the content of iopamidol in nanoparticles can be increased, and meanwhile the problems that the contrast medium is released and leaked too early in the particle circulation process can be effectively avoided. The nanoparticles formed by lipid of the contrast medium can be used as a functional material which is used as a contrast medium and a carrier of various medicines and other contrast mediums, thereby having wide clinical application prospects.
Owner:PEKING UNIV
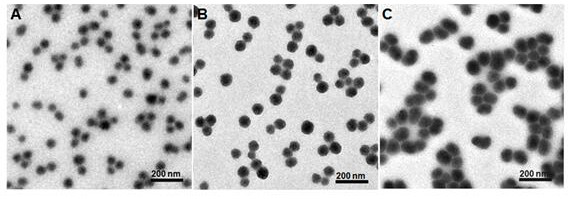
Iodine-containing nanoparticles for tumor-targeted CT imaging and preparation method thereof
ActiveCN108452327BHigh biosecurityGood biocompatibilityPowder deliveryX-ray constrast preparationsTumor targetFunctional monomer
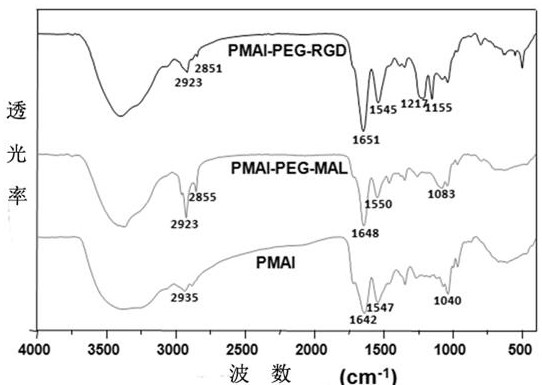
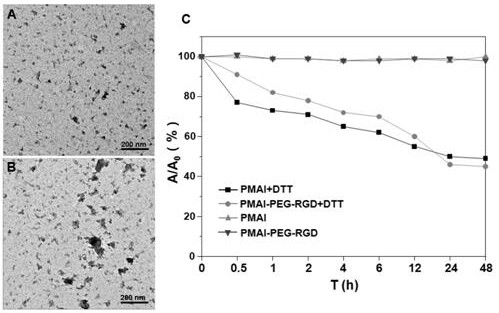
The invention relates to preparation and application of a nanometer contrast agent for tumor targeted CT imaging. The nanometer contrast agent having a tumor targeted CT imaging function is prepared by the following steps: firstly, designing and synthesizing an iodine-containing functional monomer compound which can be applied to polymerization based on a clinically-used small molecular CT contrast agent, namely, iopamidol; then, preparing iodine-containing polymer nanoparticles with appropriate particle sizes by precipitation polymerization, and performing polyethylene glycol and RGD peptidesurface modification on the iodine-containing polymer nanoparticles. The prepared iodine-containing nanoparticles can be used as a contrast agent for tumor CT imaging, so that a reliable theoretical basis and a method basis are laid for highly-sensitive diagnosis of tumors.
Owner:TIANJIN MEDICAL UNIV
Iodination process for the preparation of 3, 5-disubstituted-2, 4, 6-triiodo aromatic amines compounds
ActiveUS9382194B2Organic compound preparationCarboxylic acid amides preparationIodixanolIodination reaction
The invention discloses a improved process for the preparation of 3,5-disubstituted-2,4,6-triiodo aromatic amines of formula (II), wherein R1 and R2 are defined as herein. The compounds of formula (II) are the key intermediates for the synthesis of a series of non-ionic contrast agents such as Iopamidol, Iohexol and Iodixanol. The process comprises reacting chlorine-free iodinating reagents with 3,5-disubstituted-2,4,6-triiodo aromatic amines to obtain 3,5-disubstituted-2,4,6-triiodo aromatic amines of formula (II), wherein the molar yield of the iodination reaction can reach to 89%.
Owner:IMAX DIAGNOSTIC IMAGING HLDG
Manufacture of a triiodinated contrast agent
InactiveCN107445858AHigh purityReduce contentOrganic compound preparationCarboxylic acid amides optical isomer preparationHydrolysisPropanediol
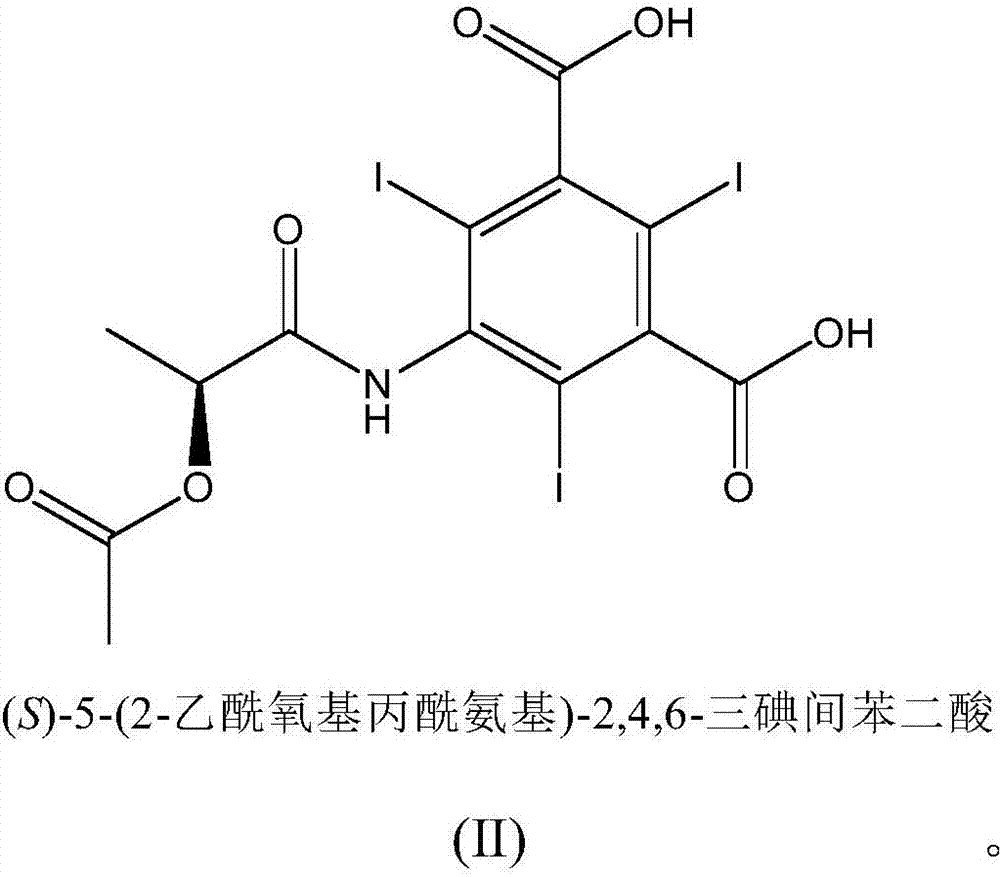
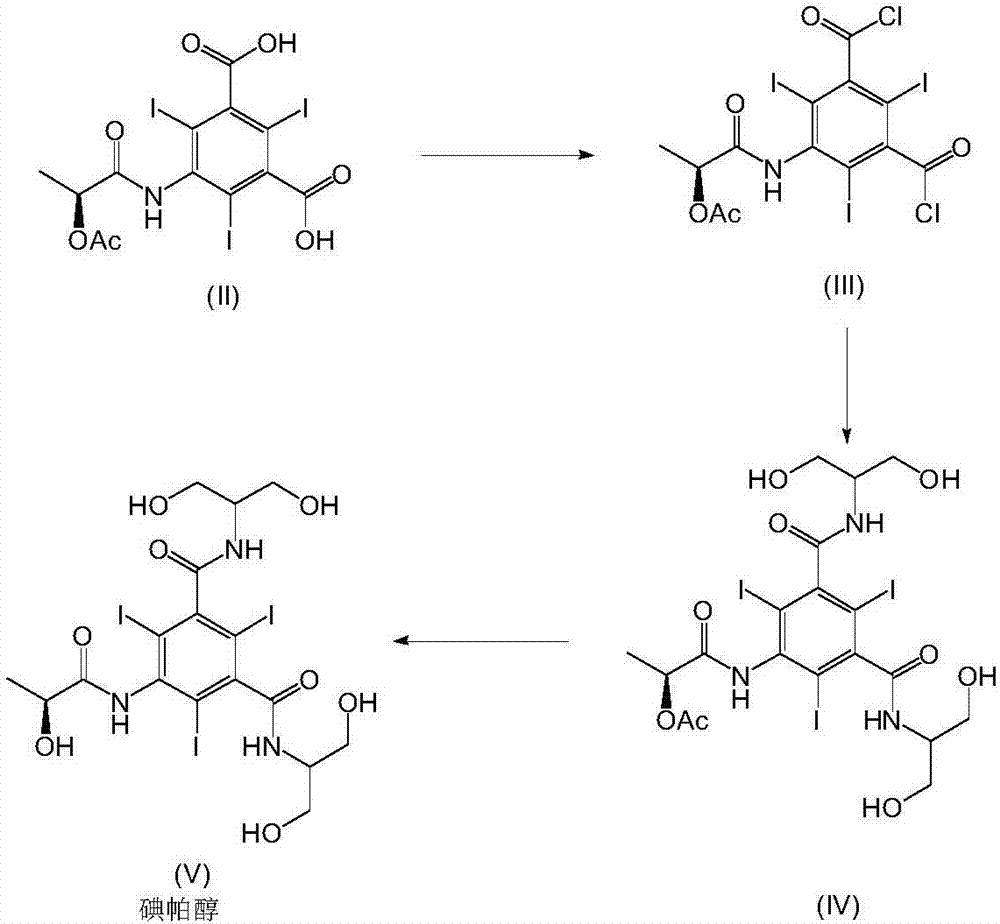
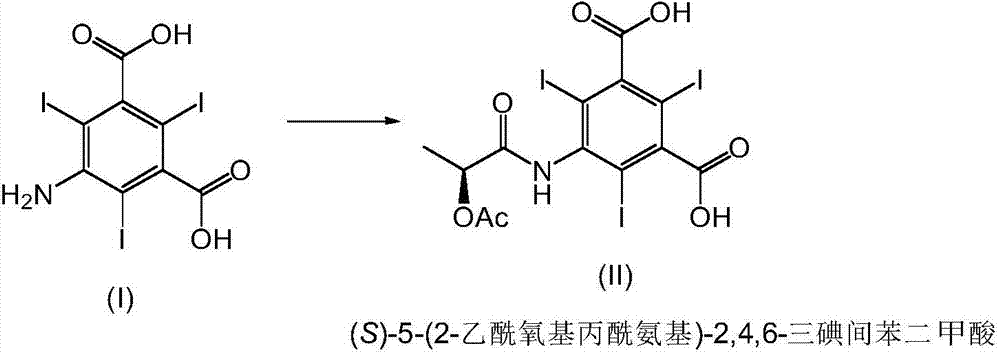
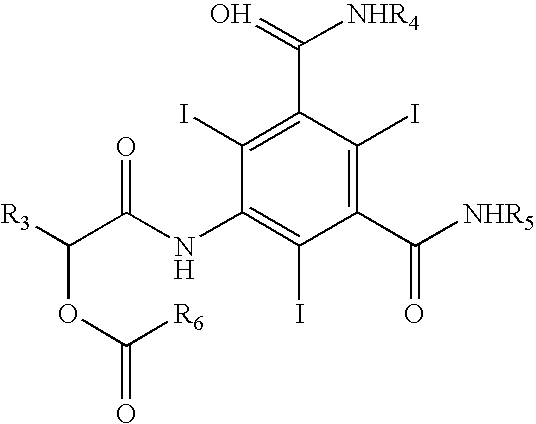
The invention relates to manufacture of a triiodinated contrast agent. The invention further relates to a new compound, (S)-5-(2-acetoxypropanamido)-2,4,6-triiodoisophthalic acid, of formula II (S)-5-(2-acetoxypropanamido)-2,4,6-triiodoisophthalic acid. Said new compound is of use for the production of triiodinated contrast agent, especially lopamidol, with low content of acetyl and hydroxyacetyl analogs. The new compound may be formed from 5-amino-2,4,6-triiodoisophtalic acid by acylating with (S)-1-chloro-1-oxopropan-2-yl acetate. The new compound may then be converted to the respective acid dichloride by reacting with a chlorinating reagent, which is a further object of the present invention, followed by the amidation with 2-amino-1,3-propanediol and acetate hydrolysis.
Owner:霍维奥恩联合有限公司
Continuous process for the preparation of (s)-2-acetyloxypropionic acid chloride
InactiveCN111548268AOrganic compound preparationCarboxylic acid esters preparationAcetic acidAcetic anhydride
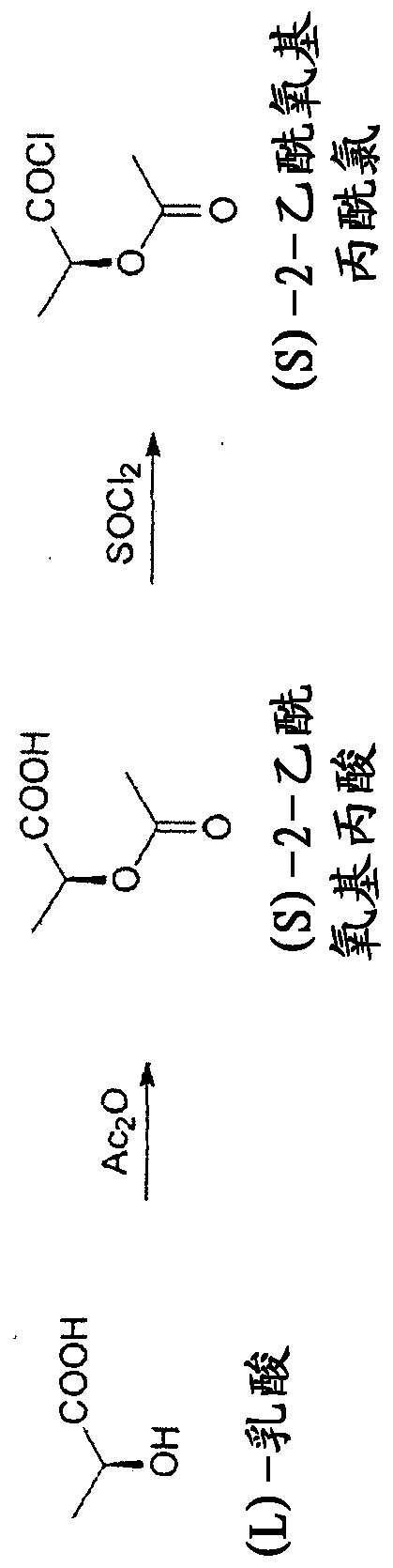
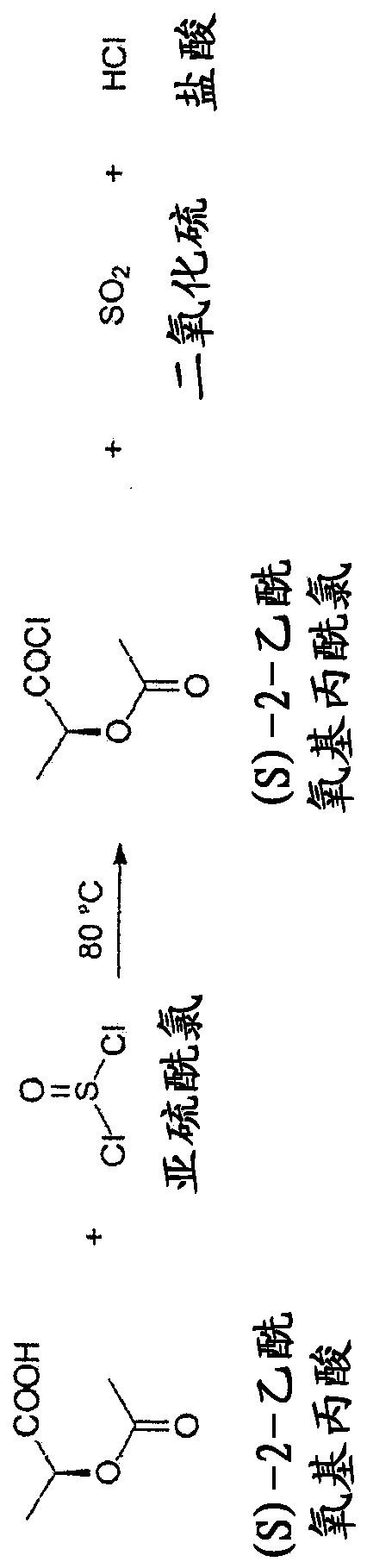
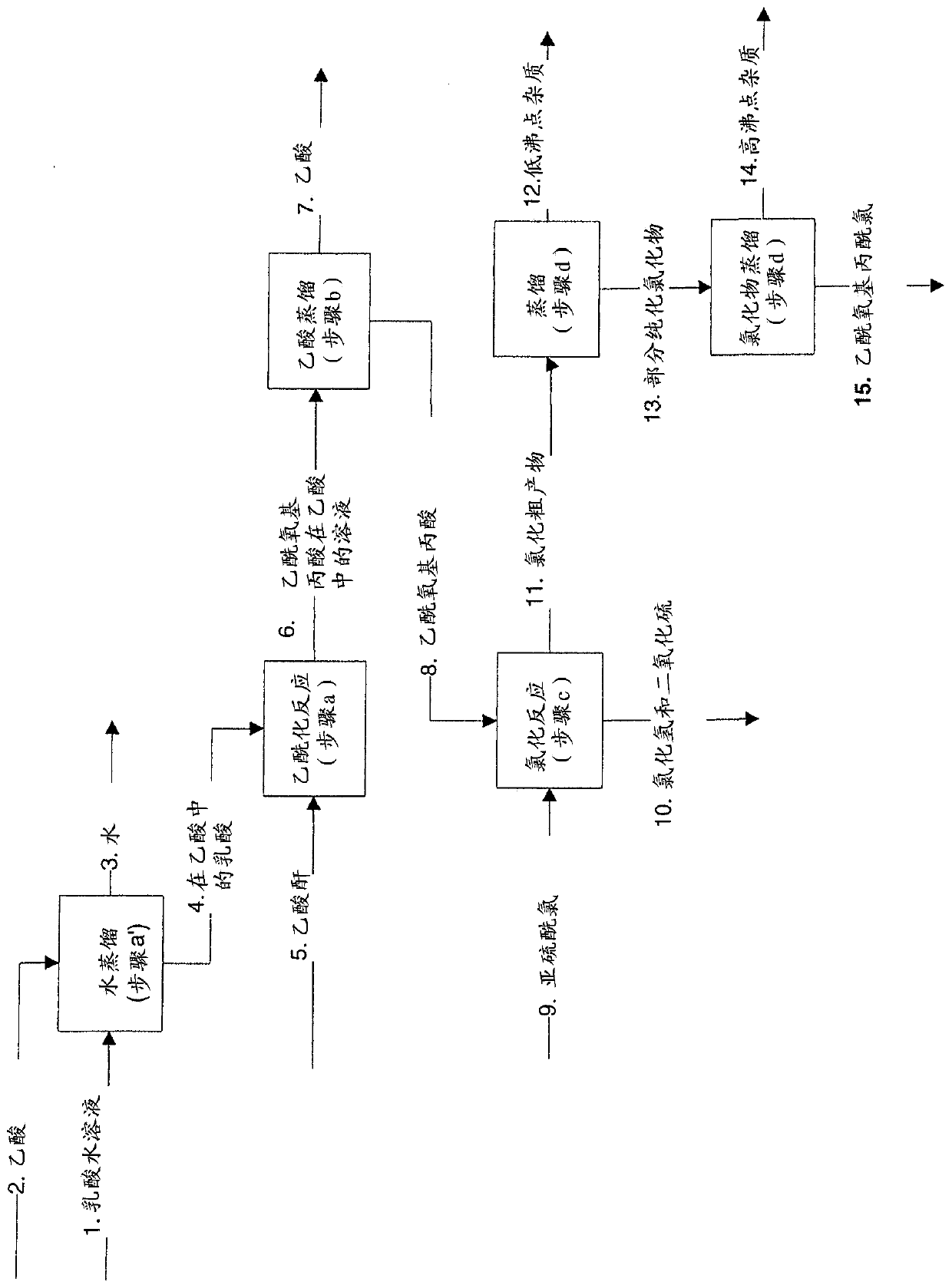
The present invention relates to a continuous method for the preparation of (S)-2-acetyloxypropionic acid from an aqueous solution of lactic acid and acetic anhydride, in acetic acid. (S)-2-acetyloxypropionic acid is used for the preparation of (S)-2-acetyloxypropionic acid chloride, an essential intermediate compound for the preparation of Iopamidol and has to be industrially produced with high purity and suitable quality for producing Iopamidol according to the Pharmacopoeia requirements. The continuous process according to the invention, comprises therefore also the chlorination steps of (S)-2-acetyloxypropionic acid with thionyl chloride to give the corresponding (S)-2-acetyloxypropionic acid chloride which is further distilled to give the suitable purity characteristics for its use for the preparation of non-ionic iodinated contrast agents as Iopamidol.
Owner:BRACCO IMAGINIG SPA
Process for the preparation of iopamidol
ActiveUS9950991B2Efficiently recyclableImprove processing yieldOrganic compound preparationCarboxylic acid amides preparationPotassium hydroxideAlkaline hydrolysis
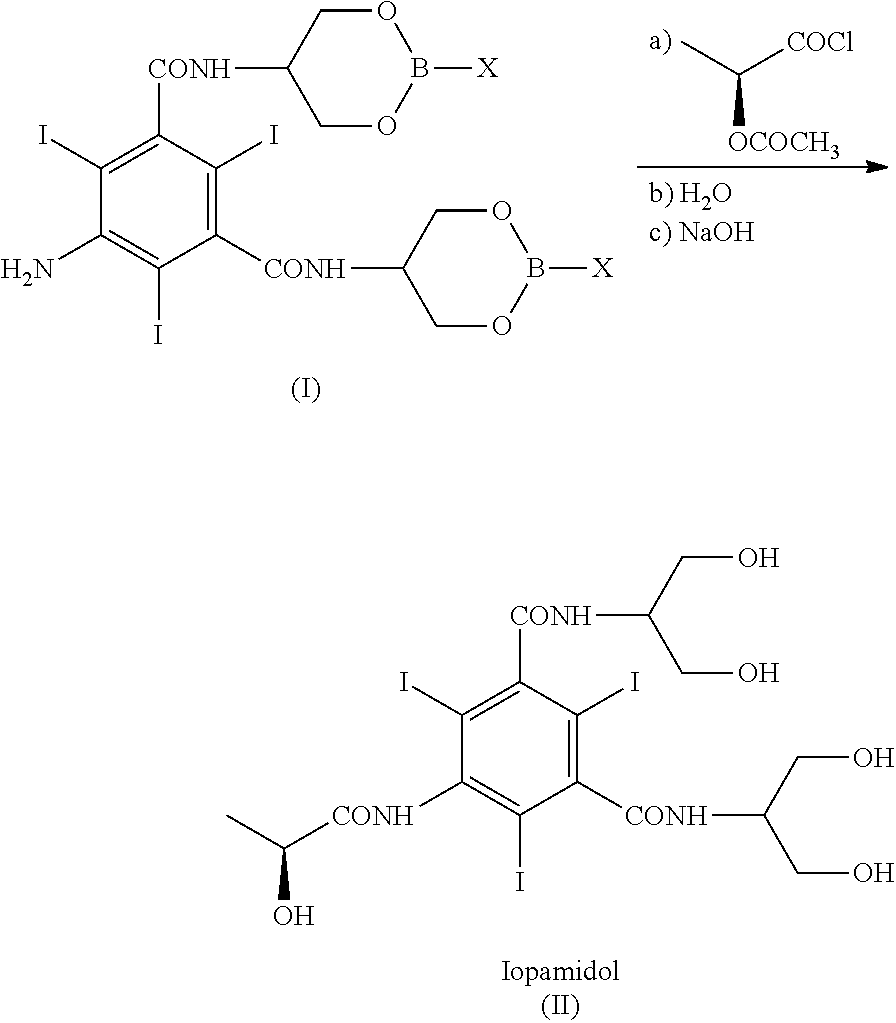
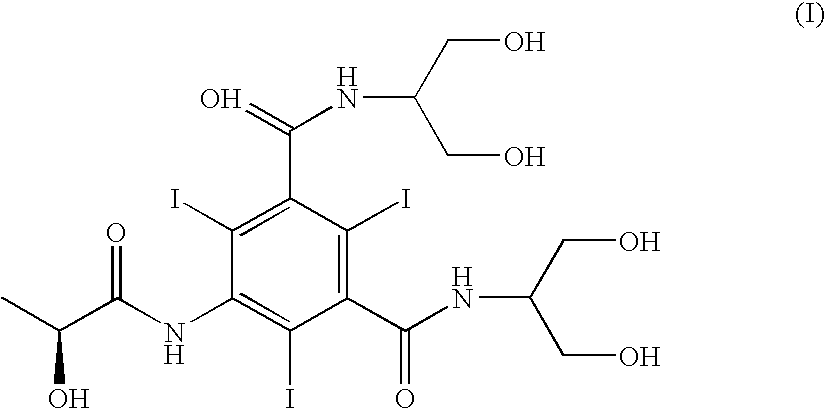
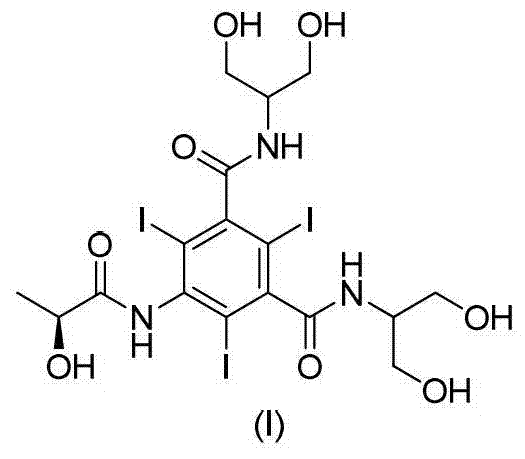
The present invention discloses a process for the preparation of Iopamidol of formula (II) and comprising the following steps: a) reacting the Compound (I) wherein X is OR2 or R3, and wherein R2 and R3 are a Ci-C6 linear or branched alkyl, C3-C6 cycloalkyl, C6 aryl, optionally substituted with a group selected from the group consisting of methyl, ethyl, n-propyl, i-propyl, n-butyl, sec-butyl, t-butyl and phenyl, with the acylating agent (S)-2-(acetyloxy)propanoyl chloride in a reaction medium to provide the acetyloxy derivative of Compound (I); b) hydrolyzing the intermediate from step a) with an aqueous solution at a pH comprised from 0 to 7, by adding water or a diluted alkaline solution such as sodium hydroxide or potassium hydroxide, freeing the hydroxyls from the boron-containing protective groups, obtaining the N—(S)-2-(acetyloxy)propanoyl derivative of Compound (II); c) alkaline hydrolysis to restore the (S)-2-(hydroxy)propanoyl group and to obtain Iopamidol (II) and optional recovery of the boron derivative from the solution obtained in step b). The boron-containing protective group is versatile, efficient and recyclable. A one-pot synthesis, without intermediate isolation is provided, leading to a decreasing of recovered and recycled solvents and a significant increasing in the yield, representing a significant advantage in terms of cost-effectiveness of the entire process and environmental awareness.
Owner:BRACCO IMAGINIG SPA
Process for the preparation of iopamidol and the new intermediates therein
InactiveUS20060106253A1High optical purityOrganic compound preparationOrganic chemistry methodsHydrogenAlkoxy group
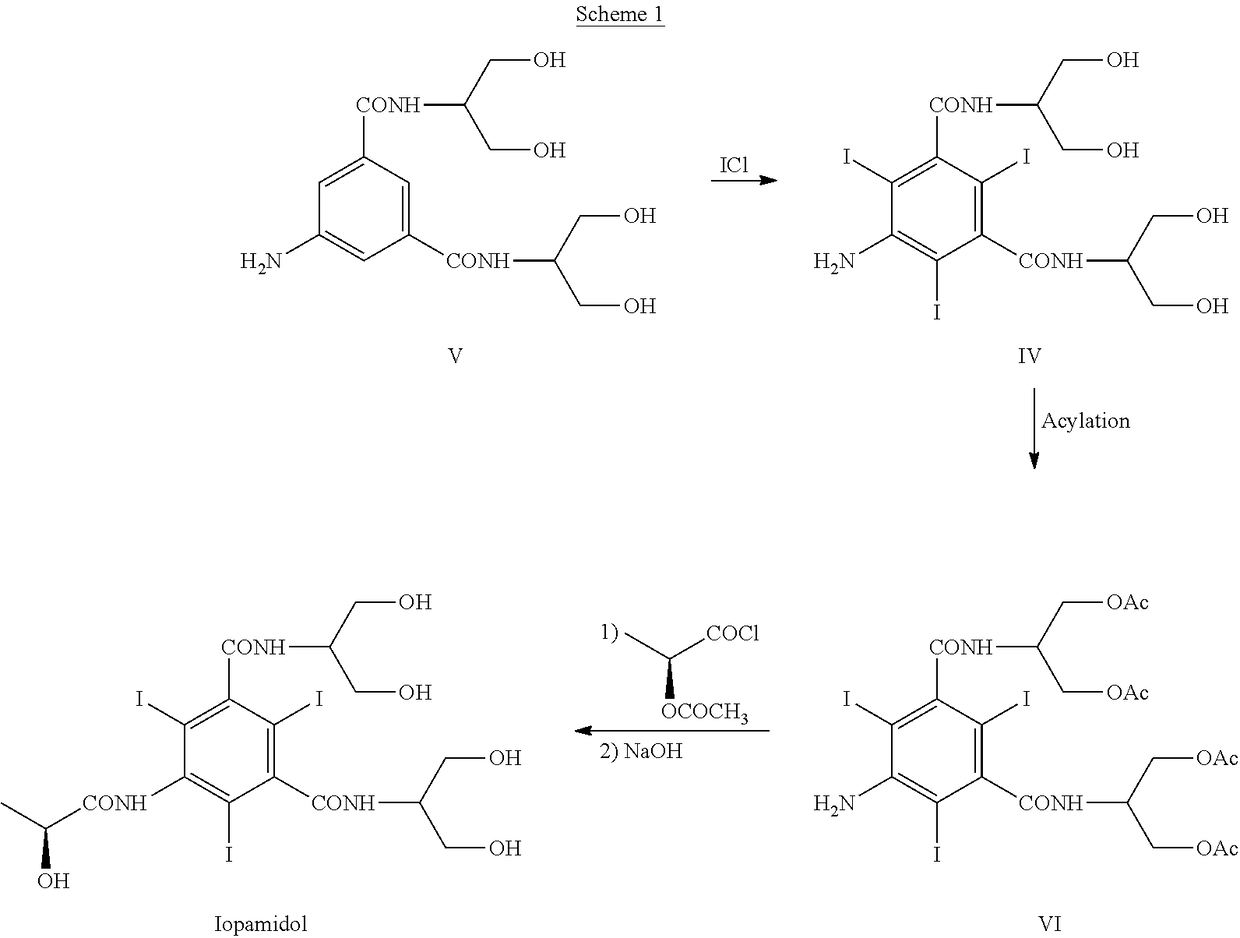
A process for the preparation of (S)-N,N′-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-5-[(2-hydroxy-1-oxo-propyl)amino]-2,4,6-triiodo-1,3-benzendicarboxamide (iopamidol) starting from 5-amino-N,N′-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-2,4,6-triiodo-1,3-benzenedicarboxamide (II) which process comprises a) reacting the compound of formula (II) with a suitable protecting agent, to give a compound of formula (III) wherein R is a group of formula A or B wherein R1 is a hydrogen atom, a C1÷C4 straight or branched alkoxy group, R2 is hydrogen, a C1÷C4 straight or branched alkoxy group and R3 ?is a C1÷C4 straight or branched alkyl group, a trifuoromethyl or a trichloromethyl group; b) acylating the amino group in position 5 of the intermediate compound of formula (III), by reaction with a (S)-2-(acetyloxy)propanoyl chloride to give a compound of formula (IV) wherein R is as defined above; and c) removing all the acyl groups present in the compound of formula (IV) under basic conditions, with prior cleavage of the cyclic protections of the hydroxy groups in the carboxamido substituents under acidic conditions, when R is a group of formula A carboxamido hydroxy groups under acidic conditions. The invention also refers to the new intermediates of formula (III) and (IV) wherein —R is a group A.
Owner:BRACCO IMAGINIG SPA
Method for determining content of 2-chloropropionic acid in iopanol
ActiveCN110687226AReduce sensitivityShort detection timeComponent separationPropanoic acidPhysical chemistry
The invention provides a method for determining the content of 2-chloropropionic acid in iopalol. S1, taking a reference solution of 2-chloropropionic acid, and using water and acid-ethanol (3:17) solution as a solvent, the 2-chloropropane acid reference solution as a reference solution is prepared; and taking the drug substance, and using water and acid-ethanol (3:17) solution as a solvent, the sample test solution is prepared; S2, two parts of 2-chloropropionic acid reference solution are prepared in parallel, and the peak areas of 2-chloropropionic acid in the two reference solutions are obtained respectively by HS-GC-MS detection method; S3, the peak area of 2-chloropropionic acid in the sample test solution is obtained by HS-GC-MS detection method, and the content of 2-chloropropionicacid in the sample test solution is calculated by external standard method. The invention has the advantages of short detection time, strong specificity, good accuracy, high precision and good repeatability.
Owner:上海明捷医药科技有限公司
Process for the preparation of iopamidol and the new intermediated therein
InactiveUS7034183B2High optical purityOrganic compound preparationOrganic chemistry methodsHydrogenHydrogen atom
A process for the preparation of (S)-N,N′-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-5-[(2-hydroxy-1-oxo-propyl)amino]-2,4,6-triiodo-1,3-benzendicarboxamide (iopamidol) starting from 5-amino-N,N′-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-2,4,6-triiodo-1,3-benzenedicarboxamide (II) which process comprises a) reacting the compound of formula (II) with a suitable protecting agent, to give a compound of formula (III) wherein R is a group of formula A or B wherein R1 is a hydrogen atom, a C1÷C4 straight or branched alkoxy group, R2 is hydrogen, a C1÷C4 straight or branched alkoxy group and R3 ?is a C1÷C4 straight or branched alkyl group, a trifuoromethyl or a trichloromethyl group; b) acylating the amino group in position 5 of the intermediate compound of formula (III), by reaction with a (S)-2-(acetyloxy)propanoyl chloride to give a compound of formula (IV) wherein R is as defined above; and c) removing all the acyl groups present in the compound of formula (IV) under basic conditions, with prior cleavage of the cyclic protections of the hydroxy groups in the carboxamido substituents under acidic conditions, when R is a group of formula A carboxamido hydroxy groups under acidic conditions. The invention also refers to the new intermediates of formula (III) and (IV) wherein —R is a group A.
Owner:BRACCO IMAGINIG SPA
Exosome purification method
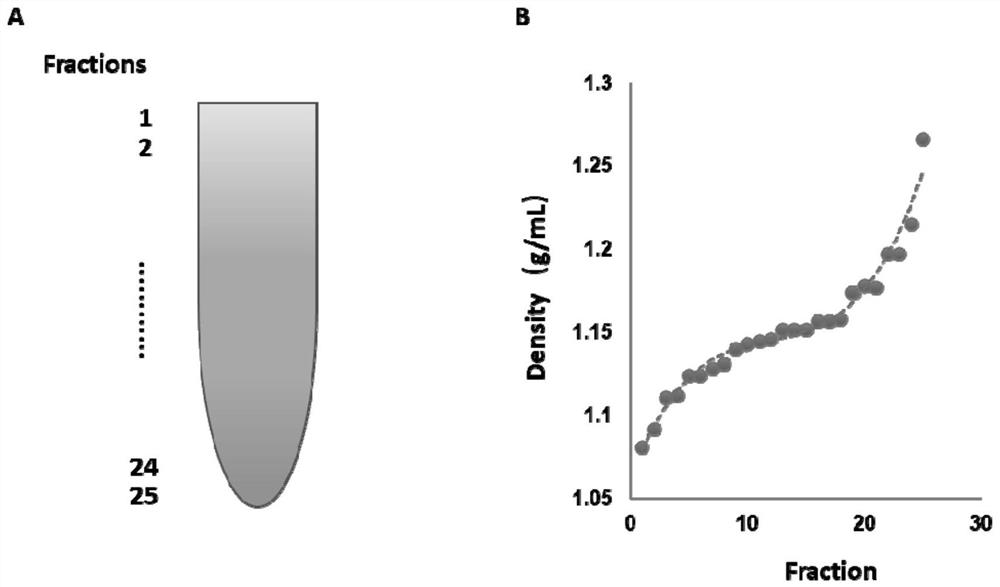
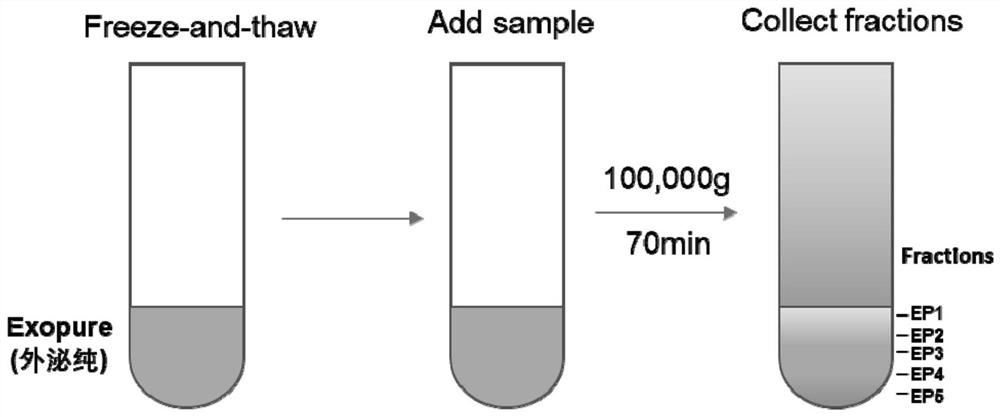
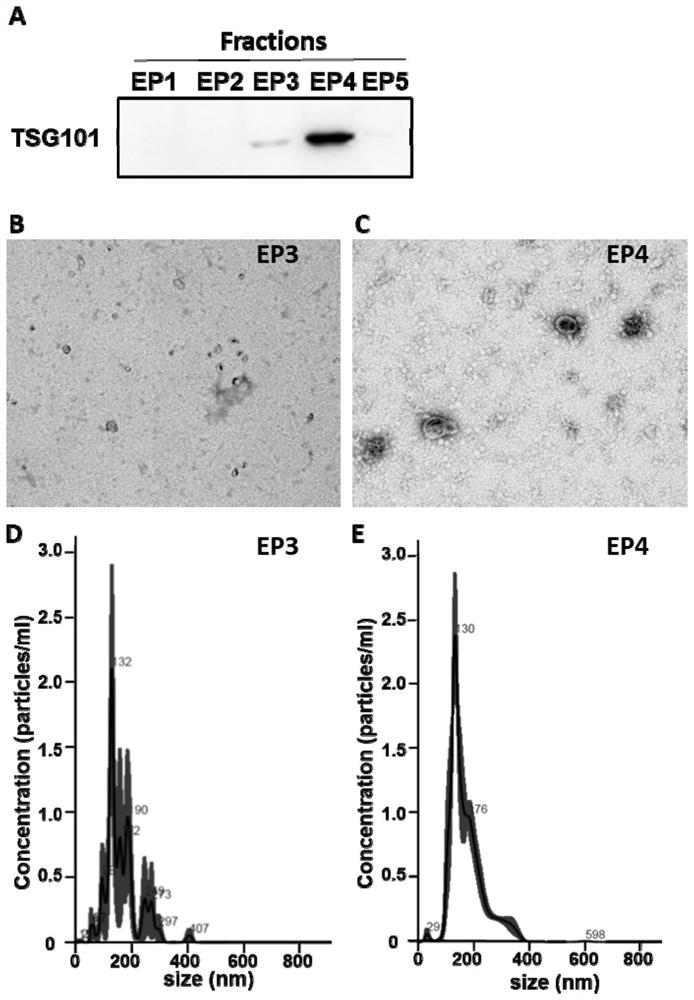
The invention provides an exosome purification method. The exosome purification method comprises the following steps: (1) centrifuging a cell culture solution rich in exosomes; (2) centrifuging supernate obtained in the step (1) and a 40% (w / v) iopamidol solution together to obtain an exosome crude extract, wherein the volume ratio of the supernate obtained in the step (1) to iopamidol is (500-600):1; (3) diluting the exosome crude extract by 4-6 times with a buffer solution, and performing centrifuging together with the exosomes, wherein the exosomes are located at the lower part of the exosome crude extract; the volume ratio of the exosome crude extract to the exosome is 2:(1-1.5); and the exosome is a 25-30% (w / v) iopamidol solution; and (4) respectively collecting products obtained after centrifugal layering in the step (3). According to the purification method disclosed by the invention, the iopamidol with a certain concentration is subjected to ultracentrifugation, so that a continuous density gradient can be quickly formed; and the purification method can be used for separating the exosome with relatively high purity and is beneficial to improving the purity of the obtained exosome.
Owner:微纳核酸生物医药广东有限公司
Synthesis of iopamidol and preparation of its intermediates
ActiveCN103382160BEasy to separateReduce difficultyOrganic compound preparationCarboxylic acid amides preparationAcyl groupEthyl group
The invention discloses a method for preparation of (S)-N,N'-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-5-[(2-hydroxy-1-oxopropyl)amino]-2,4,6-triiodo-1,3-benzenedicarboxamide (iopamidol) shown in the formula I from 5-amino-N,N'-bis[2-hydroxy-1-(hydroxymethyl)ethyl]-2,4,6-triiodo-1,3-benzenedicarboxamide shown in the formula II. The method comprises the following steps that a, the compound shown in the formula II and a mixed anhydride as an appropriate protective agent undergo a reaction to produce a mixed ester shown in the formula III; b, the mixed ester shown in the formula III and (S)-2-(acetoxy)propionyl chloride undergo a reaction so that an amino group at the 5th site is acylated and a compound shown in the formula IV is obtained; and c, the compound shown in the formula IV undergoes a hydrolysis reaction under acidic or alkaline conditions or undergoes an alcoholysis reaction so that all acyl groups of the compound shown in the formula IV are removed and the iopamidol shown in the formula III is obtained. The invention relates to a synthesis intermediate of the iopamidol shown in the formula III.
Owner:ZHEJIANG HISYN PHARMA
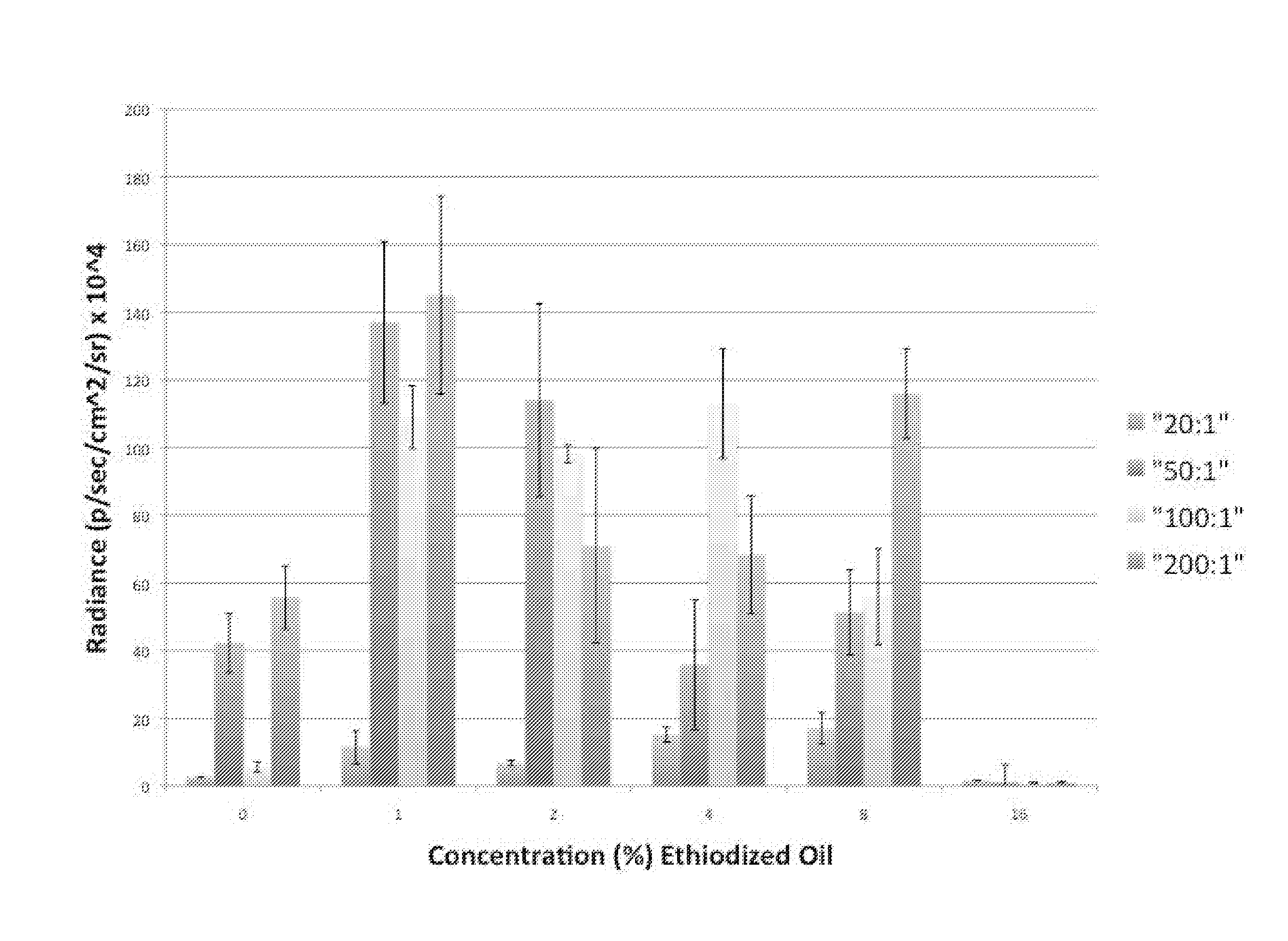
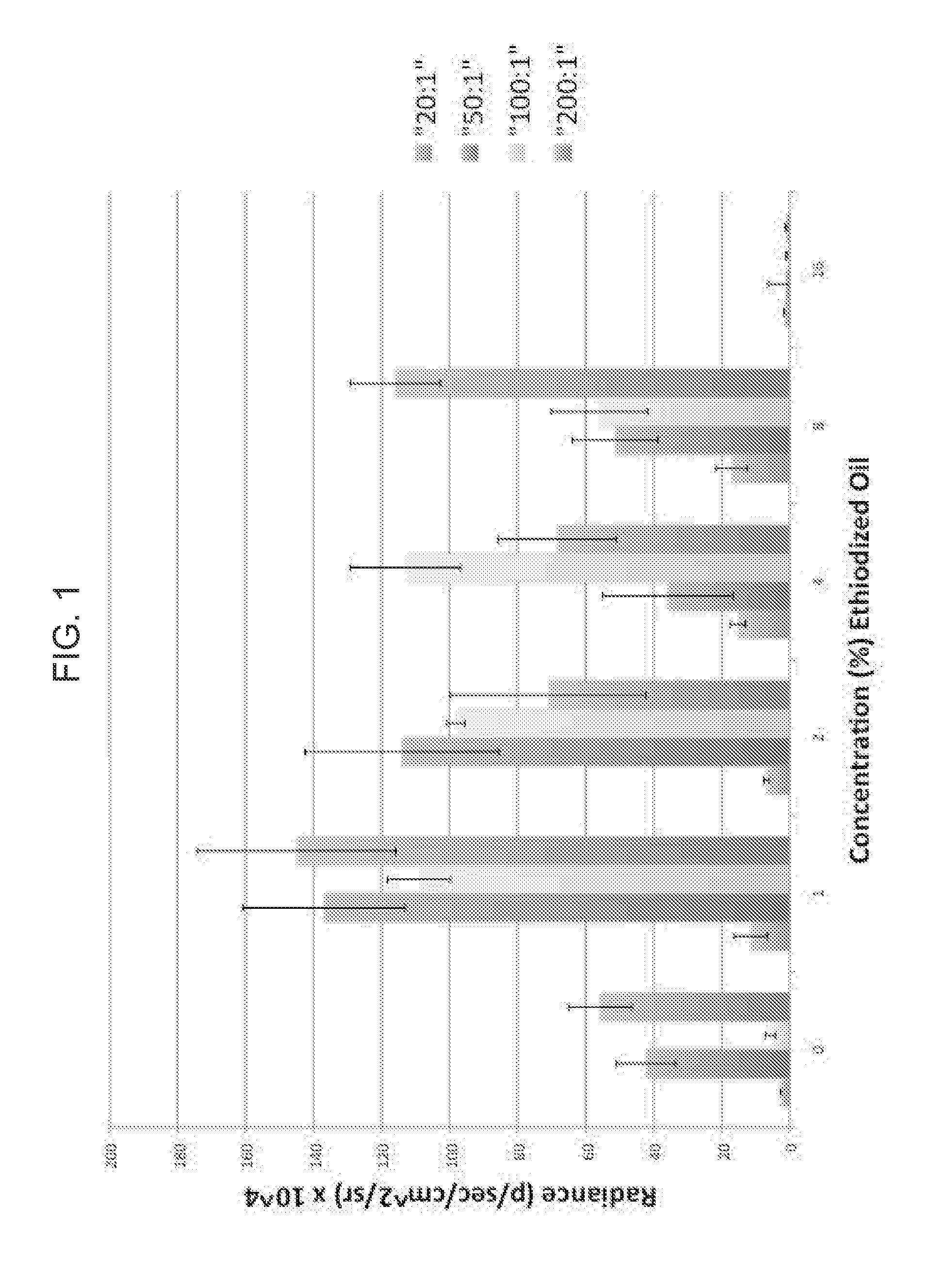
Nonviral gene delivery vector iopamidol, protamine, ethiodized oil reagent (VIPER)
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
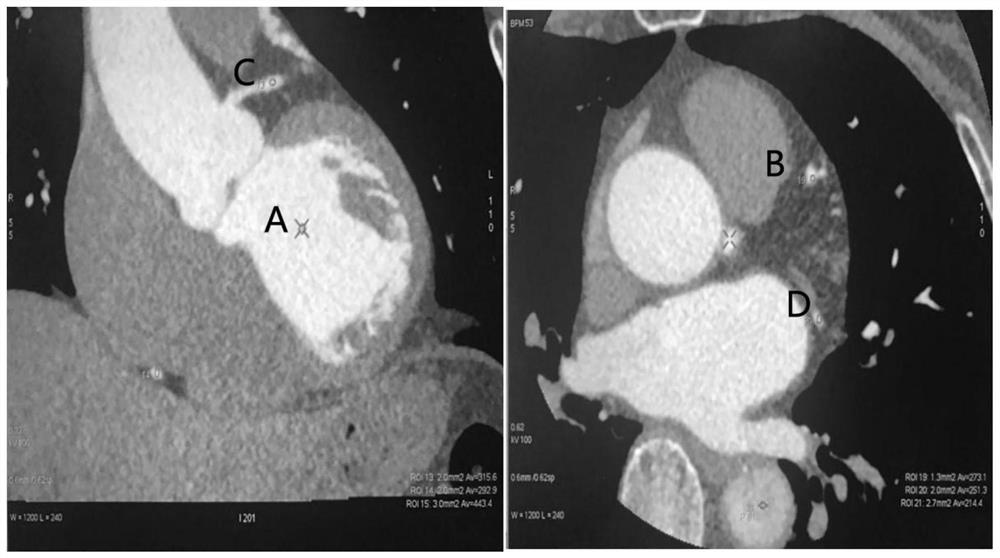
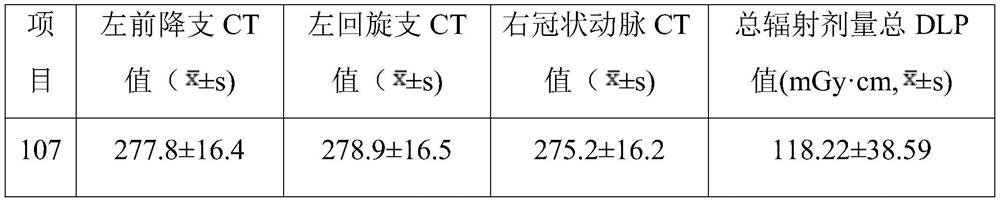
Application of ultra-low-amount contrast agent in coronary artery imaging of renal insufficiency patient
PendingCN113331848ASolve the problem that predisposes to contrast-induced nephropathyAchieving a definitive diagnosisComputerised tomographsTomographyNephrosisCoronary heart disease
The invention discloses application of an ultra-low-amount contrast agent in coronary artery imaging of a renal insufficiency patient, and belongs to the field of imaging diagnosis medicine application. The contrast agent is iopamidol 370, and the ultra-low amount is 18 ml. According to the application, the problem that contrast agent-induced nephropathy is easily caused by using a conventional dose (50-70ml) of a contrast agent for coronary artery imaging of general patients with suspicious coronary heart disease and coronary artery imaging of patients with renal insufficiency is effectively solved, and particularly, the influence of the contrast agent on renal function is greatly reduced; and the specific diagnosis and treatment of the coronary heart disease by special patient groups are realized.
Owner:宋培记
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com