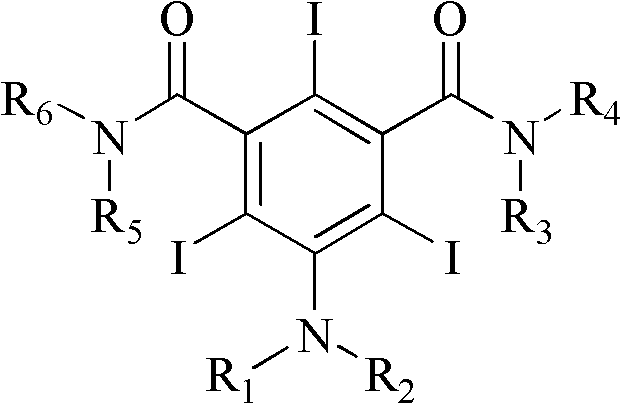
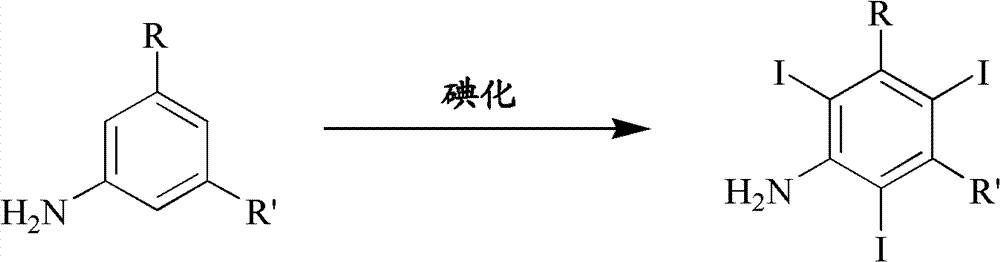
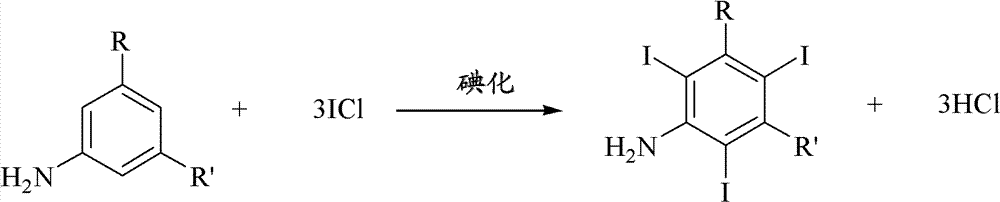
Iodination method for preparing 3,5-disubstituted-2,4,6-triiodo aromatic amine compound
A compound and mixture technology, applied in the field of chlorine-free iodination reagent system, can solve the problem of less mention of iodine recovery and utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0086] An example of the preparation method of the novel iodination reagent system among the present invention is, to the water or recovery reaction mother liquor that is 2.5 / 1 relative to the substrate mass ratio of formula (I), drop into relative to the substrate molar ratio is 1.28 / 1 1 elemental iodine, potassium iodate relative to the substrate molar ratio of 0.59 / 1 and sodium sulfate relative to the substrate molar ratio of 0.12 / 1, and finally the 98% concentration relative to the substrate molar ratio of 0.6 / 1 Add sulfuric acid slowly, stir for 1-1.5h, and the system temperature should always be lower than 50°C, preferably 38-42°C.
[0087] An example of the method for adding the substrate solution or suspension of formula (I) in the present invention to the iodinating reagent system is to continue adding within 4 hours, gradually slowing down the adding speed while maintaining good stirring. The preferred method is to add 3 / 4 of the substrate in the first 2h, and add 1 / ...
Embodiment 1
[0109] Preparation of 5-amino-N, N'-bis[2-hydroxyl-1-hydroxymethyl-ethyl]-2,4,6-triiodo-isophthalamide
[0110] In a 1000mL three-necked flask, add water (100mL), add iodine (45.1g, 0.178mol), potassium iodate (15.6g, 0.073mol), anhydrous sodium sulfate (2.1g, 0.015mol) under mechanical stirring, dropwise 15% sulfuric acid (50 g, 0.076 mol). After stirring at 40°C for 2h, an aqueous solution of 5-amino-N,N'-bis[2-hydroxy-1-hydroxymethyl-ethyl]-isophthalamide (380mL, 0.124mol ), the dropwise addition was completed within 4 hours. After reacting at 80°C for 12 hours, potassium iodate (2.2 g, 0.010 mol) was added, and the reaction was continued for 20 hours while keeping warm. After the reaction is finished, the temperature rises to sublimate the remaining elemental iodine in the reaction system and trap it in the iodine trap.
[0111] Cool down to 20°C and filter with suction. The filter cake was suspended in water (250 mL) and stirred at 80 °C for 1 h. Cool down to 10°C, a...
Embodiment 2
[0115] Preparation of 5-amino-N, N'-bis[2-hydroxyl-1-hydroxymethyl-ethyl]-2,4,6-triiodo-isophthalamide
[0116] In a 1000mL three-necked flask, add water (120mL), add iodine (59.5g, 0.234mol) under mechanical stirring, potassium iodate (25.1g, 0.117mol), anhydrous sodium sulfate (3g, 0.021mol), drop 15 % sulfuric acid (66 g, 0.101 mol). After stirring at 40°C for 2h, an aqueous solution of 5-amino-N,N'-bis[2-hydroxy-1-hydroxymethyl-ethyl]-isophthalamide (473mL, 0.168mol ), the dropwise addition was completed within 4 hours. After reacting at 80° C. for 12 h, potassium iodate (3 g, 0.014 mol) was added, and the reaction was continued for 24 h while keeping warm. After the reaction is finished, the temperature rises to sublimate the remaining elemental iodine in the reaction system and trap it in the iodine trap.
[0117] Cool down to 20°C and filter with suction. The filter cake was suspended in water (600 mL), stirred at 80°C for 2h, cooled to 10°C, stirred for 2h, and fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com