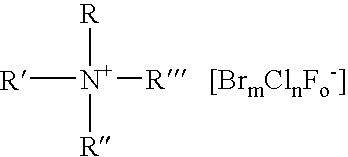Patents
Literature
107results about "Inter-halogen compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
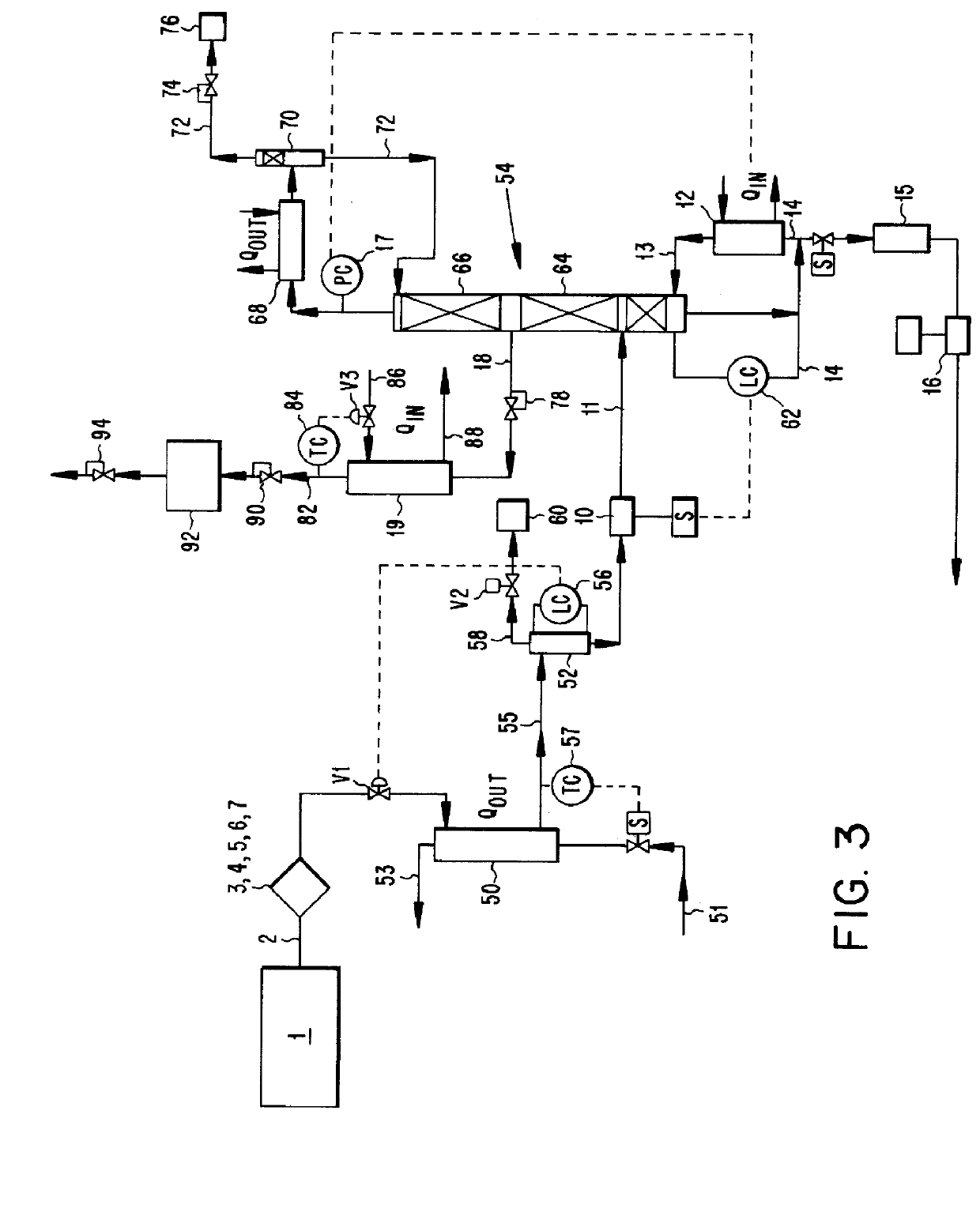
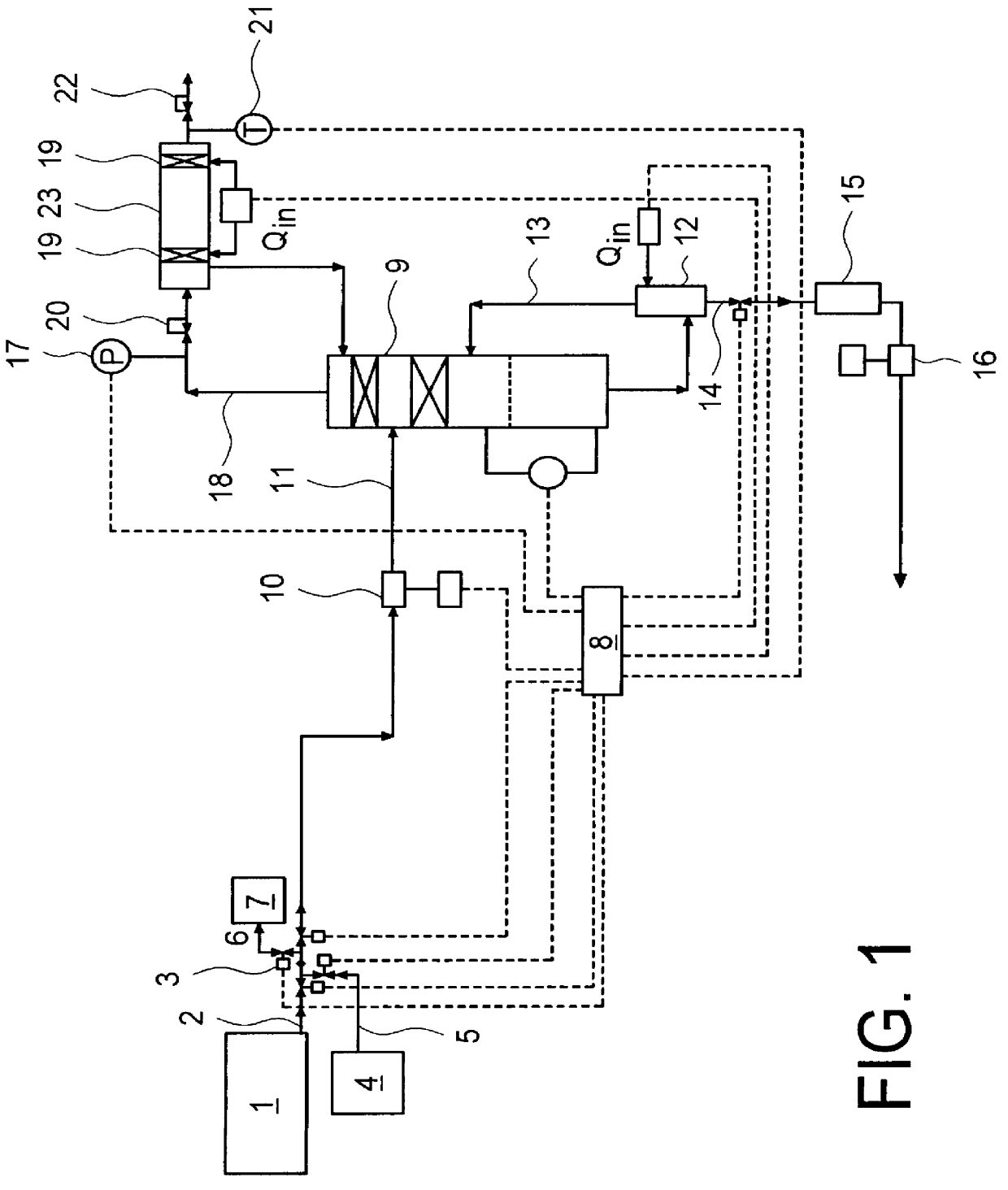
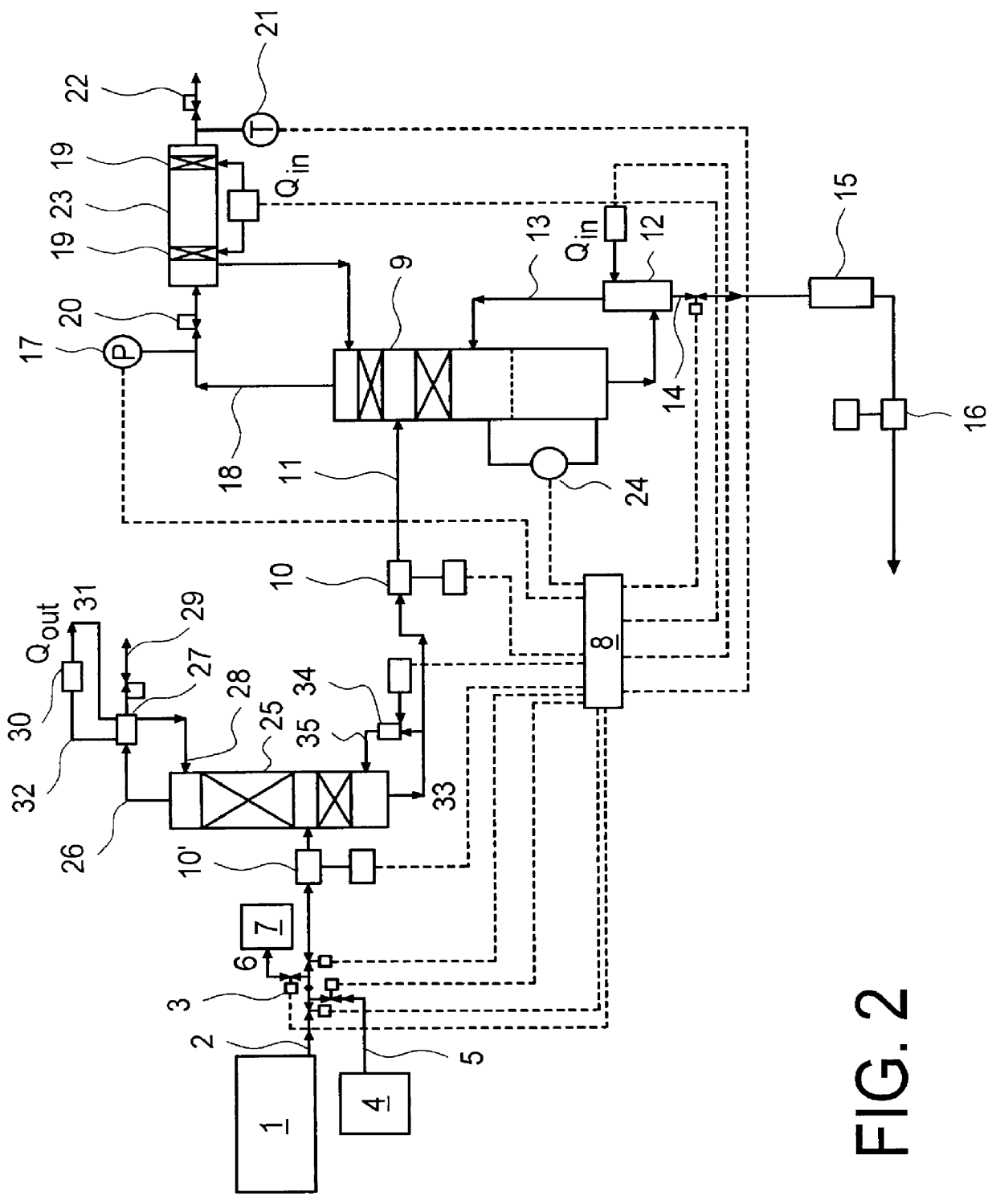
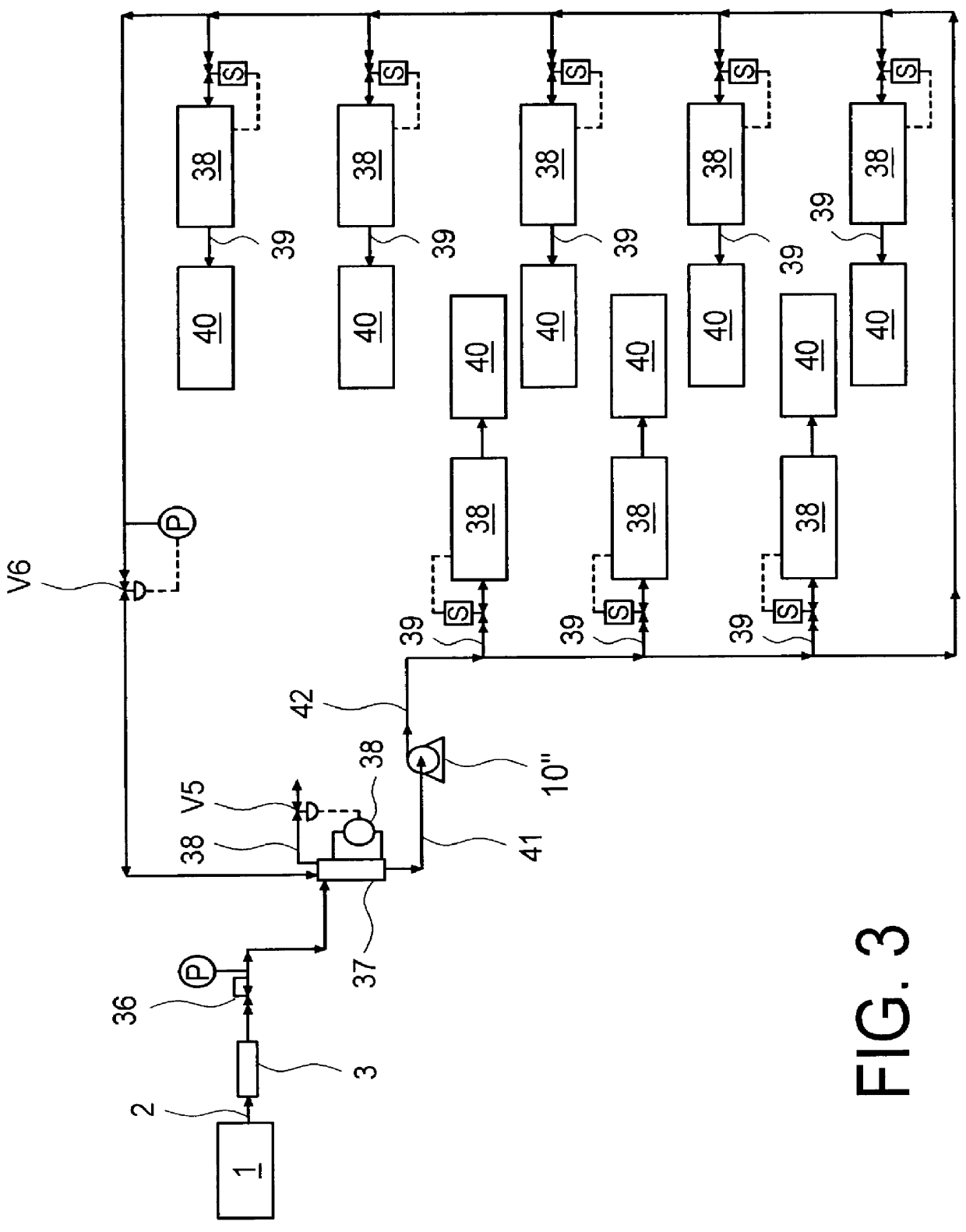
Composition for the production of chlorine dioxide using non-iodo interhalides or polyhalides and methods of making and using the same
ActiveUS7087190B2Reduce microbial countQuick buildBiocideLiquid degasificationChlorine dioxideCHLORITE ION
A composition for the generation of chlorine dioxide including at least one non-iodo interhalide, polyhalide or salt thereof having the formulaBrmClnFoXpwherein m=0–3, n=0–4, o=0–3, p=0–2, X is a cationic moiety and with the provisos that m+n+o cannot be zero; if m+n+p<2, or mixtures thereof, and at least one source of chlorite ions.
Owner:ECOLAB USA INC
Method of preparing a biocide comprising stabilized hypochlorite and a bromide ion source and a method of controlling microbial fouling using the same
InactiveUS20050147528A1Long retentionIncreased durabilityBiocideSpecific water treatment objectivesAlkaline earth metalBromide ions
Disclosed is a method of preparing a biocide having improved durability of its biocidal activity as well as disinfection efficiency at an initial stage, comprising the steps of: (a) preparing stabilized alkali or alkaline earth metal hypochlorite having a pH at least 11 by mixing a chlorine oxidant including alkali or alkaline earth metal hypochlorite with a stabilizer in an alkali solution; (b) preparing a bromide ion source; and (c) adding the bromide ion source prepared in step (b) into the stabilized alkali or alkaline earth metal hypochlorite prepared in step (a). Also, a method of controlling the growth of microorganisms using a biocide prepared by the method of the present invention is disclosed.
Owner:ACCU LAB CO LTD
Cathode active material for non-aqueous electrolyte secondary battery and manufacturing method of the same
InactiveUS20100035155A1Increase energy densityElectrode manufacturing processesFluoride preparationMechanical millingFluoride
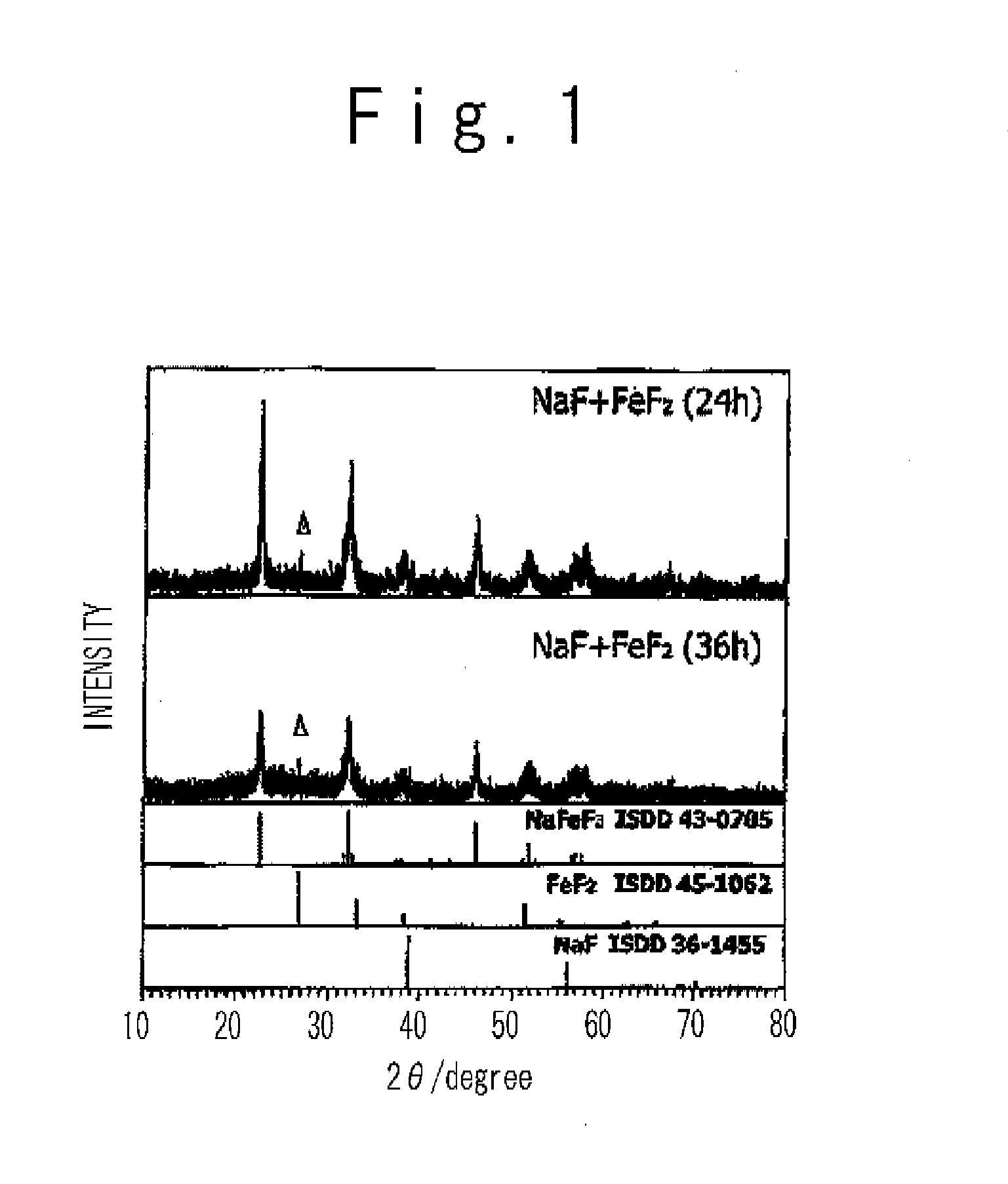
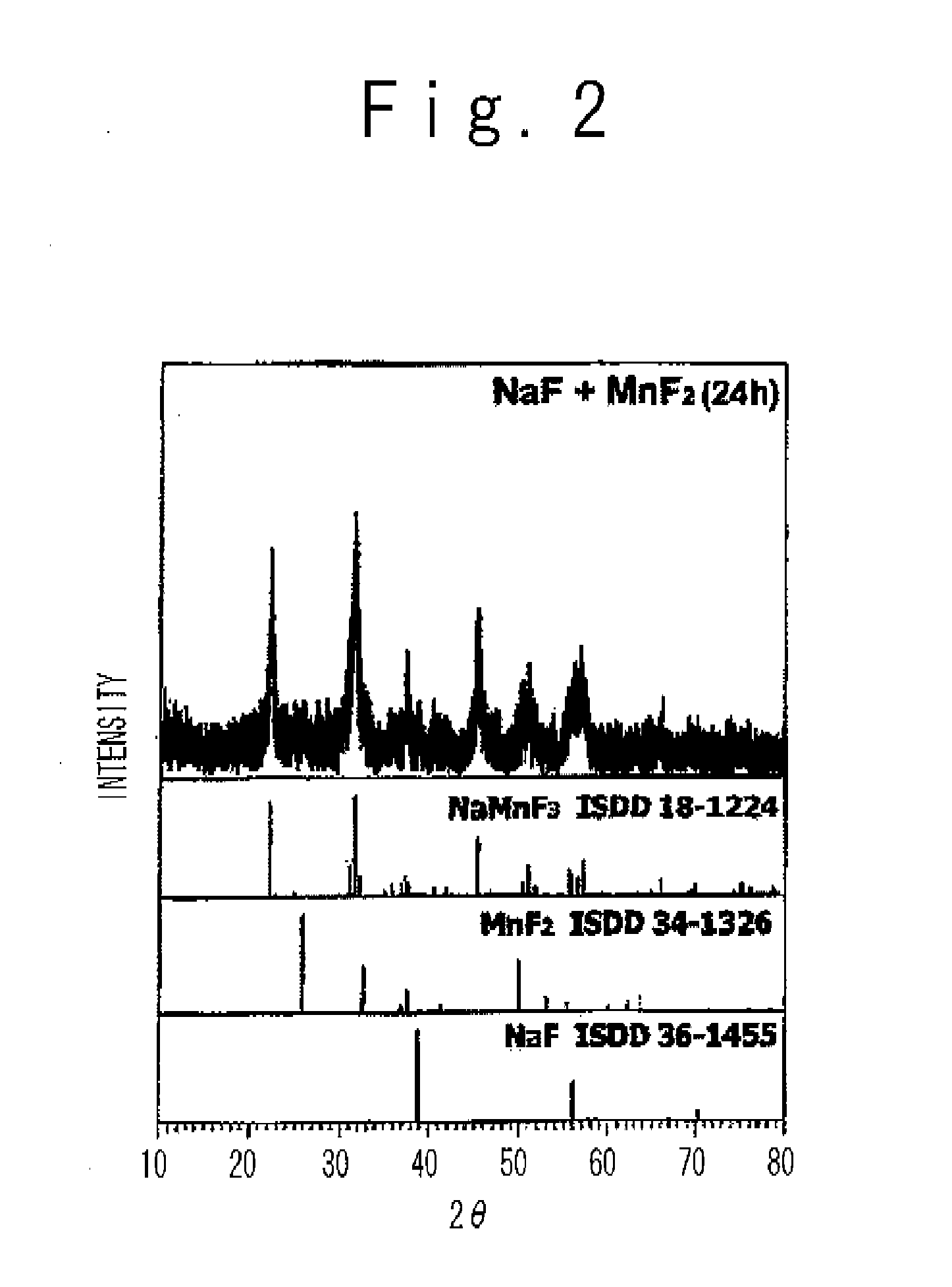
In a non-aqueous electrolyte secondary battery, in order to adjust a cathode active material in which guest cation such as Na and Li is included, alkaline metal fluoride which is expressed by a general formula AF and transition metal fluoride which is expressed by a formula M′ F2 are subjected to a mechanical milling process to produce metal fluoride compound AM′ F3. The mechanical milling process desirably uses a planetary ball mill.
Owner:MITSUBISHI HEAVY IND LTD +1
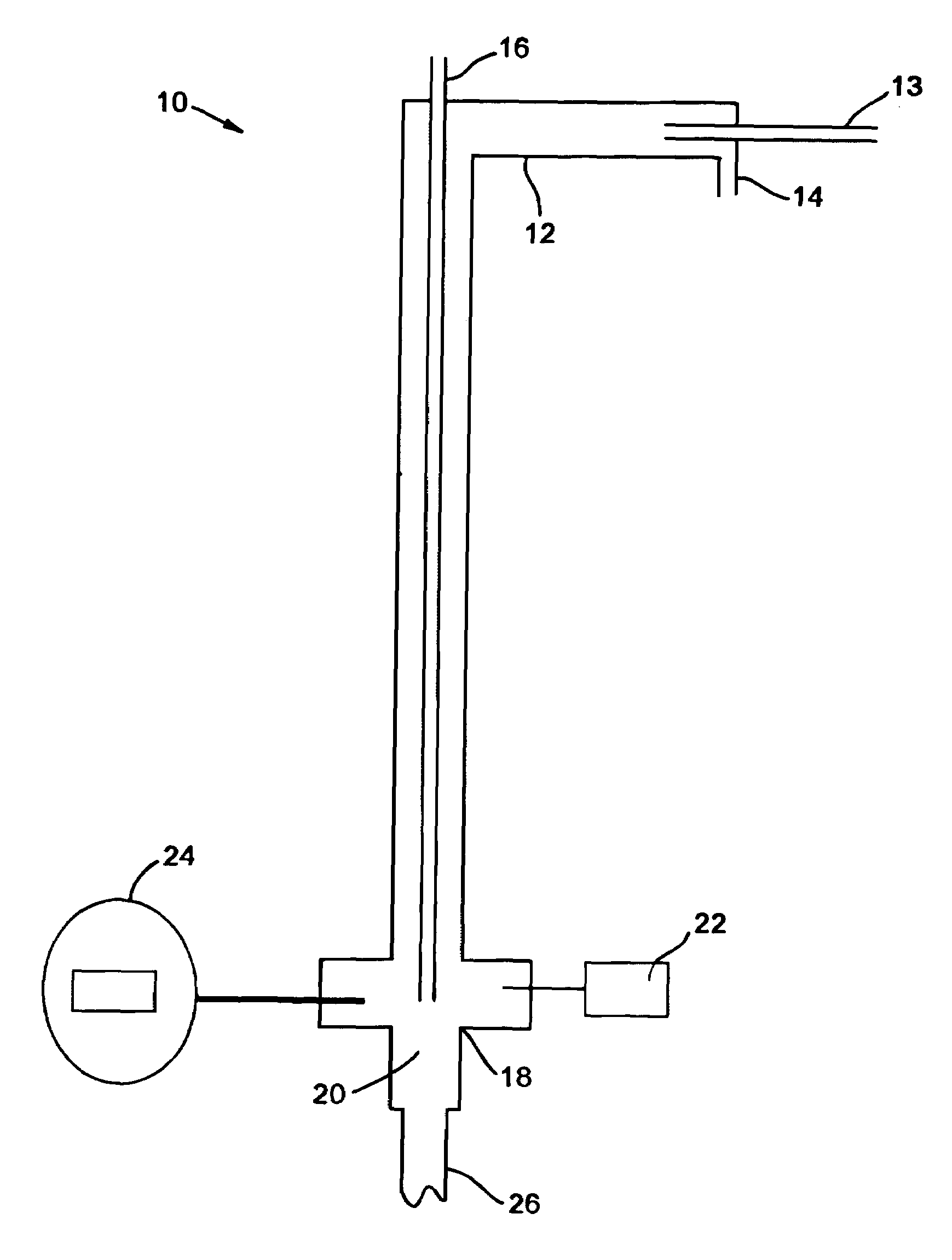
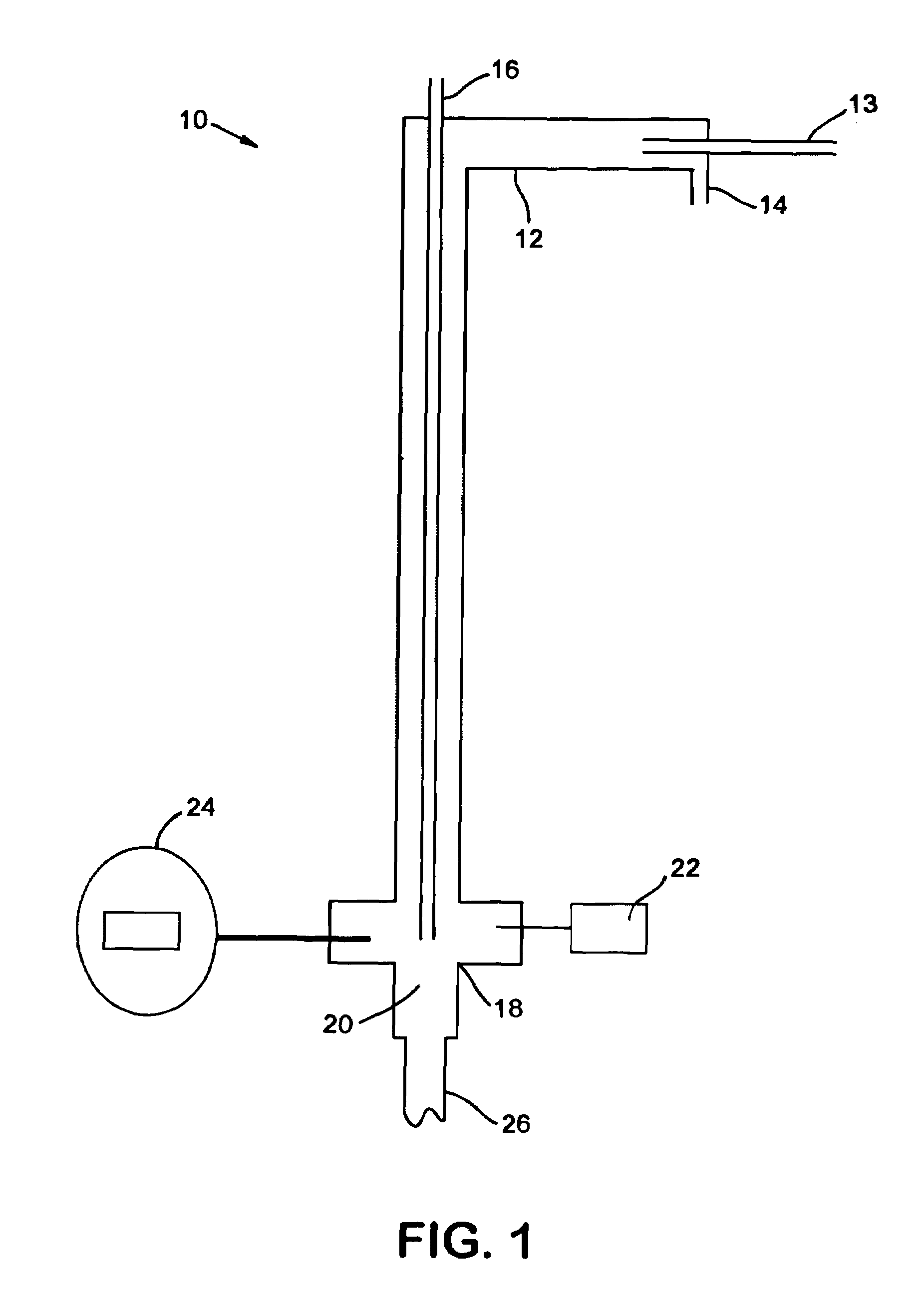
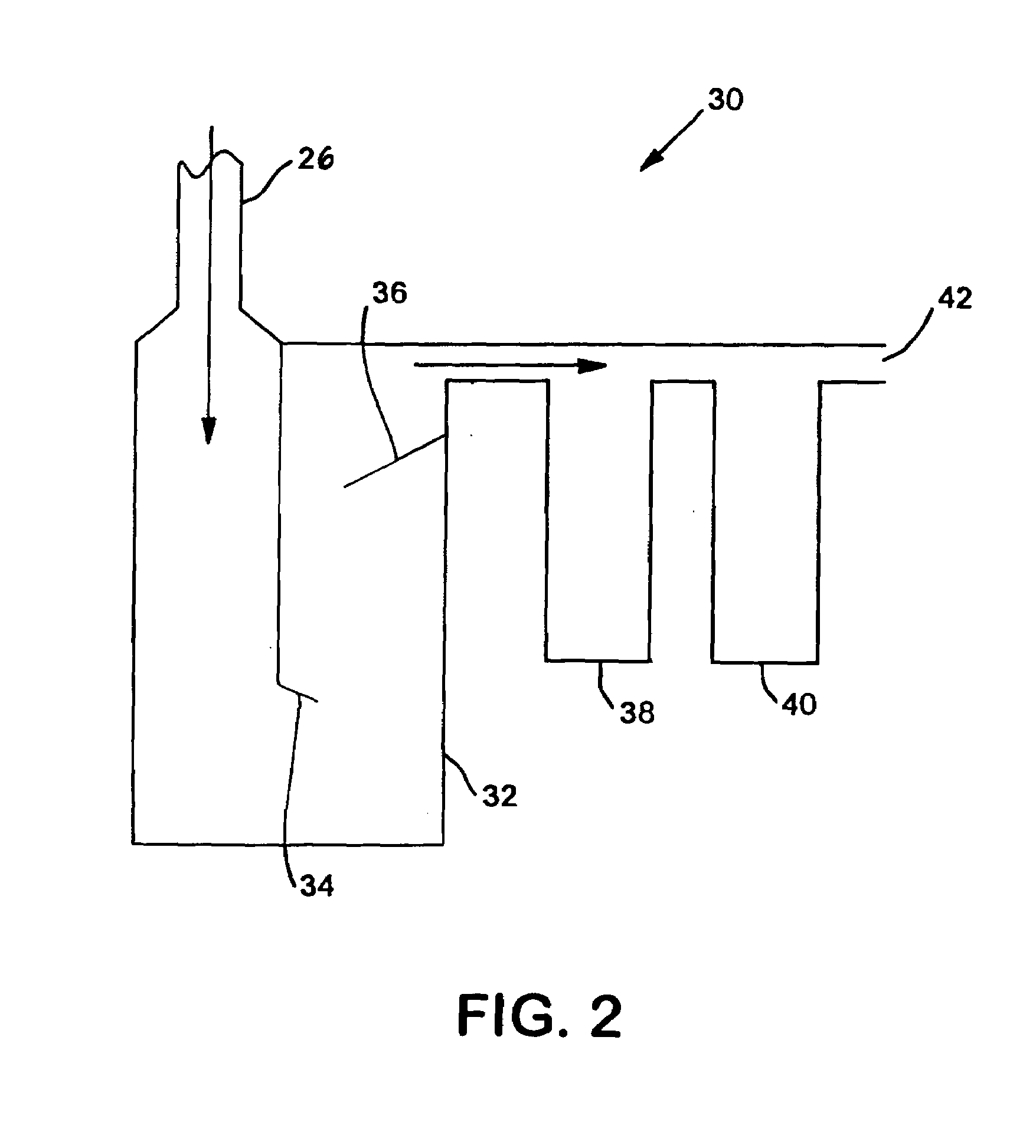
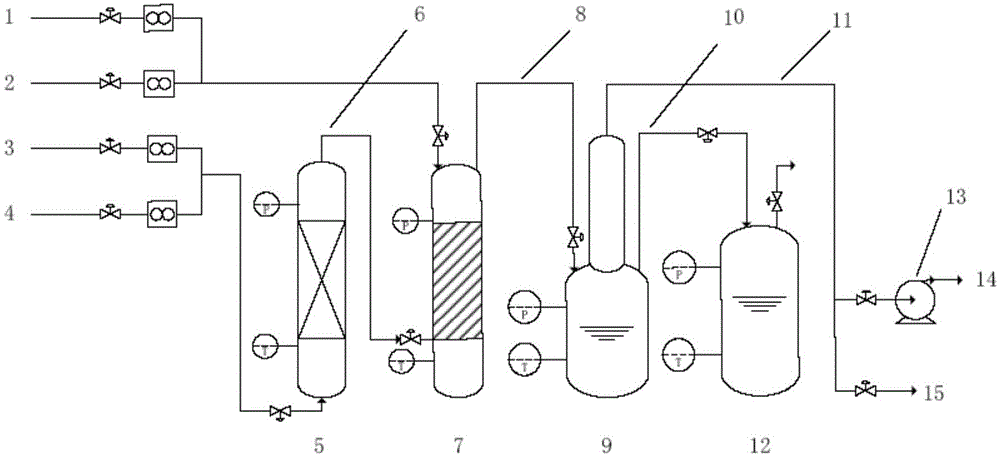
System and method for delivery of a vapor phase product to a point of use
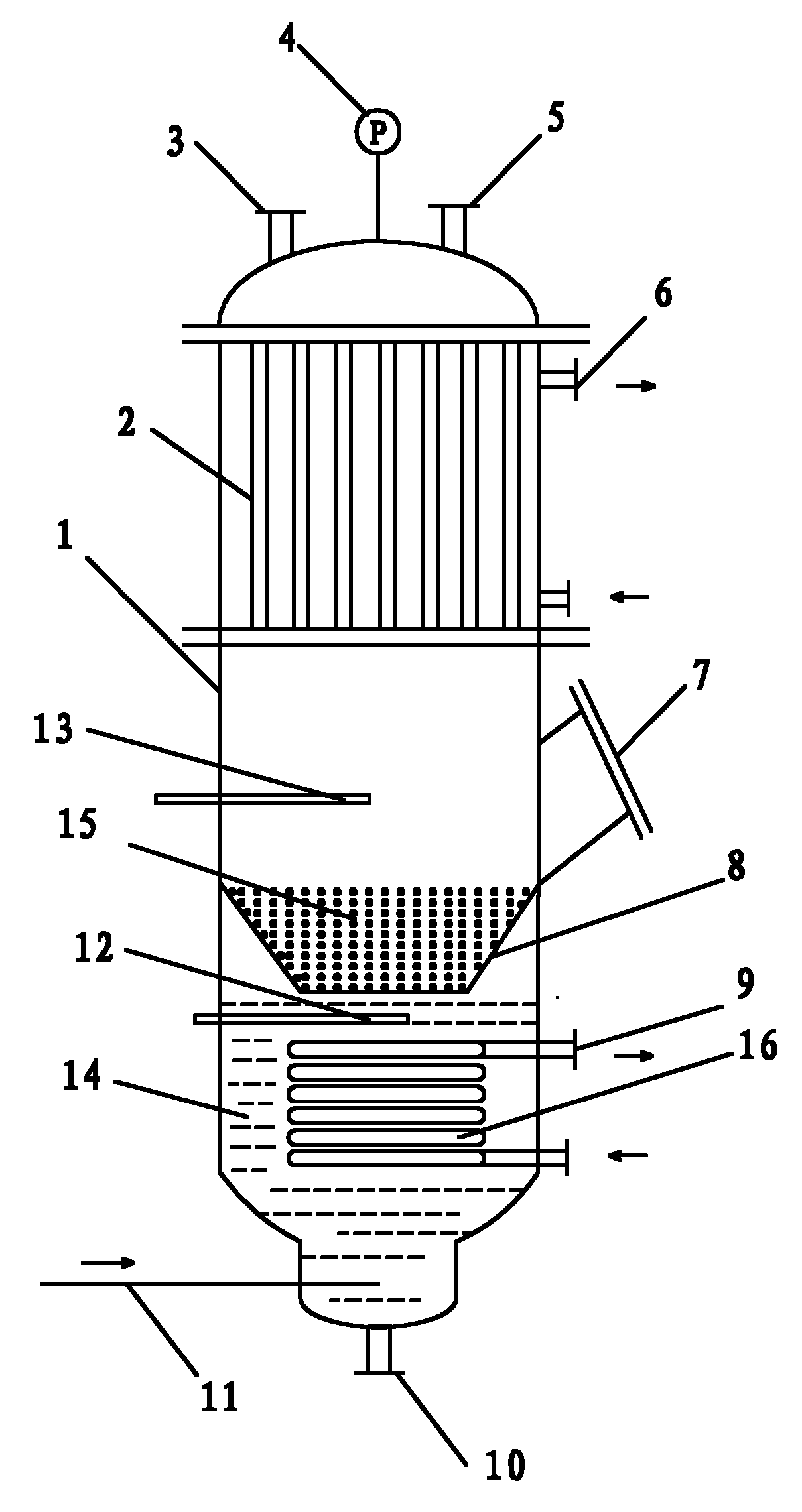
Provided are a novel system and method for delivery of a vapor phase product to a point of use, as well as a novel on-site chemical distribution system and method. The system for delivery of a vapor phase product includes a storage vessel containing a liquid chemical under its own vapor pressure, a column connected to receive the chemical in liquified state from the storage vessel, wherein the chemical is fractionated into a contaminated liquid heavy fraction and a purified light vapor fraction and a conduit connected to the column for removing the purified light vapor fraction therefrom. The system is connected to the point of use for introducing the purified vapor fraction thereto. Particular applicability is found in semiconductor manufacturing in the delivery of electronic specialty gases to one or more semiconductor processing tools.
Owner:AIR LIQUIDE AMERICA INC
System and method for delivery of a vapor phase product to a point of use
Provided are a novel system and method for delivery of a vapor phase product to a point of use, as well as a novel on-site chemical distribution system and method. The system for delivery of a vapor phase product includes a storage vessel containing a liquid chemical under its own vapor pressure, a column connected to receive the chemical in liquified state from the storage vessel, wherein the chemical is fractionated into a contaminated liquid heavy fraction and a purified light vapor fraction and a conduit connected to the column for removing the purified light vapor fraction therefrom. The system is connected to the point of use for introducing the purified vapor fraction thereto. Particular applicability is found in semiconductor manufacturing in the delivery of electronics specialty gases to one or more semiconductor processing tools.
Owner:AIR LIQUIDE AMERICA INC
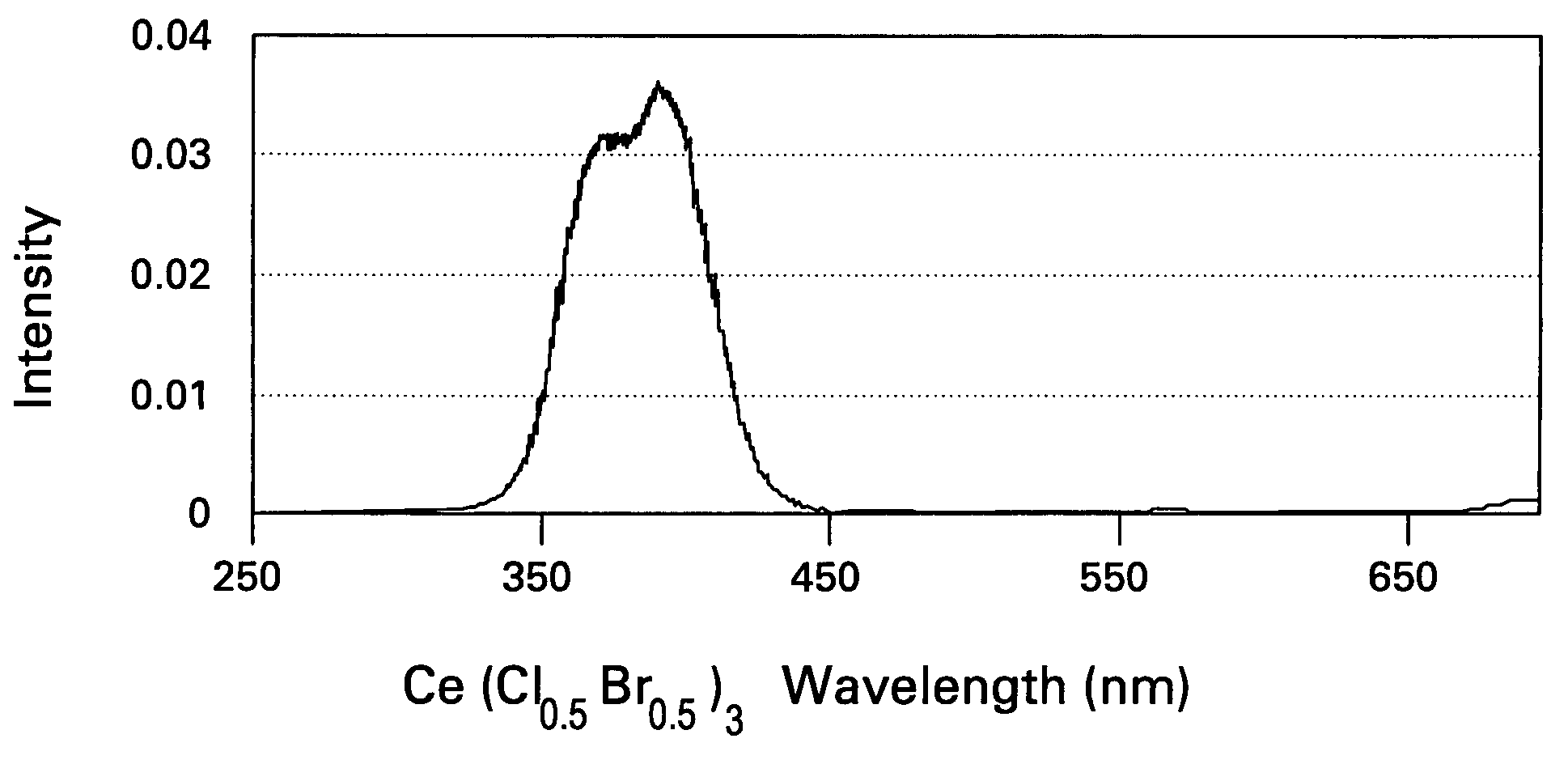
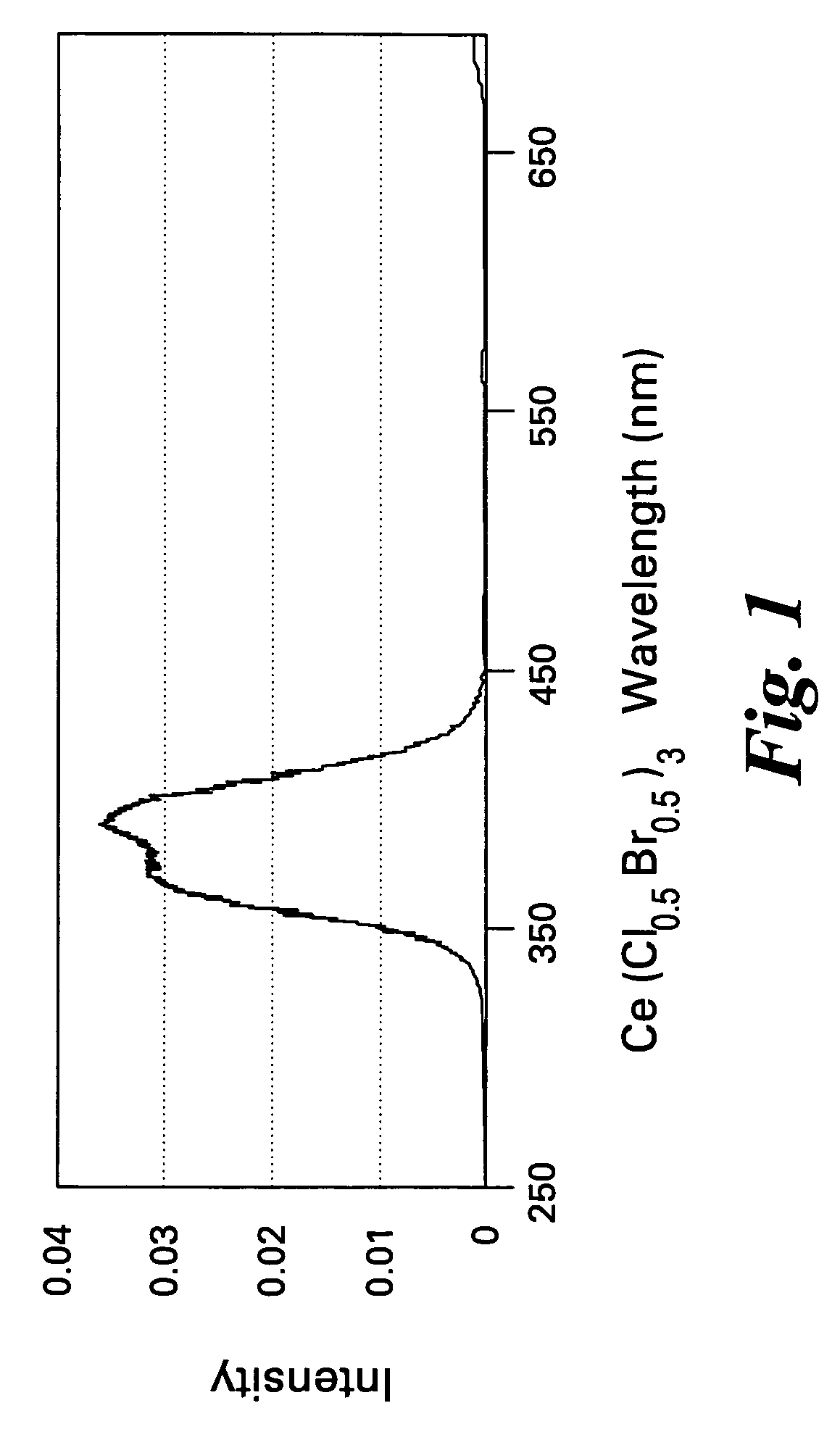
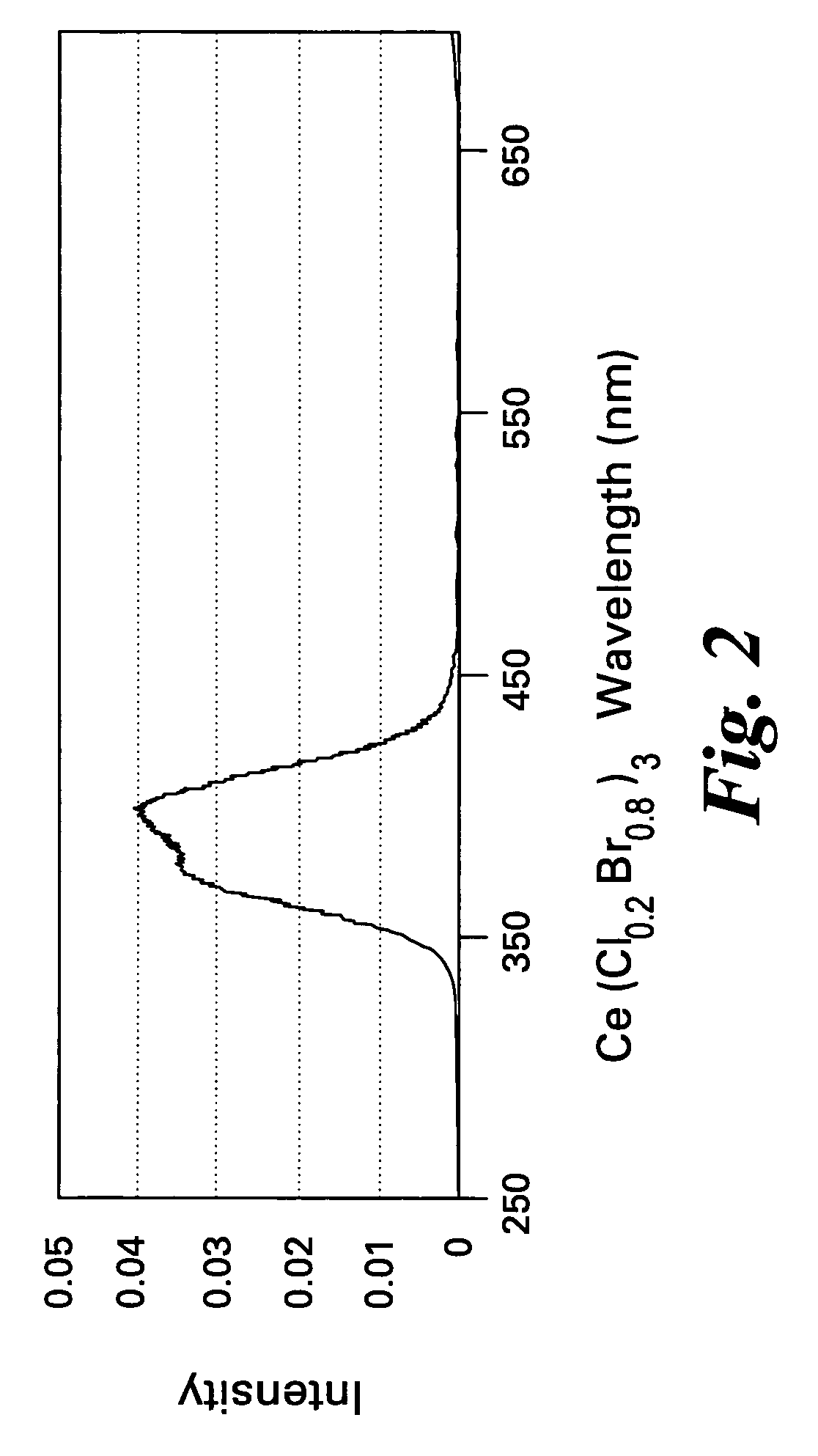
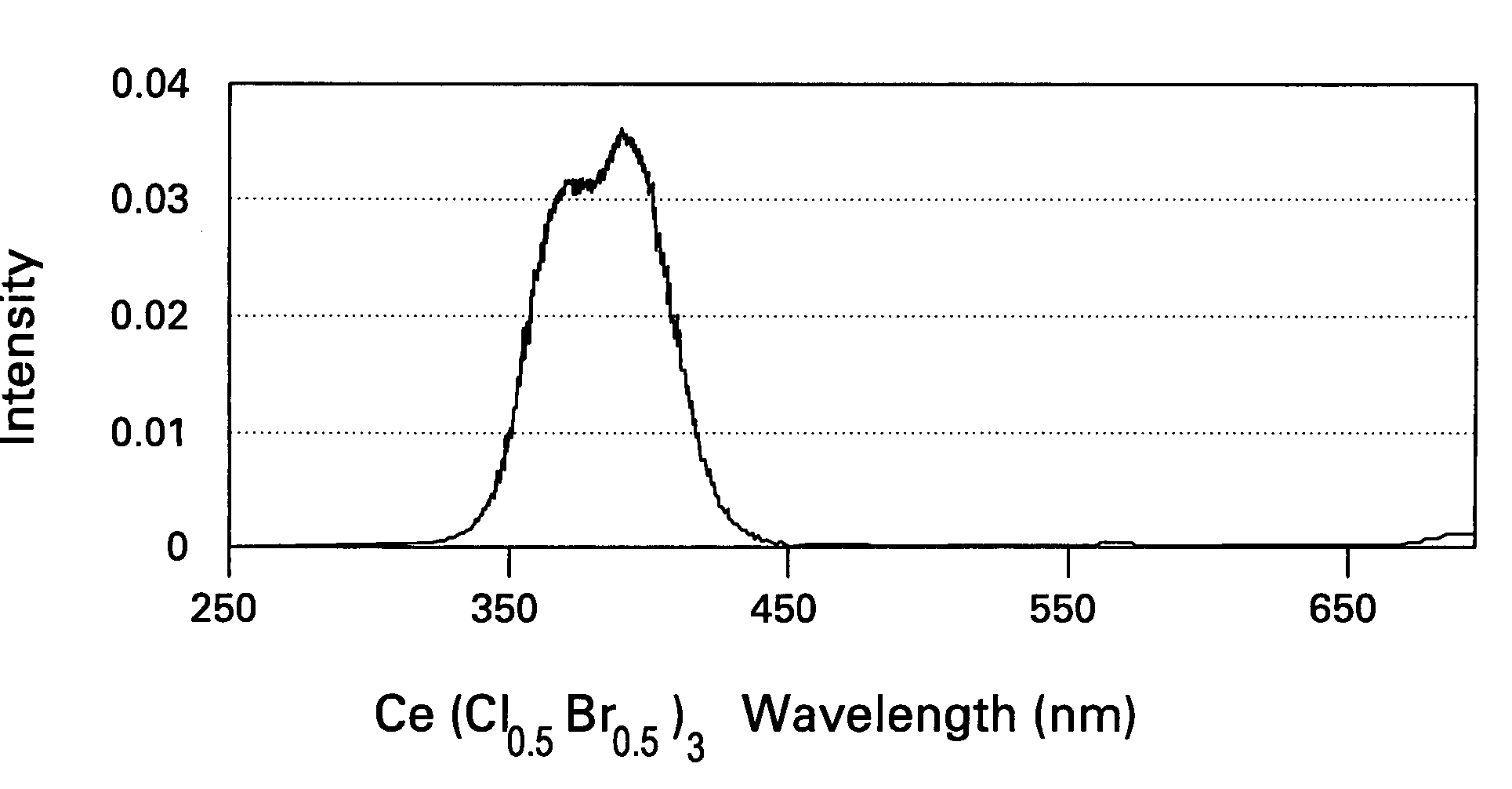
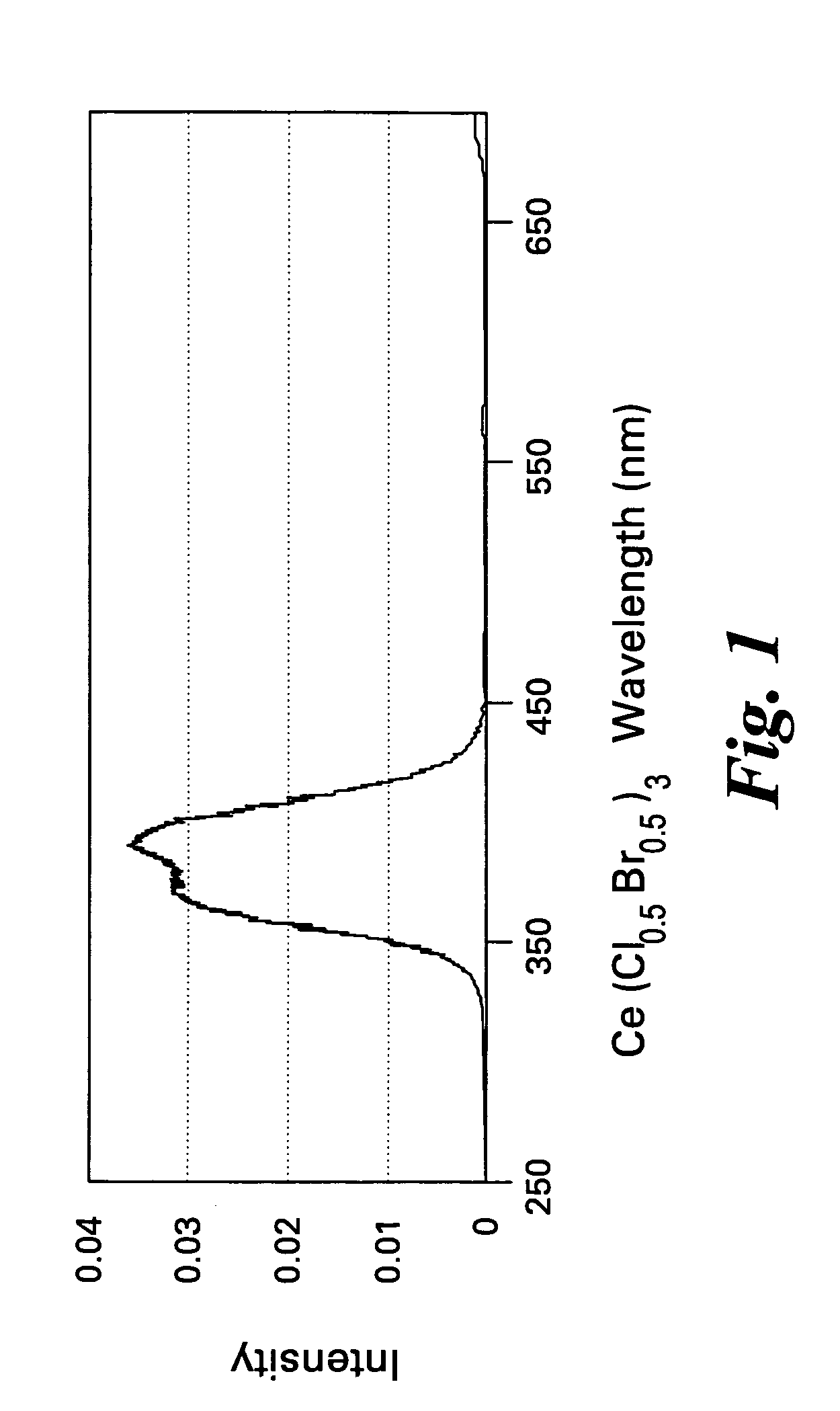
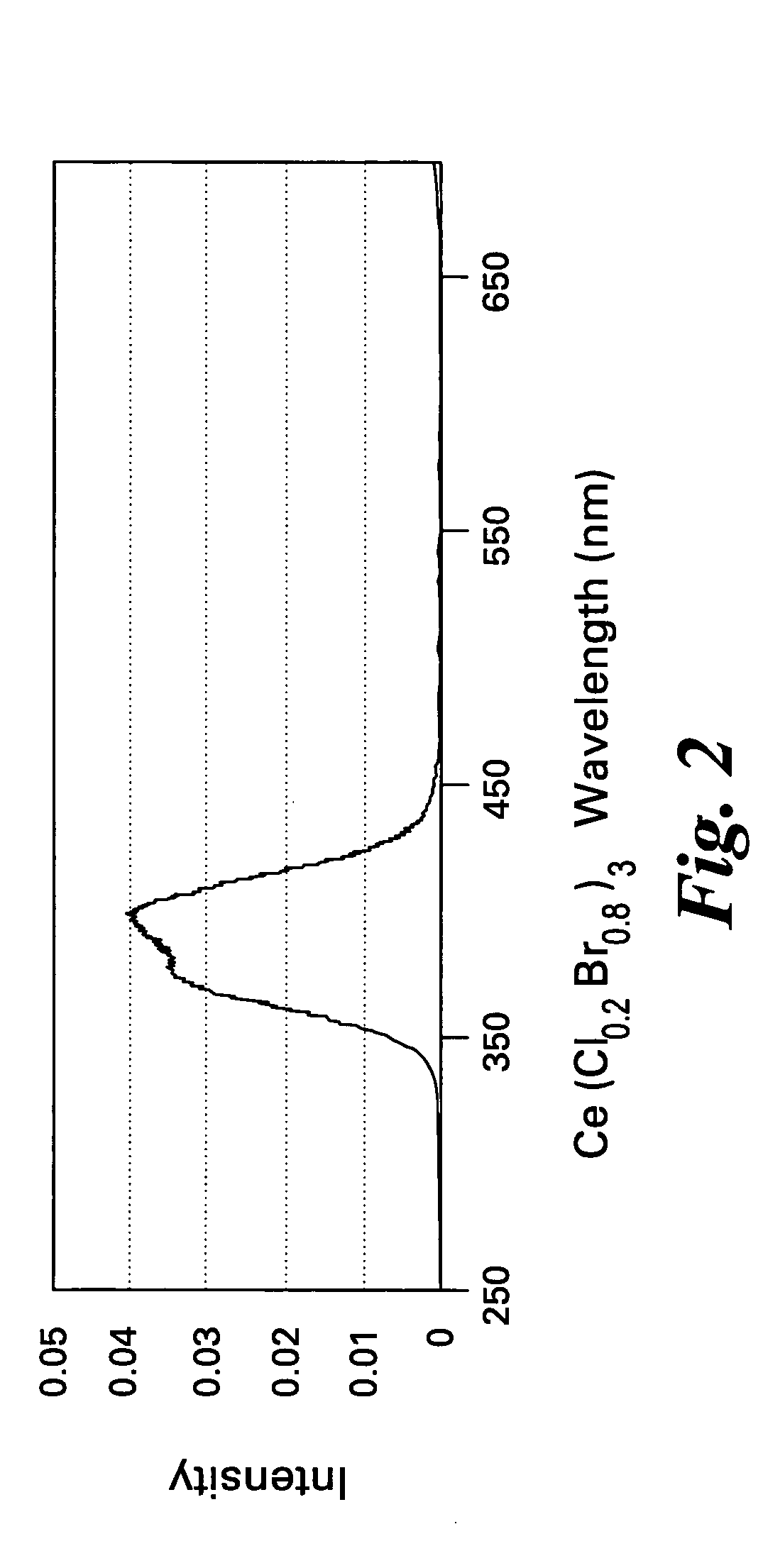
Scintillator compositions of cerium halides, and related articles and processes
ActiveUS7202477B2Material analysis by optical meansRare earth metal compoundsPhotovoltaic detectorsHigh energy
A scintillator composition is disclosed, containing a solid solution of at least two cerium halides. A radiation detector for detecting high-energy radiation is also described herein. The detector includes the scintillator composition mentioned above, along with a photodetector optically coupled to the scintillator. A method for detecting high-energy radiation with a scintillation detector is also described, wherein the scintillation crystal is based on a mixture of cerium halides.
Owner:BAKER HUGHES INC
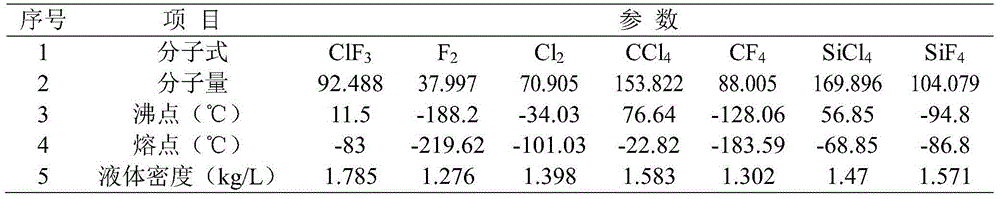
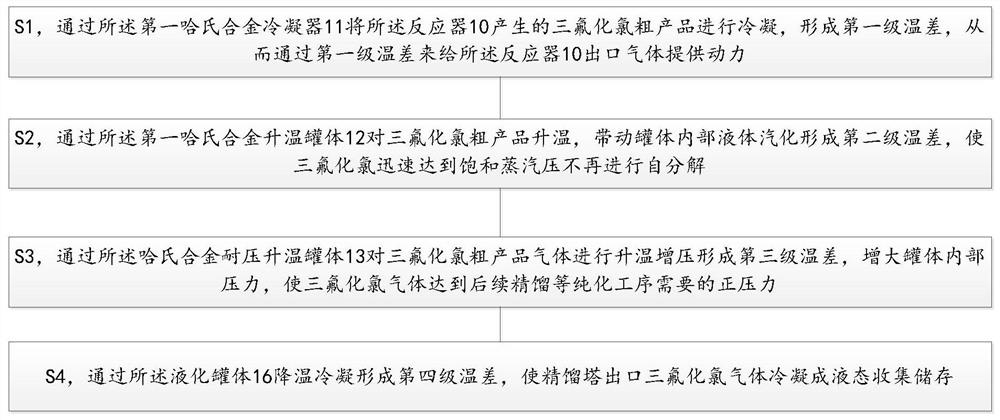
Purification method of chlorine trifluoride
ActiveCN104555927AReduce contentEasy to operateInter-halogen compoundsVacuum pumpingPurification methods
The invention discloses a purification method of chlorine trifluoride, and belongs to the field of fine chemical engineering. The purification method comprises the following steps: introducing chlorine trifluoride crude products into a tower kettle of a rectifying tower; performing vacuum-pumping on the rectifying system, starting to heat the tower kettle after the vacuum degree is stable, and performing total refluxing for 3-15 h to enable the impurity composition of which the boiling point is lower than that of chlorine trifluoride to be gathered at the top of the rectifying tower; when the temperature of the tower kettle is at a temperature of 0-5 DEG C, the temperature of the rectifying tower top is 2-3 DEG C below zero, then collecting front cut fraction at a reflux ratio of 30-300 and condensing and recovering; when the temperature of the tower kettle is 5-8 DEG C, the temperature of the rectifying tower top is 3-6 DEG C, collecting medium fraction at a reflux ratio of 10-90, and condensing and recovering; when the temperature of the tower kettle increases for 2 DEG C, that is, the temperature is 7-10 DEG C, the temperature of the rectifying tower top is 5-8 DEG C, collecting after cut fraction at a reflux ratio of 15-140, and condensing and recovering; when the temperature of the tower kettle is higher than or equal to 15 DEG C, stopping collecting after cut fraction, stopping heating, stopping vacuum-pumping, and finishing rectifying, wherein the medium fraction is the chlorine trifluoride refined product. The method is simple to operate and is high in purification efficiency.
Owner:PERIC SPECIAL GASES CO LTD
Production of gaseous chloramine
InactiveUS7070751B2Prevent scalingSimple streamlined designCombination devicesExhaust apparatusChloramine BGas phase
The present invention provides a reactor for the gas-phase reaction of commercially available gases in the presence of an inert carrier gas to form product gas. The reactor has a streamlined, compact configuration and at least one solids collection and removal system downstream of the reactor, where solids are efficiently removed from the product gas stream, leaving high purity product gas. The removal system allows for a simple reactor design, which is easy to clean and operates continuously over longer periods of time.
Owner:BRISTOL MYERS SQUIBB CO
Production of concentrated biocidal solutions
The production process comprises A) forming an acidic aqueous solution comprising alkali metal cations, bromide anions, and sulfamate anions; B) feeding into said aqueous solution a source of alkali metal cations and chlorine-containing bromide oxidant proportioned to keep the resultant aqueous medium acidic and to form an acidic product solution containing at least about 5 wt % of active bromine, and C) raising the pH of the aqueous product solution with water-soluble base to at least about 10.
Owner:ALBEMARLE CORP
Preparation method of chlorine trifluoride
ActiveCN104477849AStable reaction temperatureTimely supplementInter-halogen compoundsFine chemicalCorrosion
The invention discloses a preparation method of chlorine trifluoride and belongs to the field of fine chemical engineering. The method comprises the following steps: carrying out a bubbling reaction on liquid-phase chloride and fluorine gas at a certain temperature to obtain a product 1; then charging the product 1, a diluent gas and fluorine gas in a synthesis reactor, and reacting at a certain temperature to obtain a product 2; finally carrying out liquidation, lightweight material discharge and vaporization treatments on the product 2 to obtain chlorine trifluoride. The raw materials in the preparation method are low in price and easily available, thus avoiding the adoption of chlorine with high corrosion and high toxicity, improving safety during production, transportation and storage processes; moreover, the reactor is simple in structure and convenient for material charging and discharging; the reaction conversion rate is high; by-products can be easily separated; and the preparation method can be put into industrial production easily.
Owner:PERIC SPECIAL GASES CO LTD
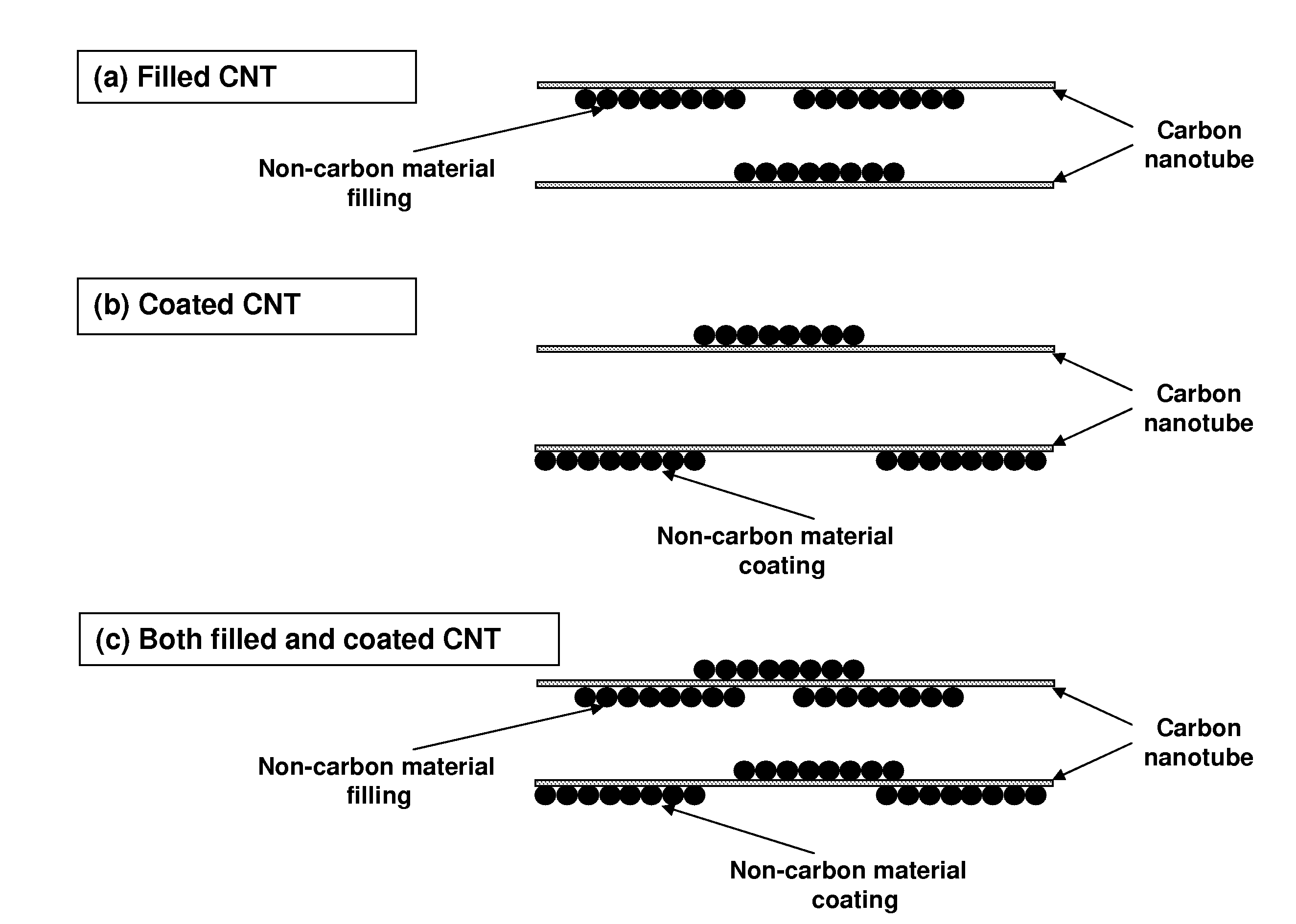
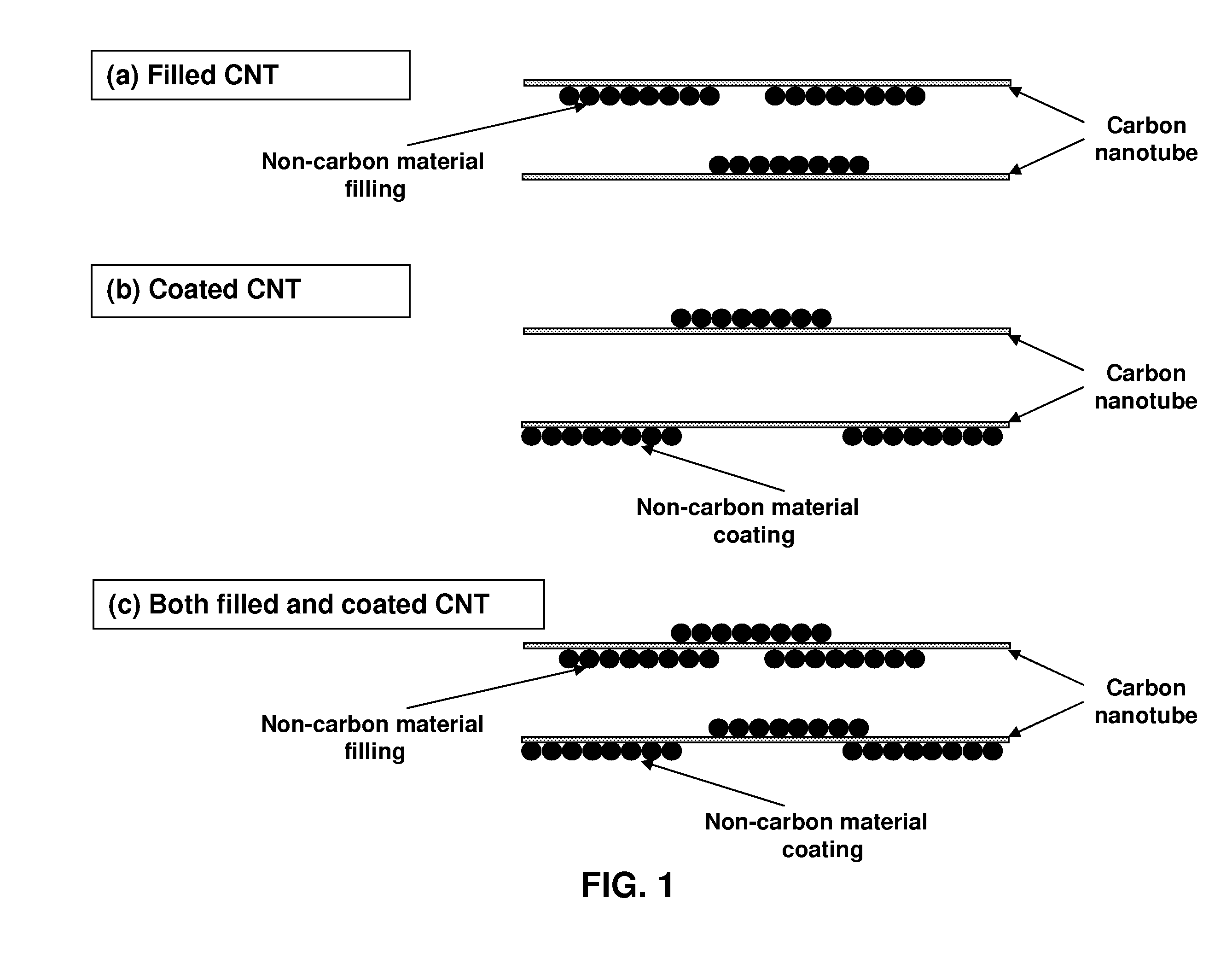
Organized carbon and non-carbon assembly and methods of making
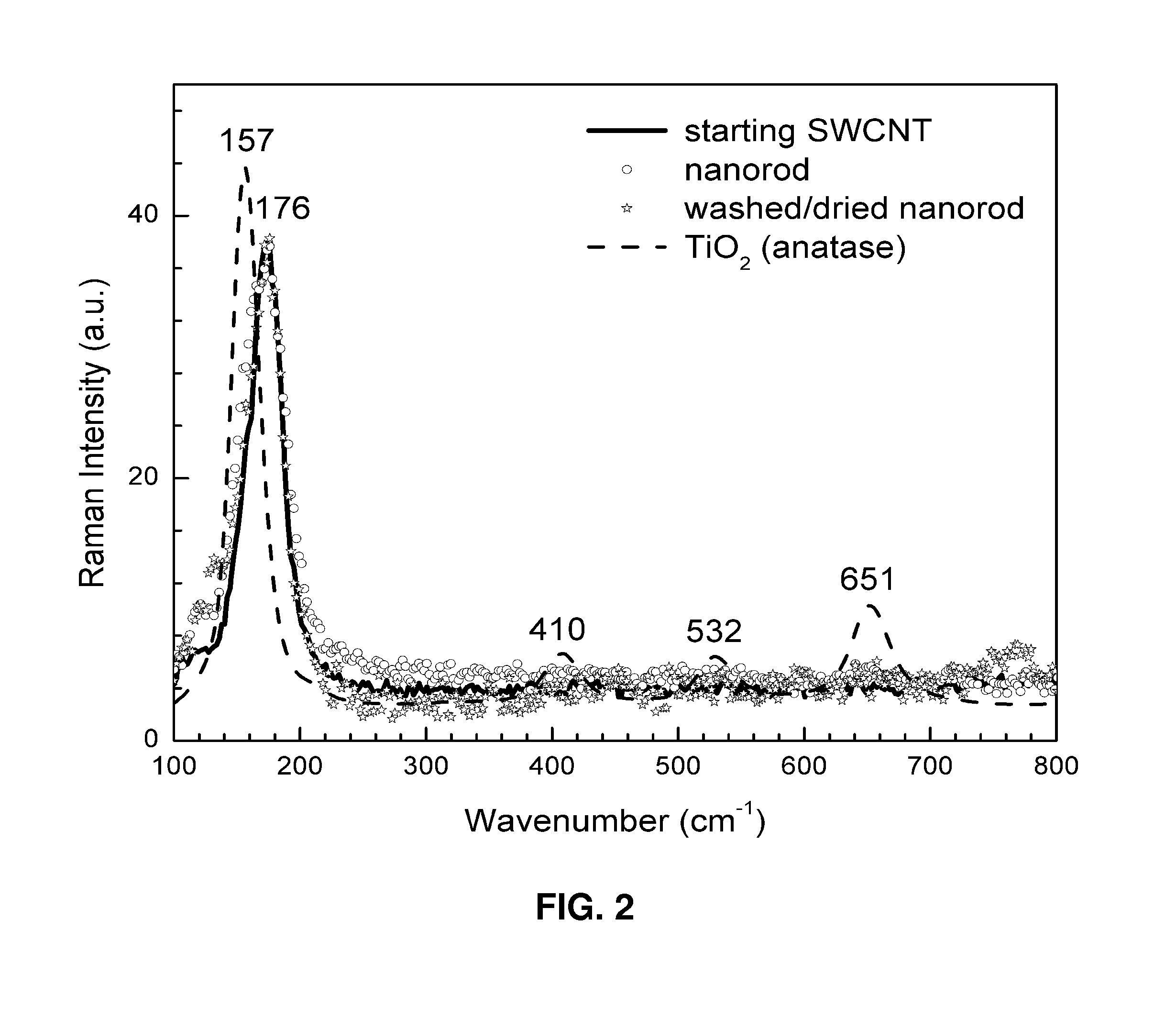
InactiveUS7938987B2Material nanotechnologyElectric discharge heatingCarbon nanotubeCarbon nanotube fet
This invention relates generally to organized assemblies of carbon and non-carbon compounds and methods of making such organized structures. In preferred embodiments, the organized structures of the instant invention take the form of nanorods or their aggregate forms. More preferably, a nanorod is made up of a carbon nanotube filled, coated, or both filled and coated by a non-carbon material. This invention is further drawn to the separation of single-wall carbon nanotubes. In particular, it relates to the separation of semiconducting single-wall carbon nanotubes from conducting (or metallic) single-wall carbon nanotubes. It also relates to the separation of single-wall carbon nanotubes according to their chirality and / or diameter.
Owner:YAZAKI CORP
Method for preparing iodine pentafluoride
ActiveCN101214927ASimple processLow reaction temperatureInter-halogen compoundsChemical reactionReaction temperature
The invention discloses a method of preparing iodine pentafluoride by chemical reaction in a negative pressure container with iodine and fluorine gas as raw materials, and iodine pentafluoride as the carrier. The method includes the following steps: firstly, the iodine is put in a reactor with iodine pentafluoride liquid; the reactor is sealed and evacuated, with pressure of -0.08 to -0.06MPa; cooling water is fed to cool the product. Then fluorine gas is fed into the reactor to have chemical reaction with the iodine, so as to form the three-phase reaction of gas, liquid and solid; the pressure in the reactor is controlled to be less than -0.02MPa and the temperature is controlled to be lower than 85 DEG C. The invention has the advantages of simple process, low reaction temperature, easiness in being controlled, stable and reliable production quality, and high yield.
Owner:RES INST OF PHYSICAL & CHEM ENG OF NUCLEAR IND
Preparation method and device of chlorine trifluoride
ActiveCN104477850ASimple production processAvoid corrosionInter-halogen compoundsBoron trifluorideReaction temperature
The invention discloses a preparation method and device of chlorine trifluoride and belongs to the field of fine chemical engineering. The method comprises the following steps: mixing chlorine gas, fluorine gas and a diluent gas, and then charging the mixed gas in a reactor filled with a catalyst, and reacting at 100-400 DEG C to obtain a product 1; then carrying out cooling, liquidation, lightweight material discharge and vaporization treatments on the product 1 to obtain the chlorine trifluoride. The device comprises a catalytic reactor, a low-temperature collector, a chlorine trifluoride storage tank and a vacuum pump, wherein the catalytic reactor, the low-temperature collector and the chlorine trifluoride storage tank are sequentially connected through pipelines. The process is low in reaction temperature, short in reaction time and high in product yield; the device has the advantages that the length of the reactor is greatly shortened and the production process of chlorine trifluoride is simplified.
Owner:PERIC SPECIAL GASES CO LTD
Preparation method and reaction equipment of iodine pentafluoride
ActiveCN101920937AHigh yieldMild responseInter-halogen compoundsIodine pentafluorideContinuous reaction
The invention discloses a preparation method of iodine pentafluoride, which has the advantages of continuous reaction, high yield, mild and complete reaction and very light pollution level and comprises the following steps: placing solid iodine above liquid iodine pentafluoride or an iodine pentafluoride solution of iodine, introducing fluorine gas in the liquid iodine pentafluoride or the iodine pentafluoride solution of iodine, and reacting solid iodine and fluorine gas to generate iodine pentafluoride which flows in the liquid iodine pentafluoride or the iodine pentafluoride solution of iodine after dissolving the iodine partially. The invention also discloses reaction equipment dedicated for the method.
Owner:FUJIAN YONGJING TECH CO LTD
Scintillator compositions of cerium halides, and related articles and processes
ActiveUS20060197023A1Material analysis by optical meansRare earth metal compoundsPhotodetectorHigh energy
A scintillator composition is disclosed, containing a solid solution of at least two cerium halides. A radiation detector for detecting high-energy radiation is also described herein. The detector includes the scintillator composition mentioned above, along with a photodetector optically coupled to the scintillator. A method for detecting high-energy radiation with a scintillation detector is also described, wherein the scintillation crystal is based on a mixture of cerium halides.
Owner:BAKER HUGHES INC
Process for the synthesis of BrSF5
InactiveUS6869582B2Shorten the overall cyclePigmenting treatmentSulfur-halogen-hydrogen-oxygen compoundsSufficient timeAlkaline earth metal
A process for producing BrSF5 includes providing a first reactant including a metal fluoride of fluorine and a metal M selected from the group consisting of alkali metals, alkaline earth metals, and Ag, providing a second reactant including BrF3, combining the first reactant and the second reactant to form a mixture, wherein the first reactant and the second reactant are allowed to contact for a period of time sufficient to produce MBrF4 in an amount stoichiometrically equivalent to a quantity of BrF3, and providing a third reactant including SF4, wherein the third reactant reacts with MBrF4. The process for producing BrSF5 can further include providing a fourth reactant including Br2, wherein the fourth reactant is provided before, during and / or after providing the first reactant, the second reactant and / or the third reactant. BrSF5 is produced in a yield of from about 50% to about 99.99% based on the amount of SF4.
Owner:VERSUM MATERIALS US LLC
Method for continuously preparing iodine pentafluoride
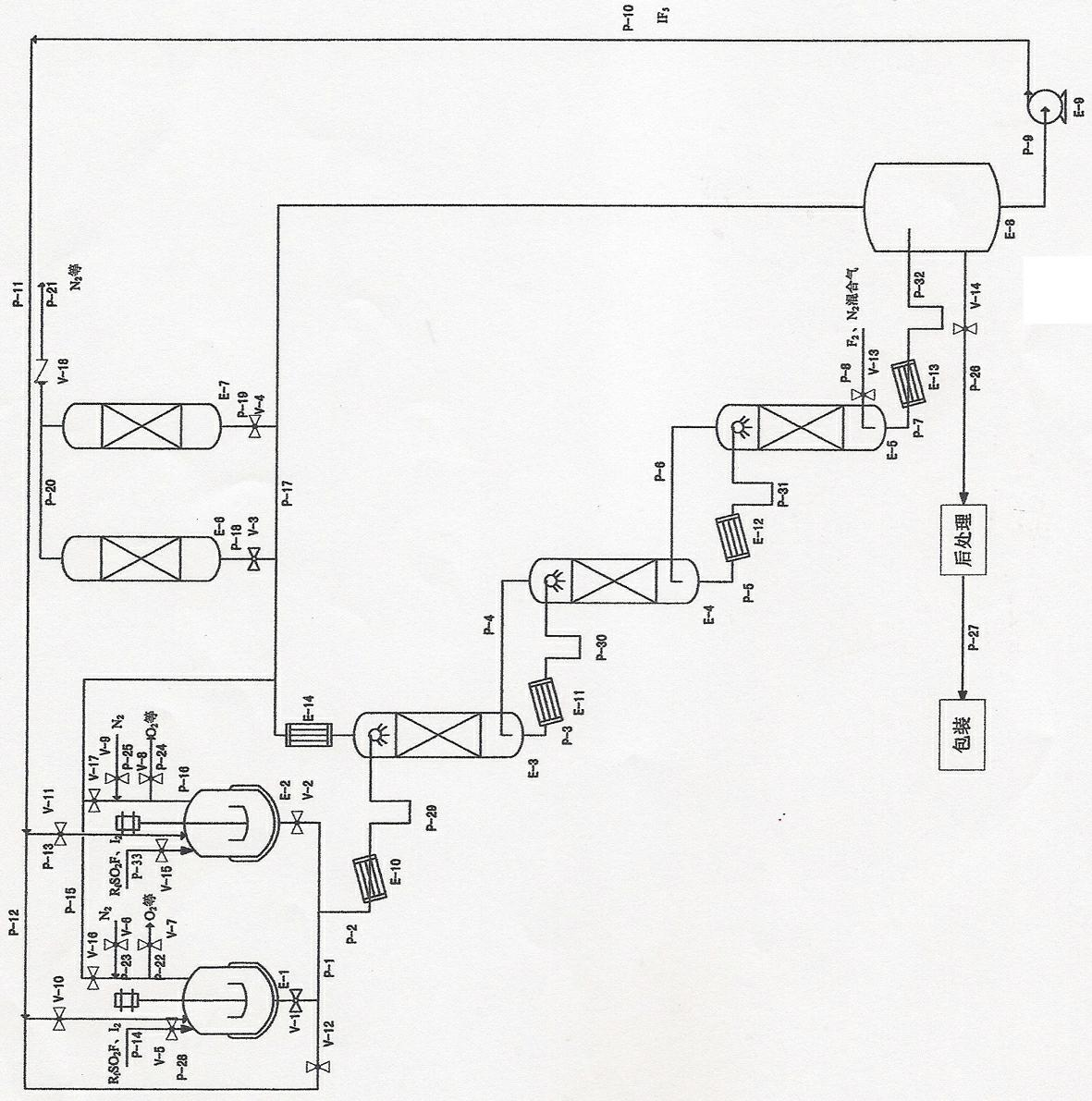
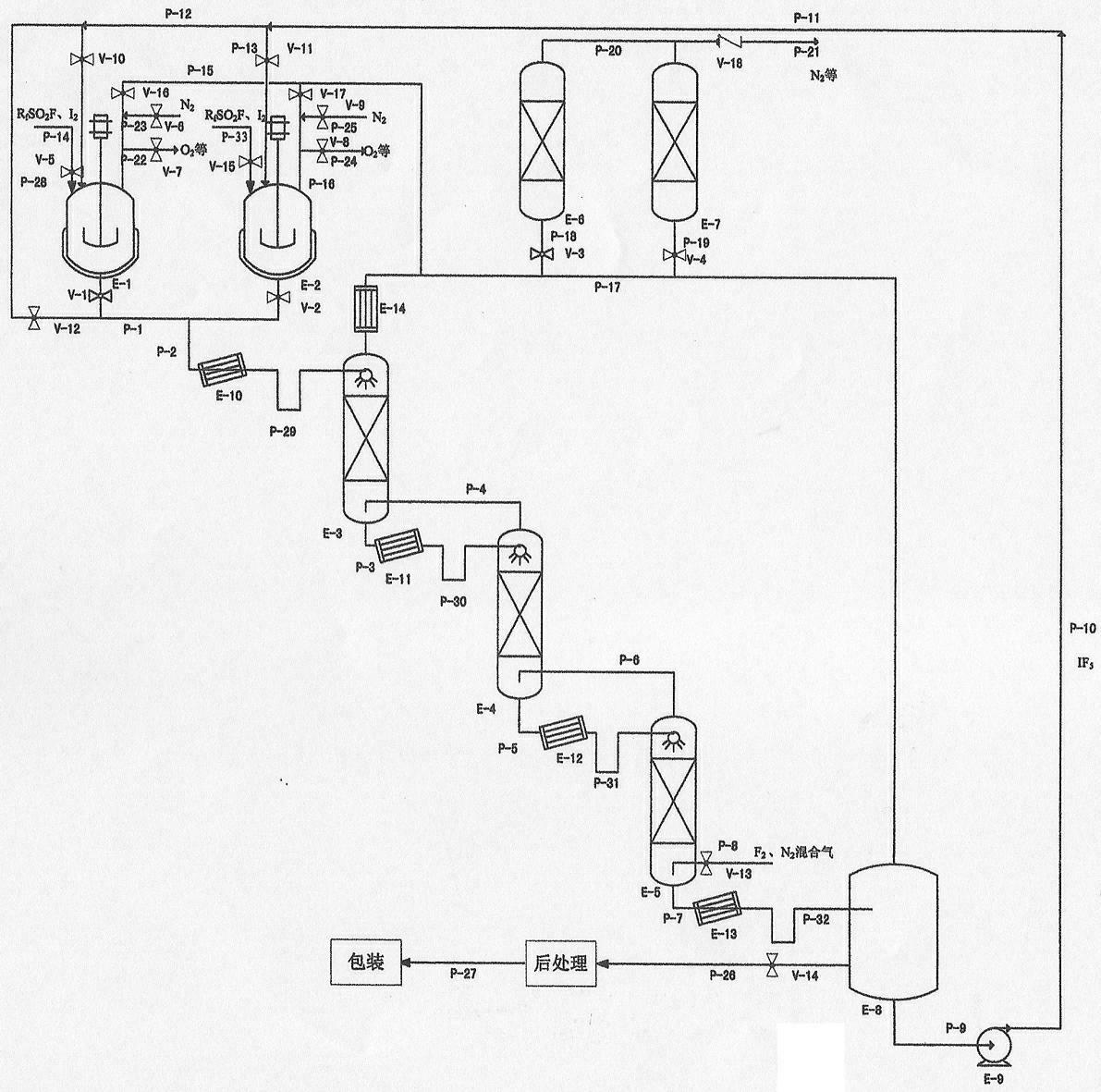
ActiveCN102556974AThe reaction temperature is easy to controlReaction temperature safetyInter-halogen compoundsProcess equipmentReaction temperature
The invention discloses a method for continuously preparing iodine pentafluoride. According to the method, a serial process flow of a multistage tower type reactor is adopted; according to process requirement, the tower number can be 1-N grades, wherein N is more than or equal to 3; in the atmosphere containing sulfuryl fluoride (RfSO2F), an iodine pentafluoride solution of iodine and fluoride gas or mixed gas of fluoride and nitrogen respectively enter a multistage reaction tower from two ends, reversely flow and generate a reaction. The method has the advantages that the reaction temperature is controllable, higher safety is ensured and the phenomenon that the iodine is excessively fluoridized at high temperature to generate IF7 is avoided; two iodine dissolving tanks are alternatively used so as to facilitate the continuous atomization; the sulfuryl fluoride atmosphere facilitates the operation of a gas-liquid reaction and is remarkably superior to the hydrogen fluoride existing atmosphere introduced in other patents, so that the purification of products is facilitated; and unreacted fluoride gas in tail gas can be adsorbed by solid salts or alkali and then be recycled. The process equipment and parts are made of general steel; and pitting-free chemical nickel-plating treatment and fluorine gas passivation treatment are used in sequence so as to effectively reduce the investment cost and facilitate the industrialization.
Owner:HUBEI ZHUOXI FLUOROCHEM
Apparatus and method for purification of corrosive gas streams
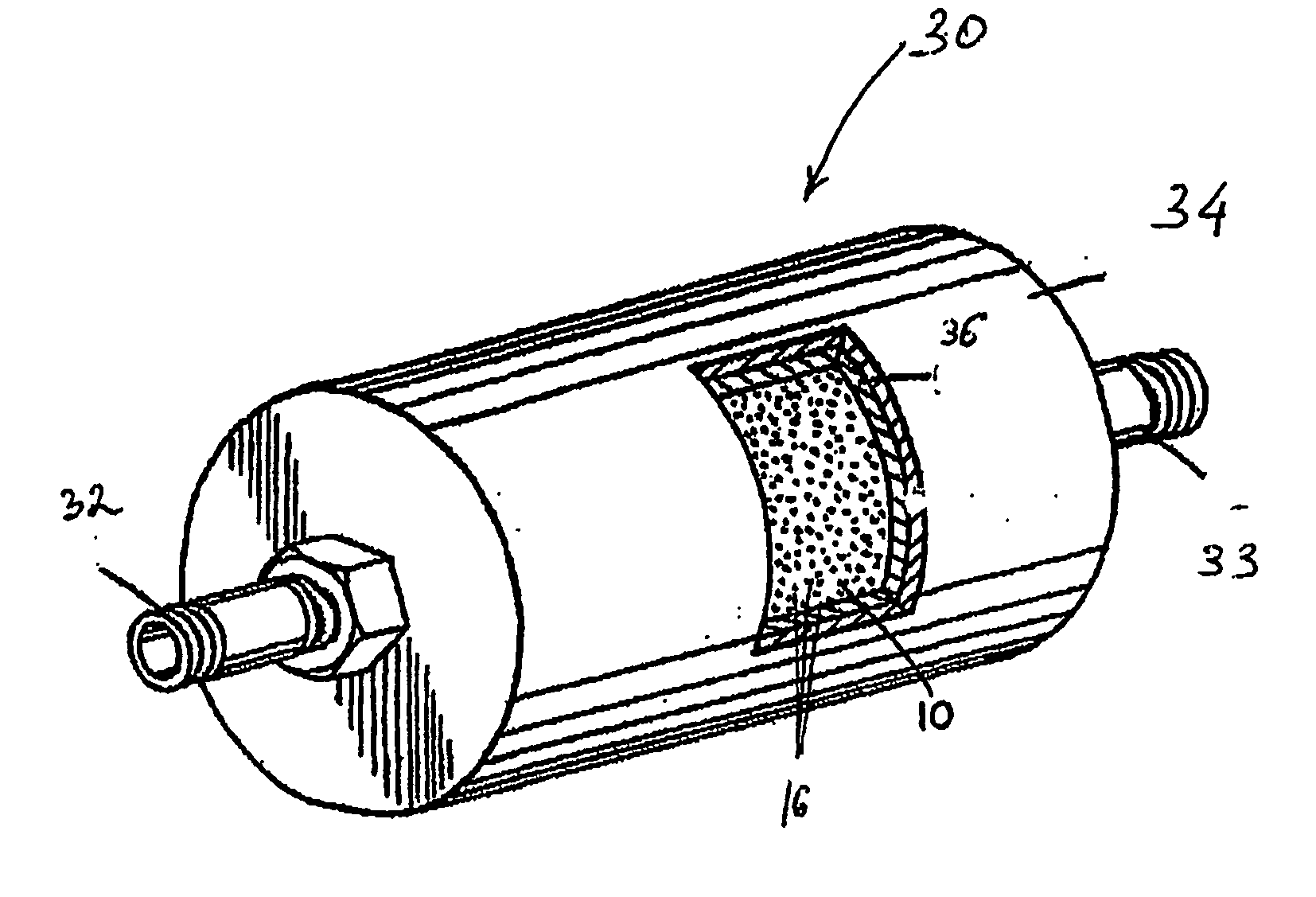
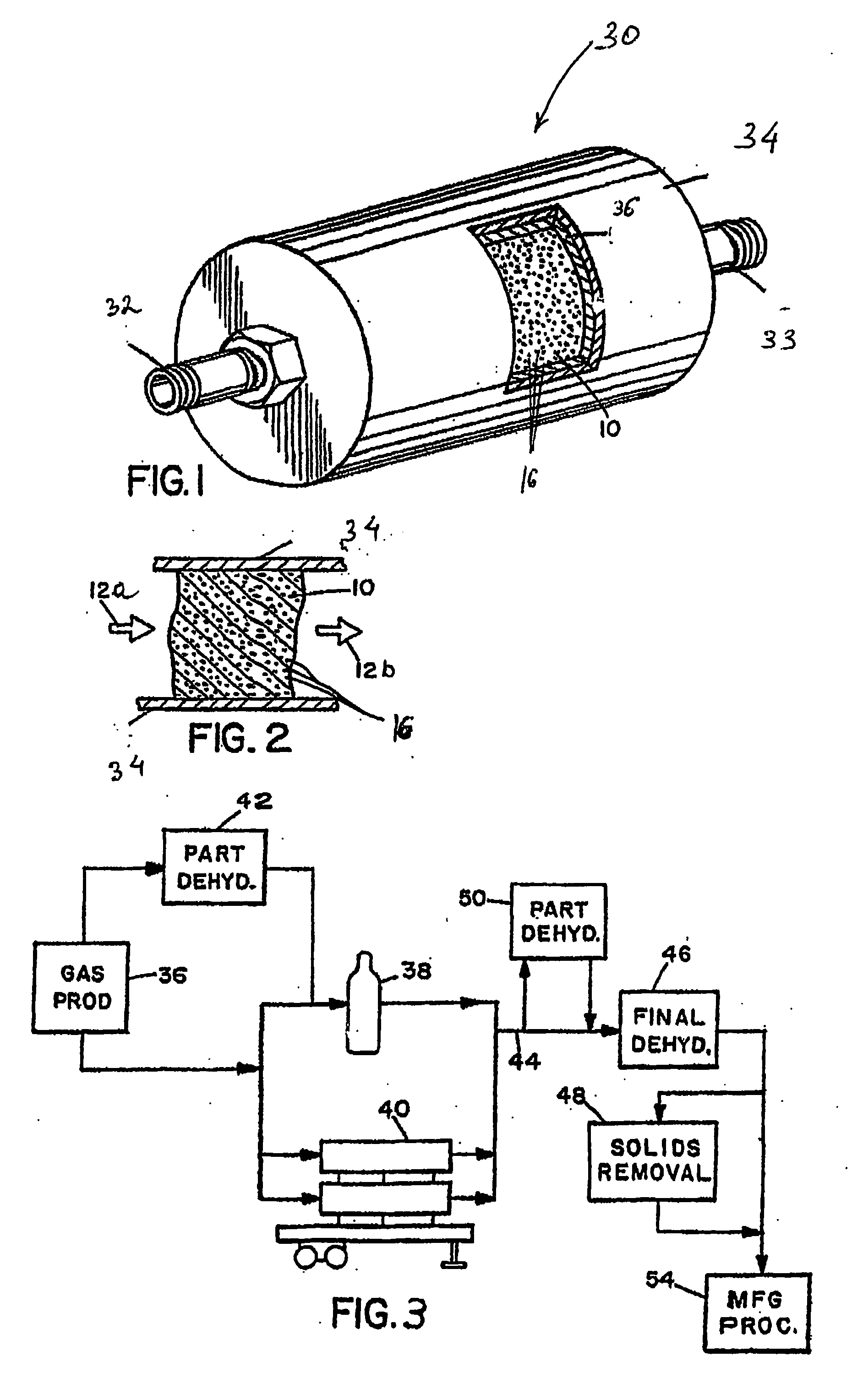
InactiveUS20070031321A1Reduce moistureWater contentMagnesium fluoridesHydrogen bromideParticulatesHalogen
A process, composition and apparatus for the removal of impurities from corrosive gases, particularly halogen-containing gases, down to about 100 ppb concentration are described. The critical component is zirconia (ZrO2), which in a variety of physical forms is capable of dehydrating such gases. The zirconia can be in the form of a coating on a substrate, as a granular bulk material, or deposited within the pores of a porous body. The zirconia is retained in a simple container which is easily installed in a gas supply line, such as to a gas- or vapor-deposition manufacturing unit. The purification process can be operated for long periods of time in the presence of these gases. The invention provides final purification to gas streams intended for gas- or vapor-deposition formation of high purity electronic, prosthetic or similar products, and can be used in combination with a preliminary dehydration process or a solid particulate removal unit upstream.
Owner:MYKROLIS CORP +1
Apparatus and method for purification of corrosive gas streams
InactiveCN1809412ALess susceptible to corrosionReduce moisture contentHydrogen bromideBromineHalogenPhysical chemistry
A process, composition and apparatus for the removal of impurities from corrosive gases, particularly halogen-containing gases, down to about 100 ppb concentration are described. The critical component is zirconia (ZrO2), which in a variety of physical forms is capable of dehydrating such gases. The zirconia can be in the form of a coating on a substrate, as a granular bulk material, or deposited within the pores of a porous body. The zirconia is retained in a simple container which is easily installed in a gas supply line, such as to a gas- or vapor-deposition manufacturing unit. The purification process can be operated for long periods of time in the presence of these gases. The invention provides final purification to gas streams intended for gas- or vapor-deposition formation of high purity electronic, prosthetic or similar products, and can be used in combination with a preliminary dehydration process or a solid particulate removal unit upstream.
Owner:ENTEGRIS INC
Method for preparing chlorine trifluoride
PendingCN112723313AFully contactedIncrease contact timeInter-halogen compoundsChemical industryPtru catalyst
The invention belongs to the technical field of inorganic fluorine chemical industry, and particularly relates to a method for preparing chlorine trifluoride, which comprises the following steps: respectively preheating fluorine gas and chlorine gas, sufficiently mixing, and reacting at normal pressure to obtain chlorine trifluoride mixed gas; condensing the chlorine trifluoride mixed gas to obtain a chlorine trifluoride crude product; and rectifying, purifying and condensing the chlorine trifluoride crude product to obtain a chlorine trifluoride product. According to the method, chlorine trifluoride can be prepared in one step under the conditions of no catalyst and normal pressure, the process operation is simple, the reaction time is short, the temperature and pressure are easy to control, the reaction process is safe, and industrial production can be realized.
Owner:SICHUAN HONGHUA IND
Electronic-grade chlorine trifluoride rectification purification system control method
ActiveCN112919419AImproved reflux ratio parameter stabilityRealize wide dynamic balance operationSolidificationLiquefactionHydrogen fluorideTemperature control
The invention provides an electronic-grade chlorine trifluoride rectification purification system control method and an electronic-grade chlorine trifluoride rectification device. The electronic-grade chlorine trifluoride rectification device comprises a secondary low-temperature rectification device which comprises a low-boiling tower and a high-boiling tower, and the econdary low-temperature rectification device comprises anextraction agent which is used for further dispersing hydrogen fluoride and chlorine trifluoride associated molecules, so that the requirements of electronic-grade chlorine trifluoride are met; the stability of reflux ratio parameters of a vapor-liquid (chlorine trifluoride-hydrogen fluoride) phase equilibrium system can be effectively improved through a tower plate temperature control method, and wide dynamic equilibrium operation under various working conditions is realized; according to the tower plate temperature control method, effective separation of chlorine trifluoride and various impurity components can be realized through a deep rectification technology, and electronic-grade chlorine trifluoride is purified.
Owner:FUJIAN DEER TECH CORP
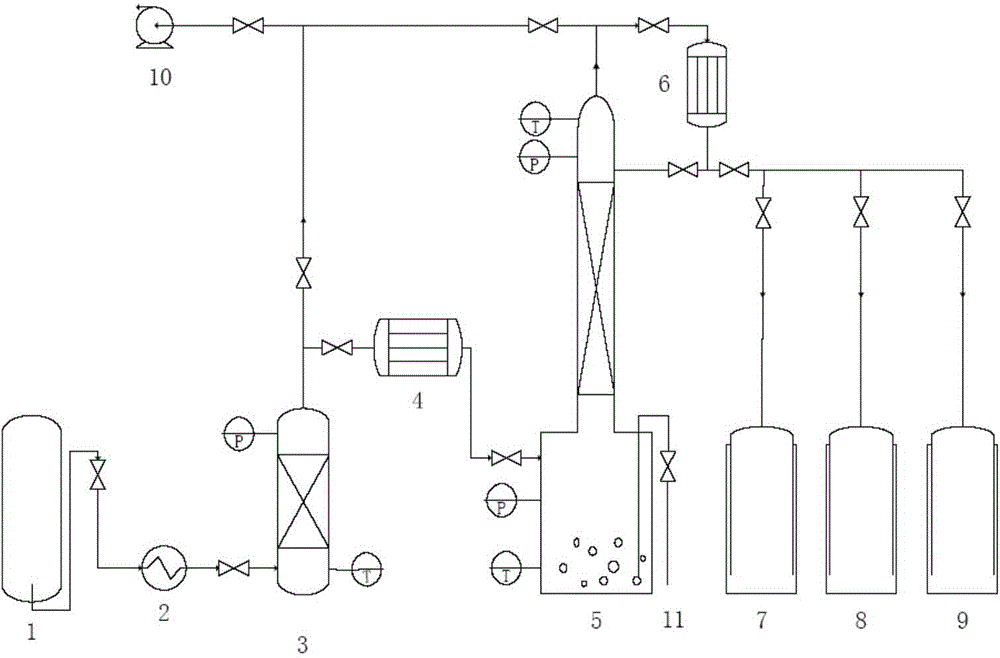
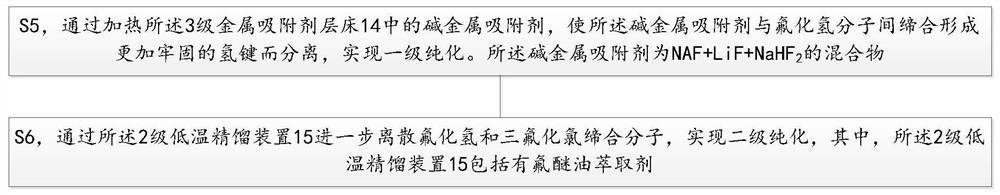
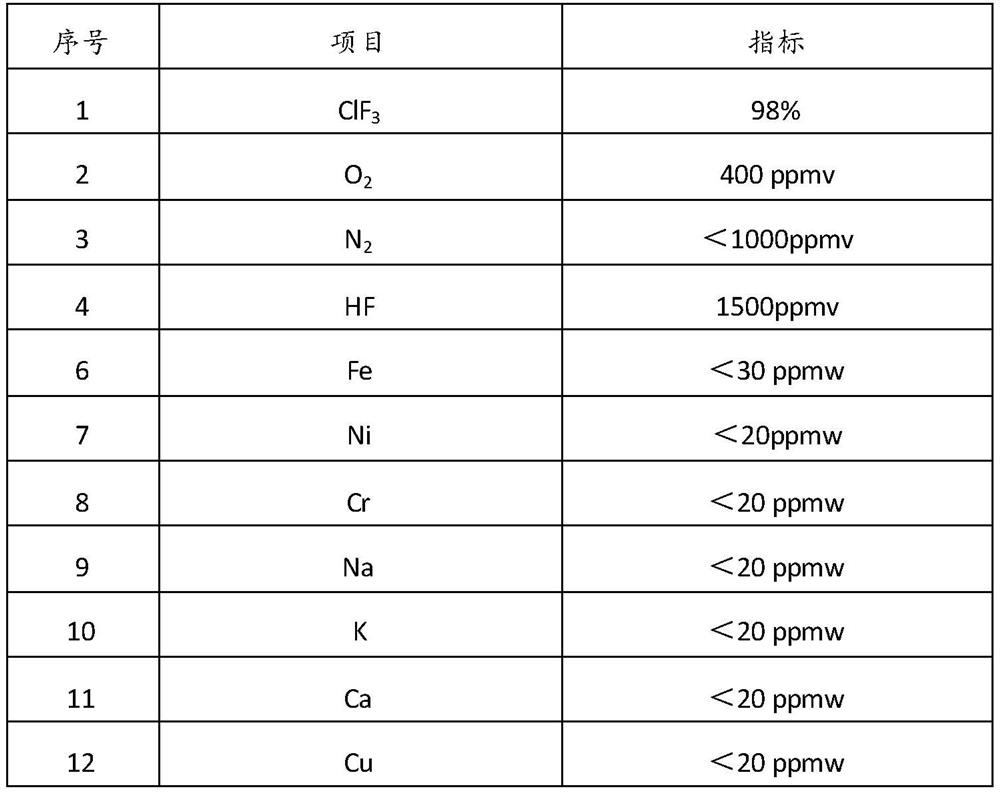
Purification method and purification system of chlorine trifluoride
The invention provides a purification method of chlorine trifluoride, which comprises the following steps: S1) pressurizing and vaporizing a chlorine trifluoride crude product, and adsorbing with an adsorbent to obtain chlorine trifluoride gas without hydrogen fluoride, wherein the adsorbent is fluoride salt, S2) condensing the chlorine trifluoride gas from which hydrogen fluoride is removed to obtain condensed chlorine trifluoride liquid, and S3) carrying out two-stage rectification on the condensed chlorine trifluoride liquid to obtain high-purity chlorine trifluoride. Compared with the prior art, the method has the advantages that adsorption condensation and two-stage rectification are combined, the chlorine trifluoride crude product is firstly subjected to adsorption to remove part ofhydrogen fluoride and then is subjected to two-stage continuous rectification, hydrogen fluoride, oxygen, nitrogen and metal ions can be removed to reach very high purity, and the removal control of metal ion impurities can be further increased, so that the purity of the chlorine trifluoride crude product is improved. The method can be continuously carried out, the operation is stable, and the 3N-purity chlorine trifluoride product used in the semiconductor industry is obtained.
Owner:SUZHOU JINHONG GAS CO LTD
Process for producing inorganic fine grains, inorganic fine grains, rare earth element-activated barium fluorohalide fluorescent substance, and radiation image conversion panel
InactiveUS20030005552A1Improve image qualityX-ray/infra-red processesMagnesium halidesRare-earth elementFluorescence
A process for producing inorganic fine grains in a definite form having a small grain size, the inorganic fine grains obtained by this process, a rare earth element-activated barium fluorohalide fluorescent substance made using the grains, and a radiation image conversion panel with a layer of the fluorescent substance. The process features adding, to a solution containing an inorganic compound, a solid matter substantially insoluble in the solution, promoting crystallization or precipitation in the solution to form crystal or precipitate, and separating out the resulting crystal or precipitate. The inorganic fine grains produced by this process are represented by the formula BaFI:xLn (Ln represents at least one of Ce, Pr, Sm, Eu, Tb, Dy, Ho, Nd, Er, Tm and Yb, and 0<x.ltoreq.0.2), have a cubic form and have a volume-average grain size of 1 to 10 .mu.m.
Owner:FUJIFILM HLDG CORP +1
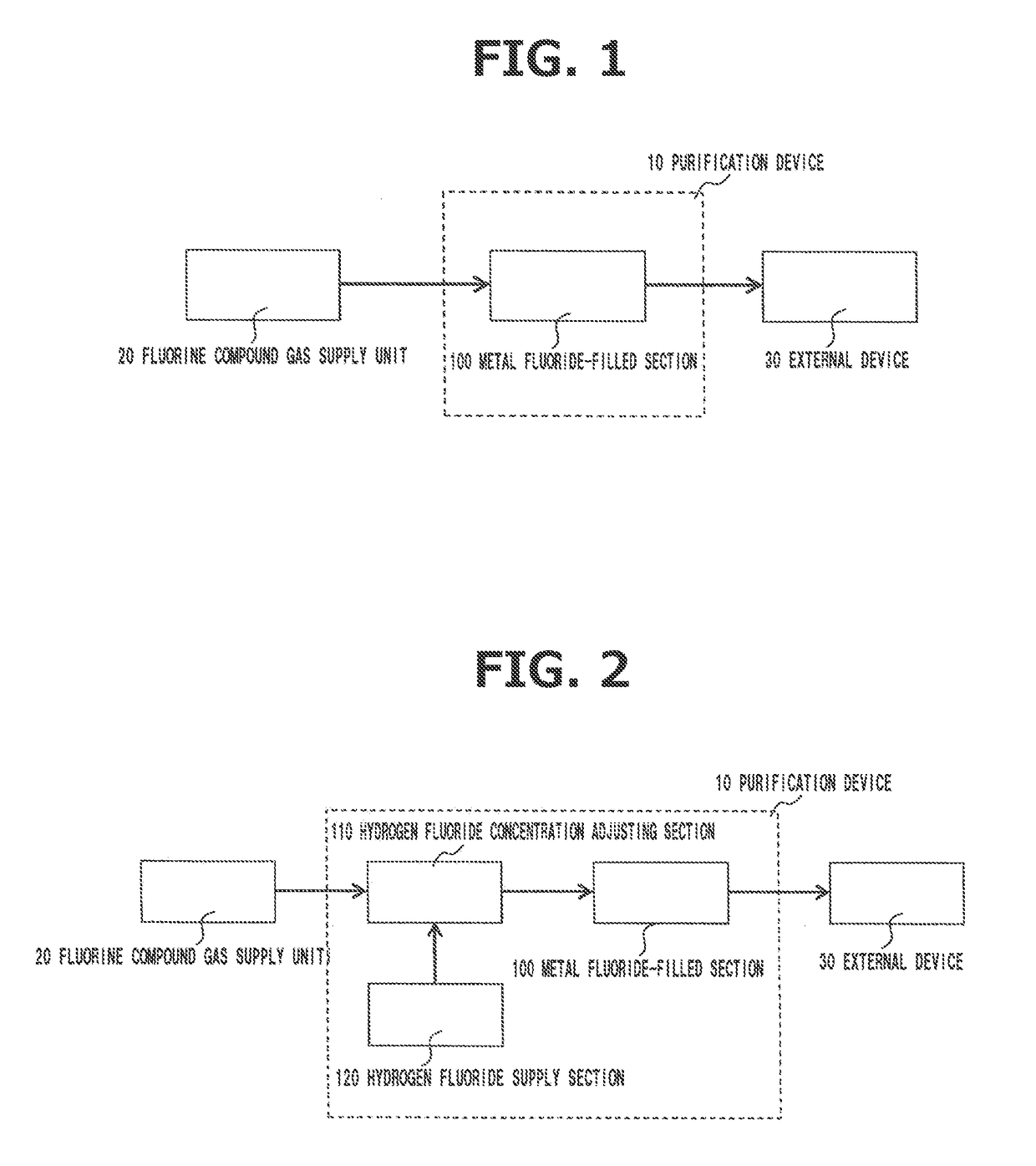
Method for Purifying Fluorine Compound Gas
ActiveUS20190046917A1Easy to disassembleSimple structureTungsten halidesGas treatmentHydrogen fluoridePurification methods
Disclosed is a purification method for removing a metal component from a fluorine compound gas containing hydrogen fluoride and a metal component. This method includes a removing step for removing the hydrogen fluoride and the metal component therefrom by bringing the fluorine compound gas into contact with a solid metal fluoride to adsorb the hydrogen fluoride and the metal component on the metal fluoride. It is preferable for the fluorine compound gas to contain at least one kind selected from the group consisting of CIF, CIF3, IF5, IF7, BrF3, BrF5, NF3, WF6, SiF4, CF4, SF6 and BF3. It is also preferable for the metal fluoride to be an alkali metal fluoride or an alkali earth metal fluoride. Surprisingly, the presence of hydrogen fluoride in a fluorine compound gas makes it possible to remove a metal component therefrom as an impurity as a result of adsorption thereof by a metal fluoride.
Owner:CENT GLASS CO LTD
Catalyst for the synthesis of CF3I and CF3CF2I
Owner:HONEYWELL INT INC
Device and method for purifying bromine trifluoride
InactiveCN107311110AImprove separation efficiencyEasy to separateInter-halogen compoundsBromine trifluoridePre treatment
The invention discloses a device and a method for purifying bromine trifluoride. The device comprises a vaporizer, an adsorption tower, a rectification purification device, a condensation collecting pipe, a front fraction collecting device, a main fraction collecting device, a rear fraction collecting device, a tail gas recycling system and a vacuum pump. The method includes steps of (i) pretreatment, (ii) adsorption purification and (iii) intermittent rectification purification. The device and the method for purifying the bromine trifluoride by means of adsorption and then intermittent rectification have the advantages that the device and the method are high in phase separation efficiency, light and heavy components can be thoroughly separated from one another, bromine trifluoride products obtained by the aid of the device and the method are high in purity, the purity of the bromine trifluoride products can reach 99.5%, and the device and the method are high in production efficiency.
Owner:天津长芦华信化工股份有限公司
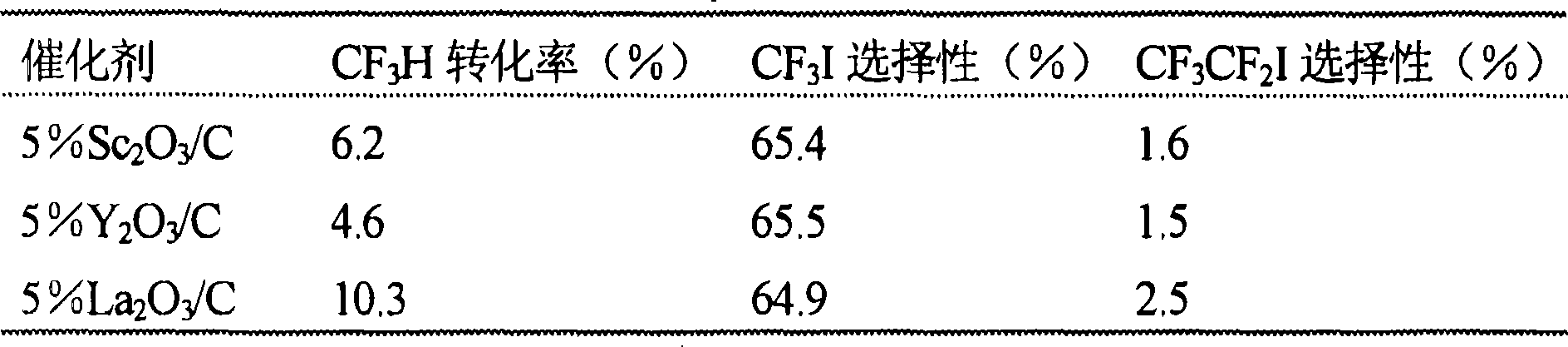
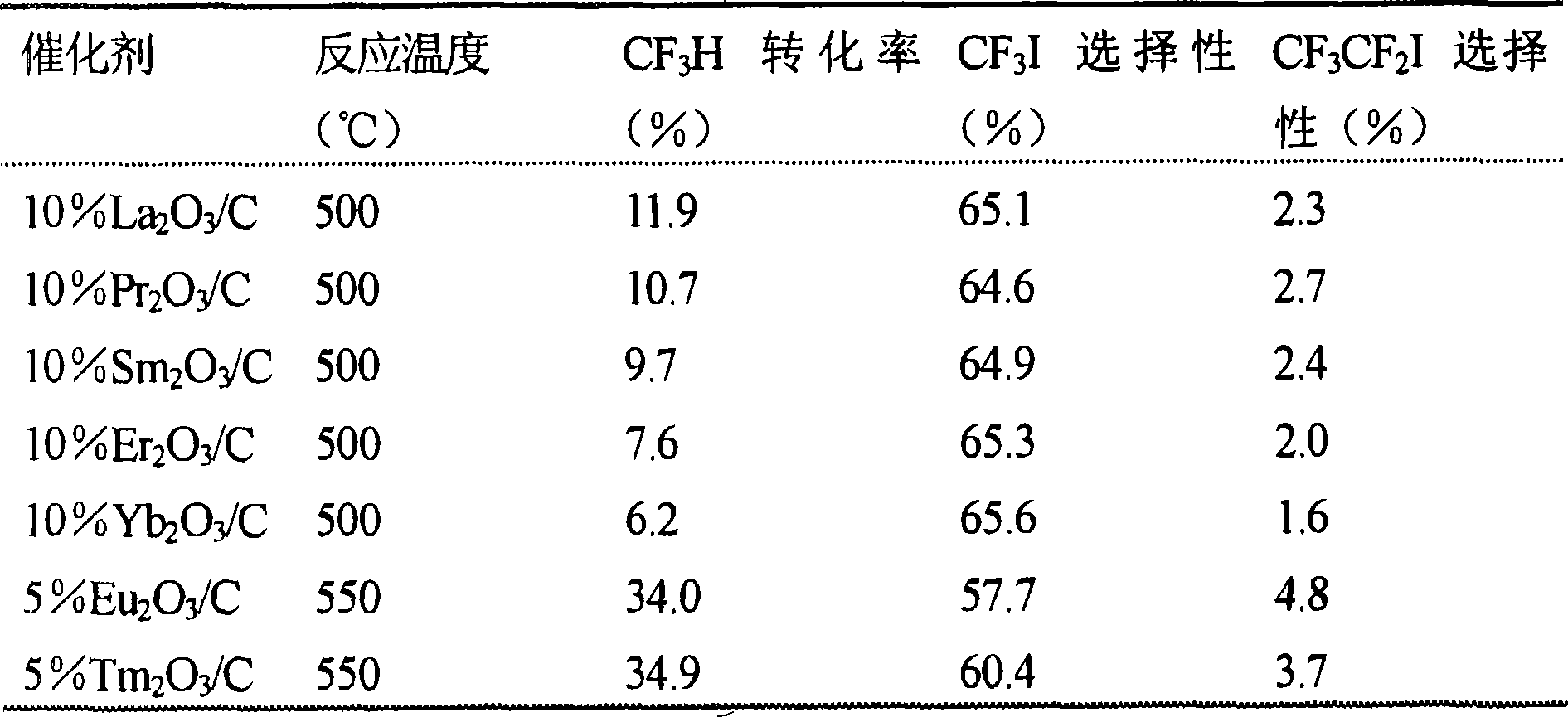
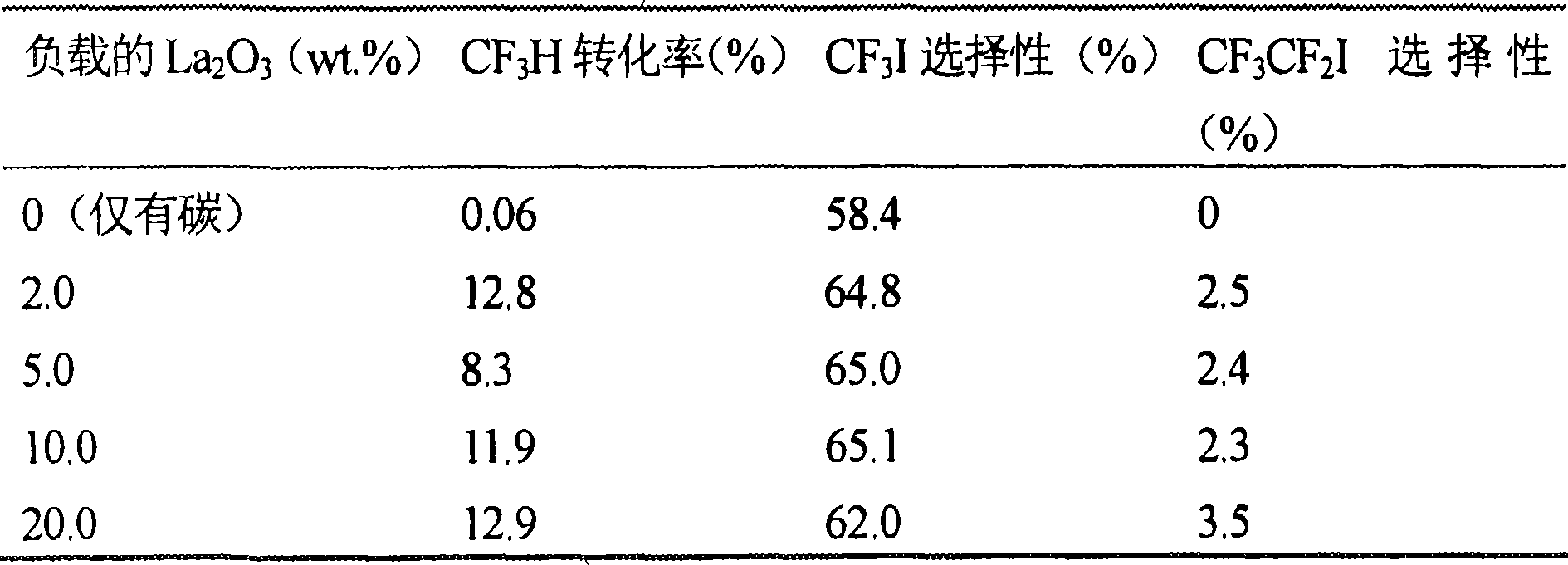
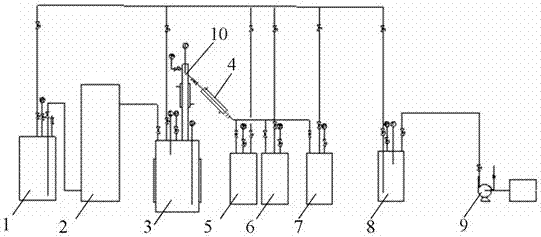
Catalyst for the synthesis of CF3I and CF3CF2I
InactiveUS20080200735A1Molecular sieve catalystPreparation by halogen replacementContact timeOrganic chemistry
A process for the preparation of a fluoroiodoalkane compound represented by the formula: CF3(CF2)n—Y, wherein n is 0 or 1. The process includes contacting A, B and C. A is represented by the formula: CF3(CF2)n—Y, wherein n is 0 or 1, and Y is selected from the group consisting of: H, Cl, Br, and COOH. B is a source of iodine, and C is a catalyst containing elements with d1s1 configuration and lanthanide elements. The process occurs at a temperature, and for a contact time, sufficient to produce the fluoroiodoalkane compound.
Owner:HONEYWELL INC
Process method for purifying iodine pentafluoride
ActiveCN103449371AReduce contentEasy to operateInter-halogen compoundsIodine pentafluorideProduct gas
The technical scheme of the invention is to provide a process method for purifying iodine pentafluoride. The process method comprises the following steps of: (i) evacuating an evaporator until the pressure is between -0.08MPa and -0.10MPa; (ii) adding an iodine pentafluoride raw material into the evaporator; (iii) heating the evaporator, and controlling the temperature of the evaporator to be 30-60 DEG C to ensure that light component impurities such as N2, O2, HF and the like in the iodine pentafluoride are gasified and separated out; (iv) controlling the temperature of the evaporator to be 90-130 DEG C to ensure that the iodine pentafluoride in the evaporator is gasified and the gasified iodine pentafluoride enters a condenser, and controlling the temperature of the condenser to be 60-80 DEG C to ensure that the iodine pentafluoride gas is condensed and liquefied; (v) removing deposited substances such as KF, FeF2 and other heavy components in an iodine pentafluoride crude product. The process method provided by the invention is simple in equipment, convenient in operation and safe in equipment running, the content of the impurities in the iodine pentafluoride is effectively reduced, and the product purity reaches more than 99.5%.
Owner:RES INST OF PHYSICAL & CHEM ENG OF NUCLEAR IND
Automatic pressure-lifting synthesis technique for bromine chloride by one-step method
The invention relates to a self-boosted one-step bromine chloride synthesis technology, which is characterized in that the synthesis reaction of the liquid bromine processed by dried pretreatment and the decompressed high purity chlorine is processed in a airtight medium pressure reactor, wherein the chlorine charging speed is 0.35 to 0.60 gram per minute. While the chlorine is charged and the synthesis reaction of bromine and chlorine is processed, the pressure in the reactor is increasing, and the temperature is rising; the pressure can be as high as 10 atm, the temperature ca be as high as 70 DEG C. When the ratio of the mole numbers of chlorine and bromine in the reactor reaches to 1.00-1.02 :1, the charging of chlorine in medium pressure reactor is stopped. After stopping the charging of chlorine, the materials in the reactor is kept to stir in 15 to 20 minutes, and the high purity bromine chloride product is obtained. The self-boosted one-step bromine chloride synthesis technology has the advantages of simple technological process, no three wastes, no need for refining process, little control point, easy operation, convenient maintenance and management and energy saving, and the synthesis product purity is more than 99.70%.
Owner:TIANJIN SEA WATER DESALINATION & COMPLEX UTILIZATION INST STATE OCEANOGRAPHI
Method for synthesizing hexafluorobutadiene through reaction rectification
InactiveCN108083972AEasy to separateImprove reaction efficiencyPreparation by dehalogenationMolecular sieve catalystsOrganic solventCatalytic distillation
The invention provides a method for synthesizing hexafluorobutadiene through reaction rectification. The method comprises the following steps of performing preparation by a catalysis distillation column, wherein the catalysis distillation column consists of a rectification section, a reaction section, a stripping section and a column kettle in sequentially connection from the tower top to the tower bottom; and a catalysis distillation element is filled in the reaction section. A preparation method comprises the following steps of uniformly mixing 1,2,3,4-tetrachloro-1,1,2,3,4,4-hexafluoro butane and organic solvents accounting for 500 to 1000 mass percent of the 1,2,3,4-tetrachloro-1,1,2,3,4,4-hexafluoro butane; continuously adding the materials into the catalysis distillation column fromthe rectification section and the stripping section for reaction.
Owner:ZHEJIANG BRITECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com