Patents
Literature
150results about "Bromine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
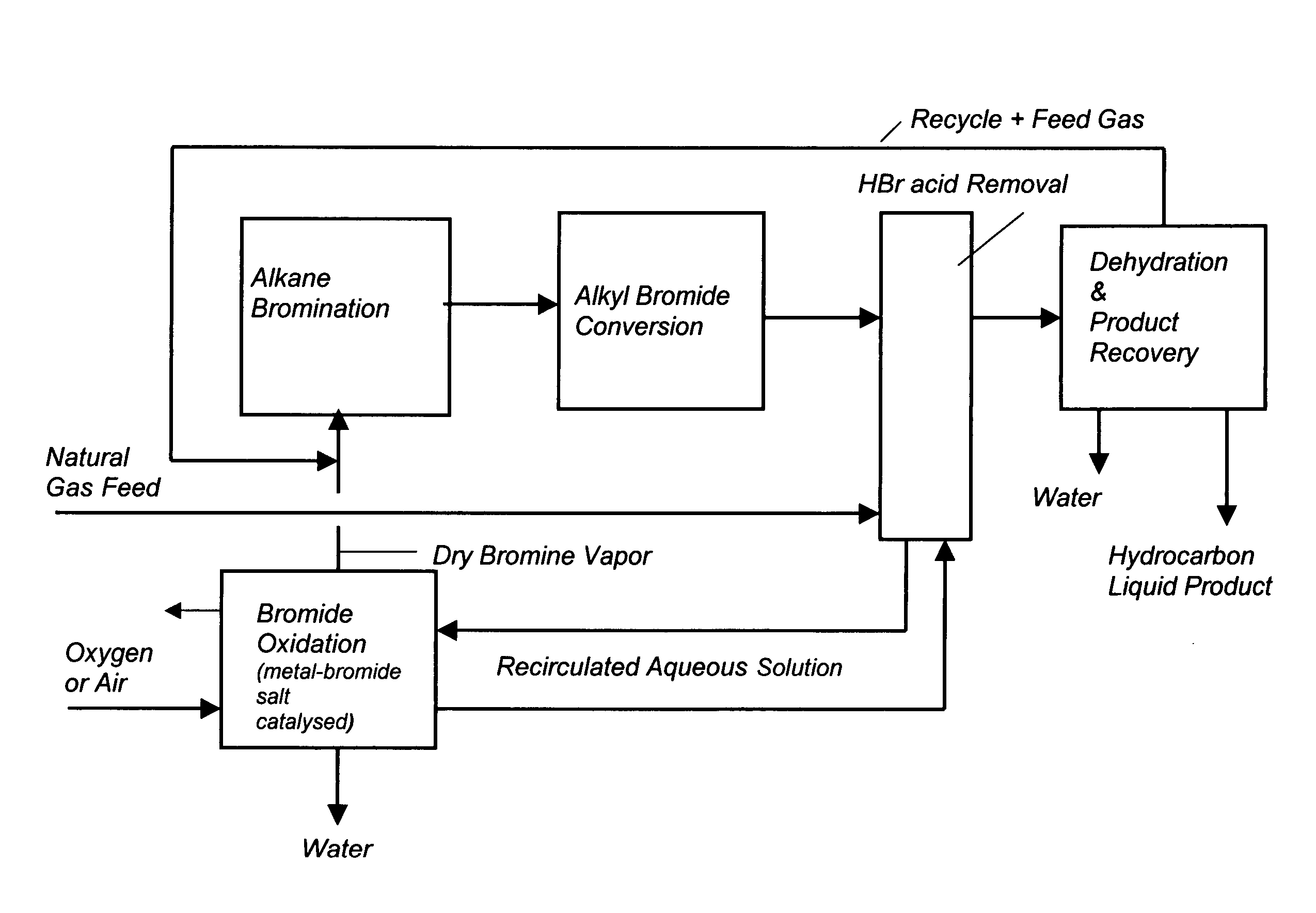
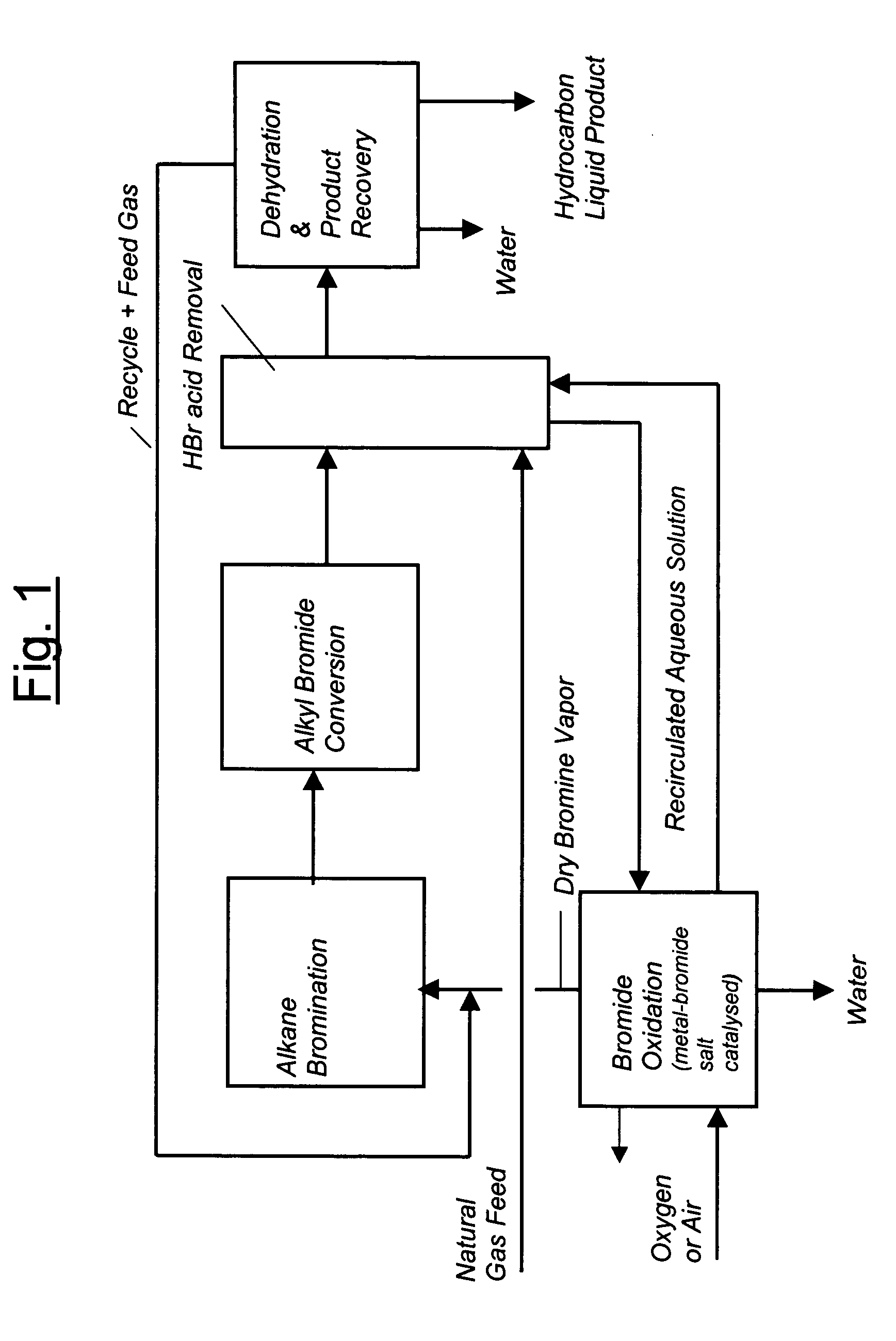
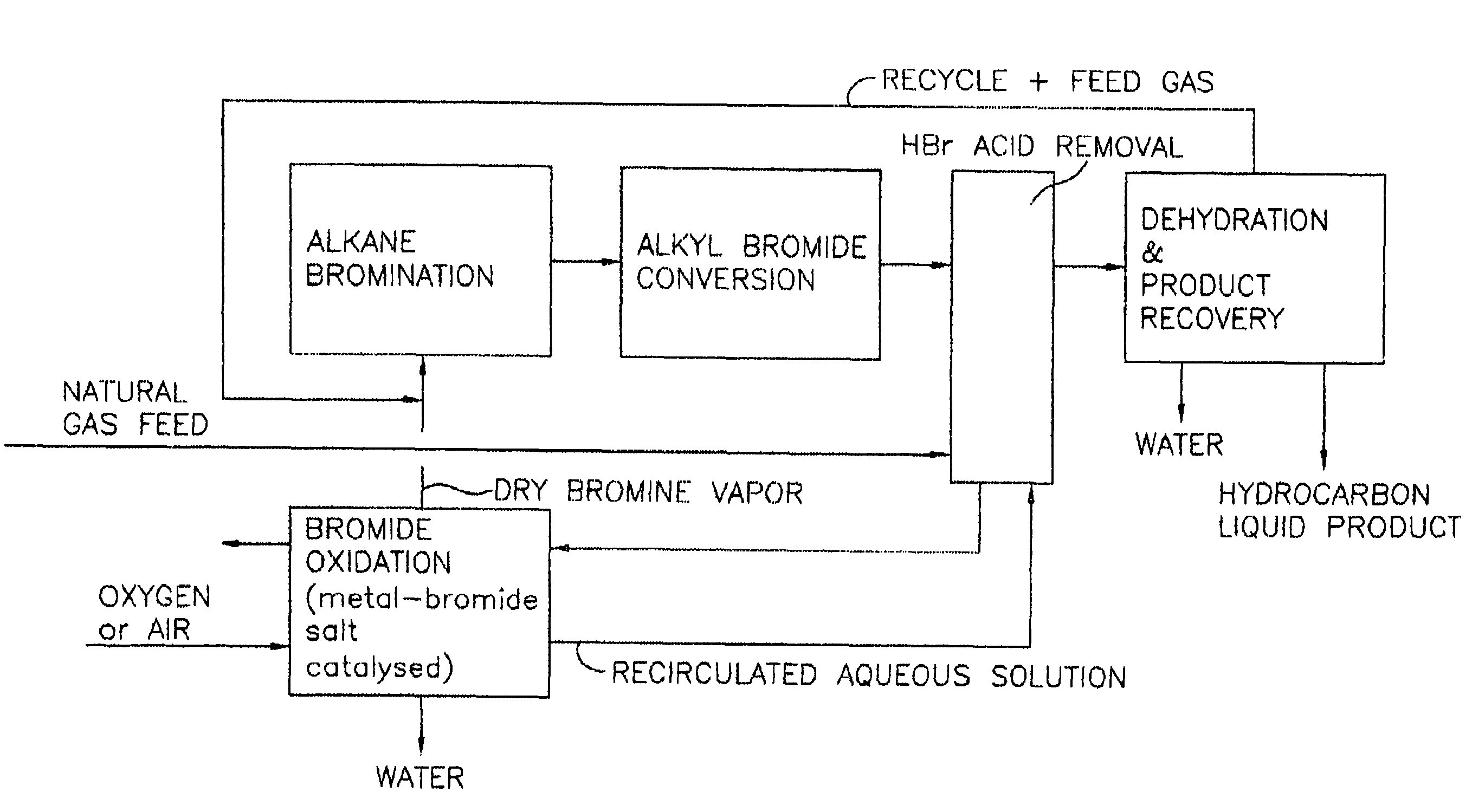
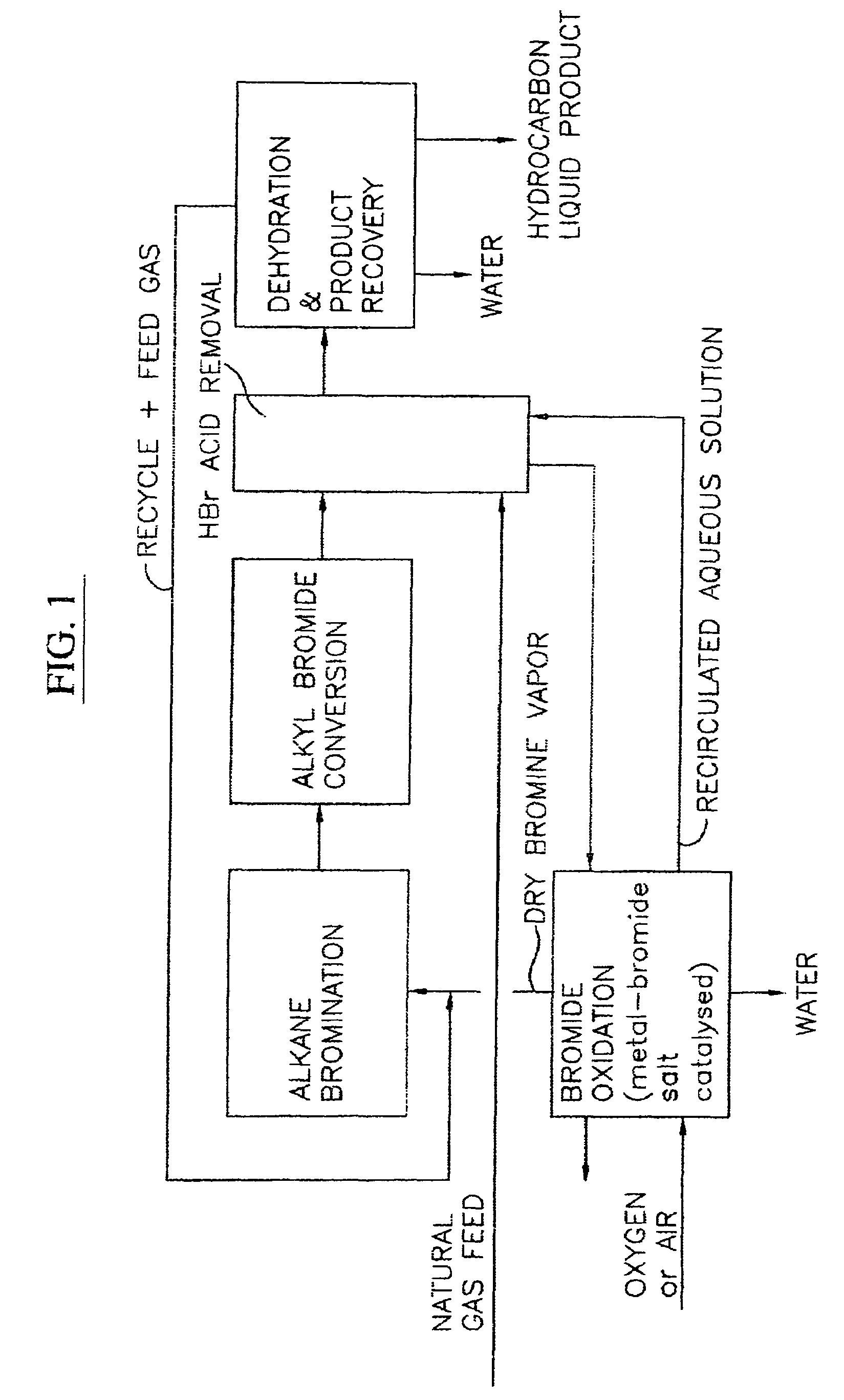
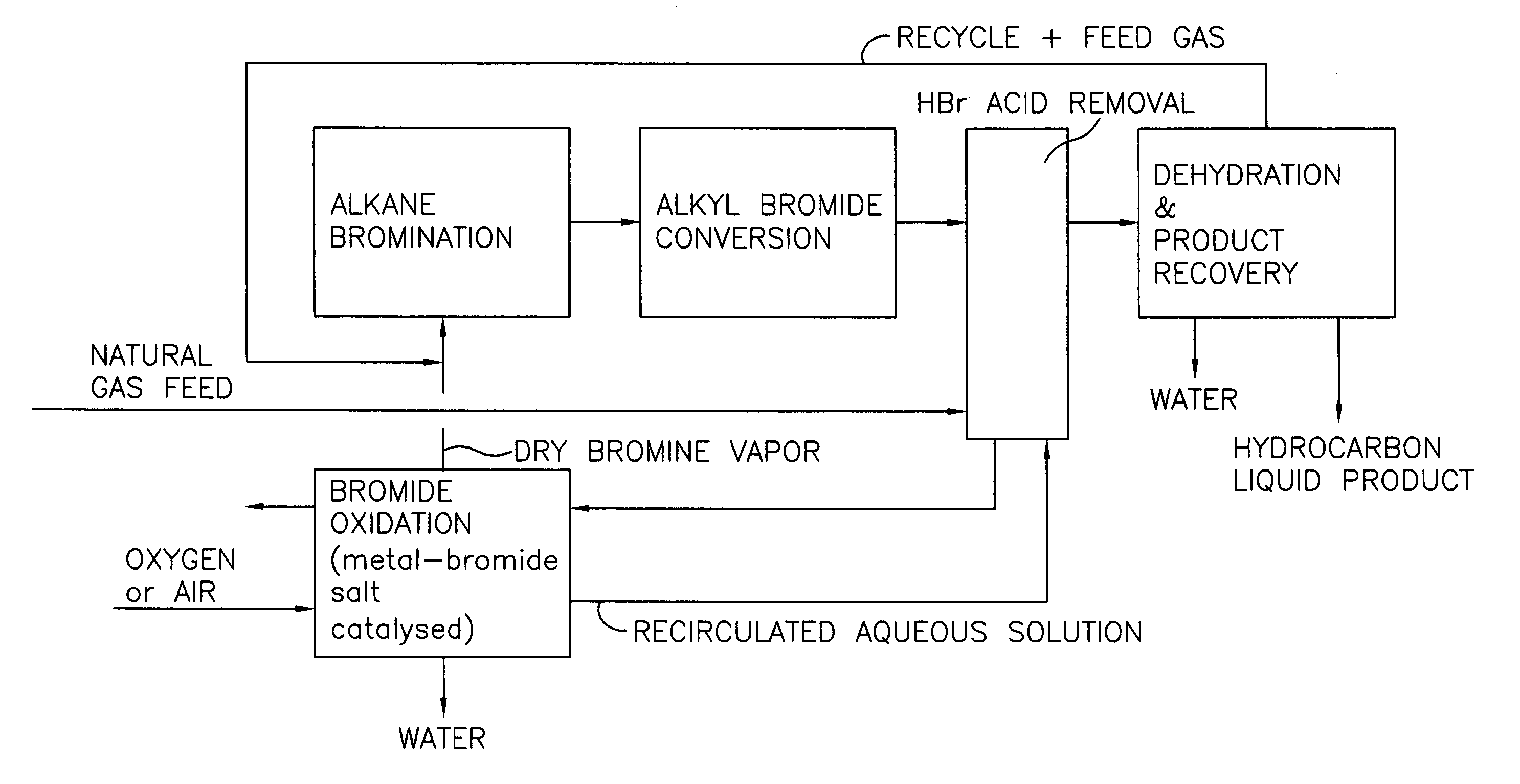
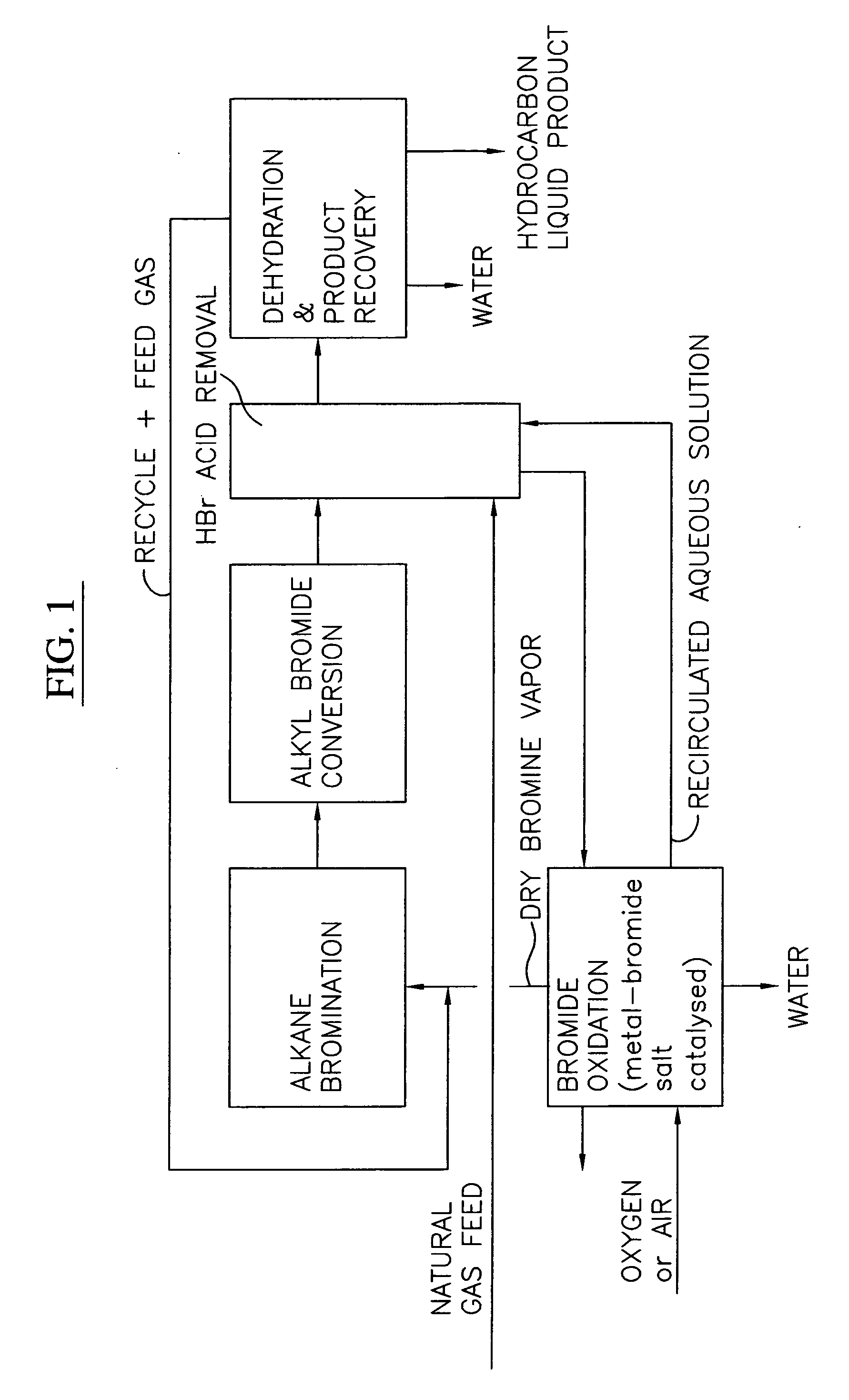
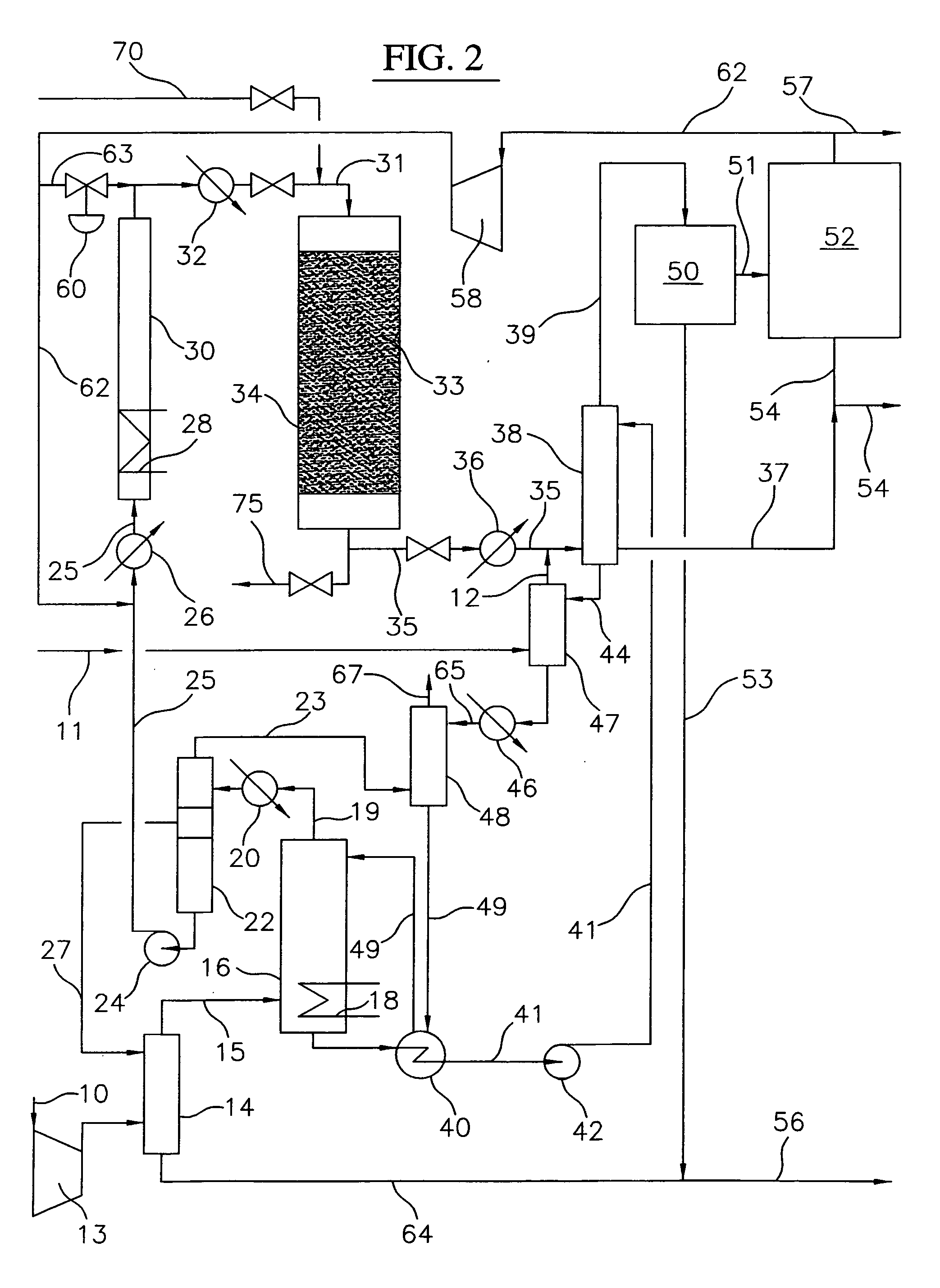
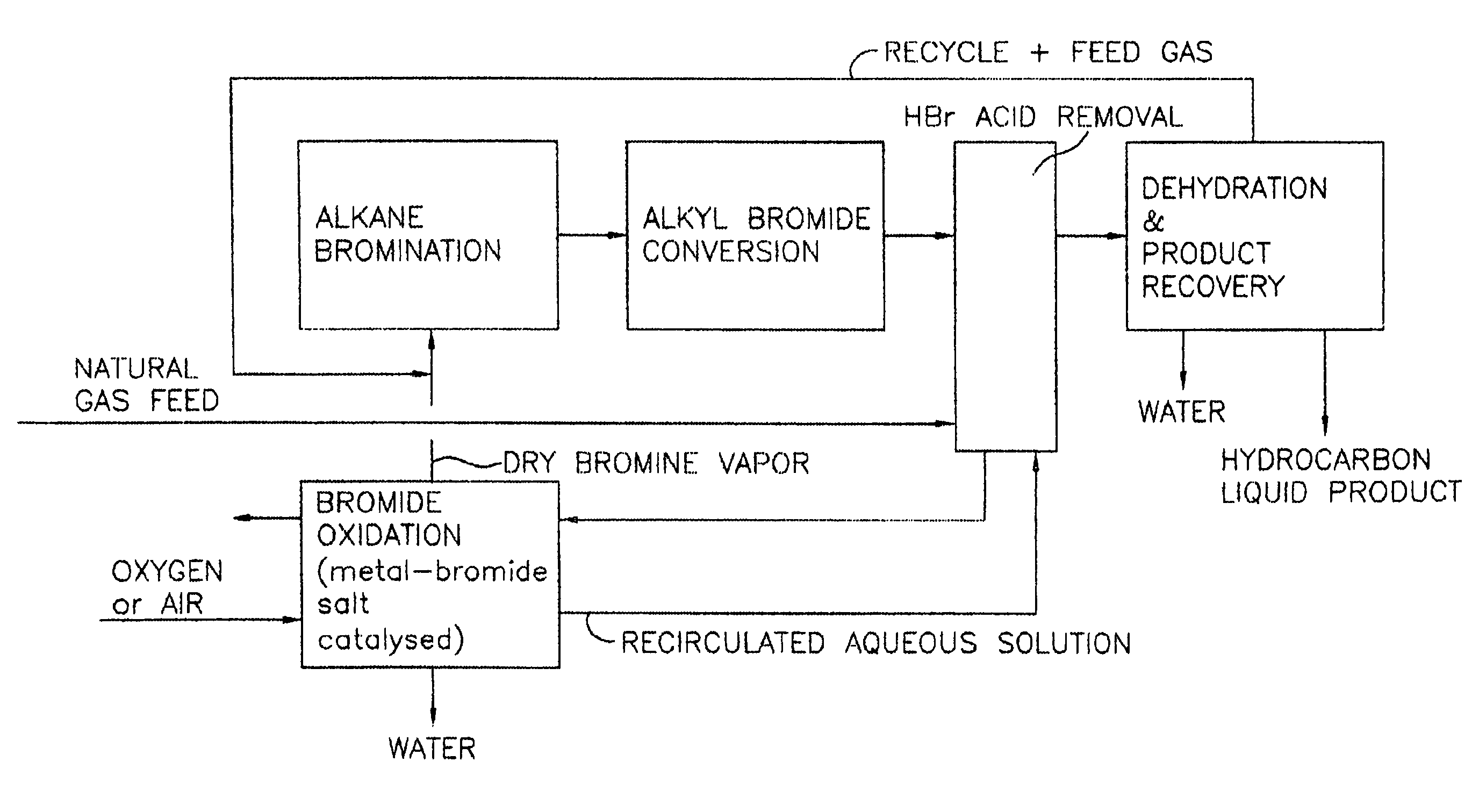
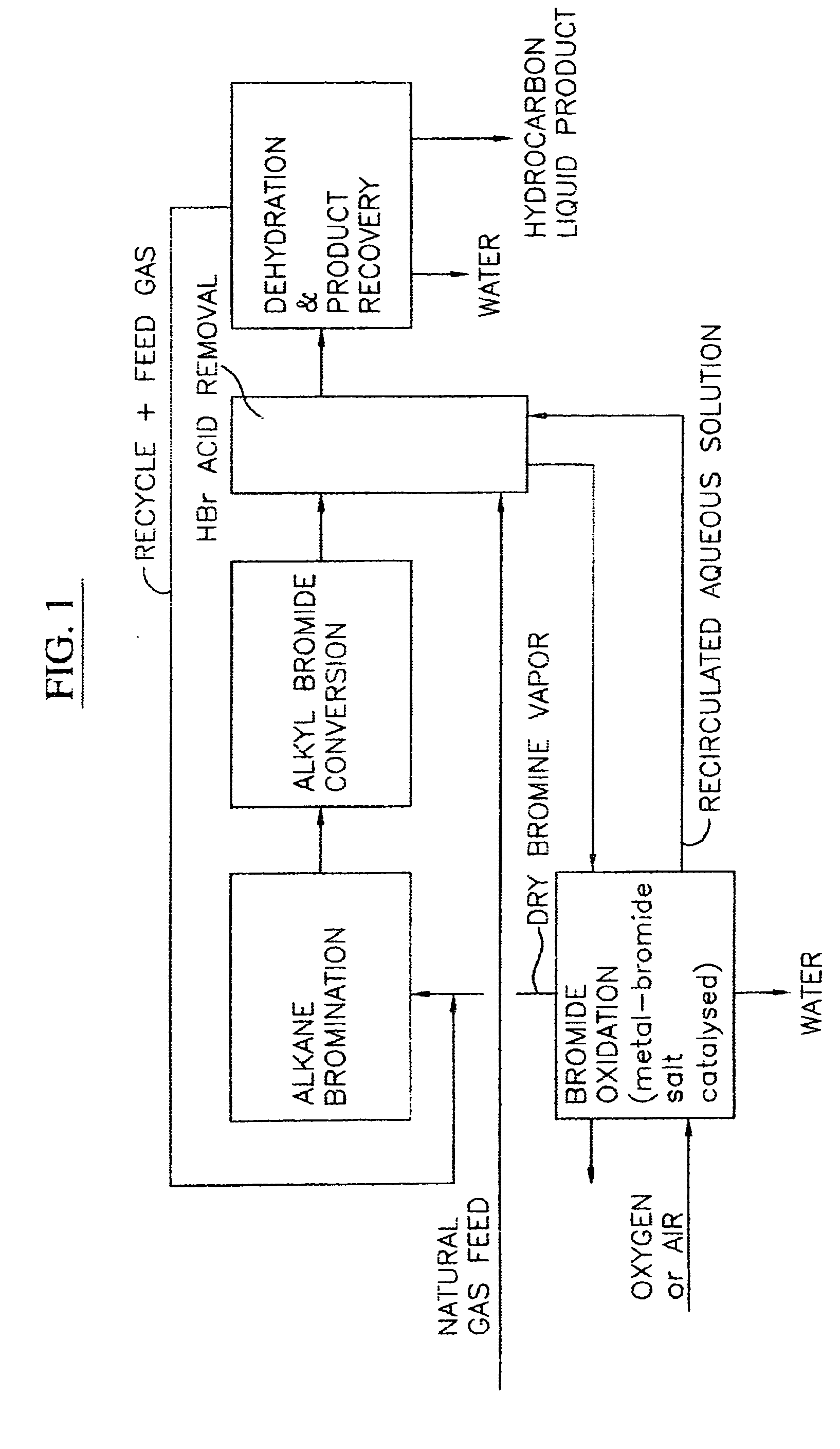
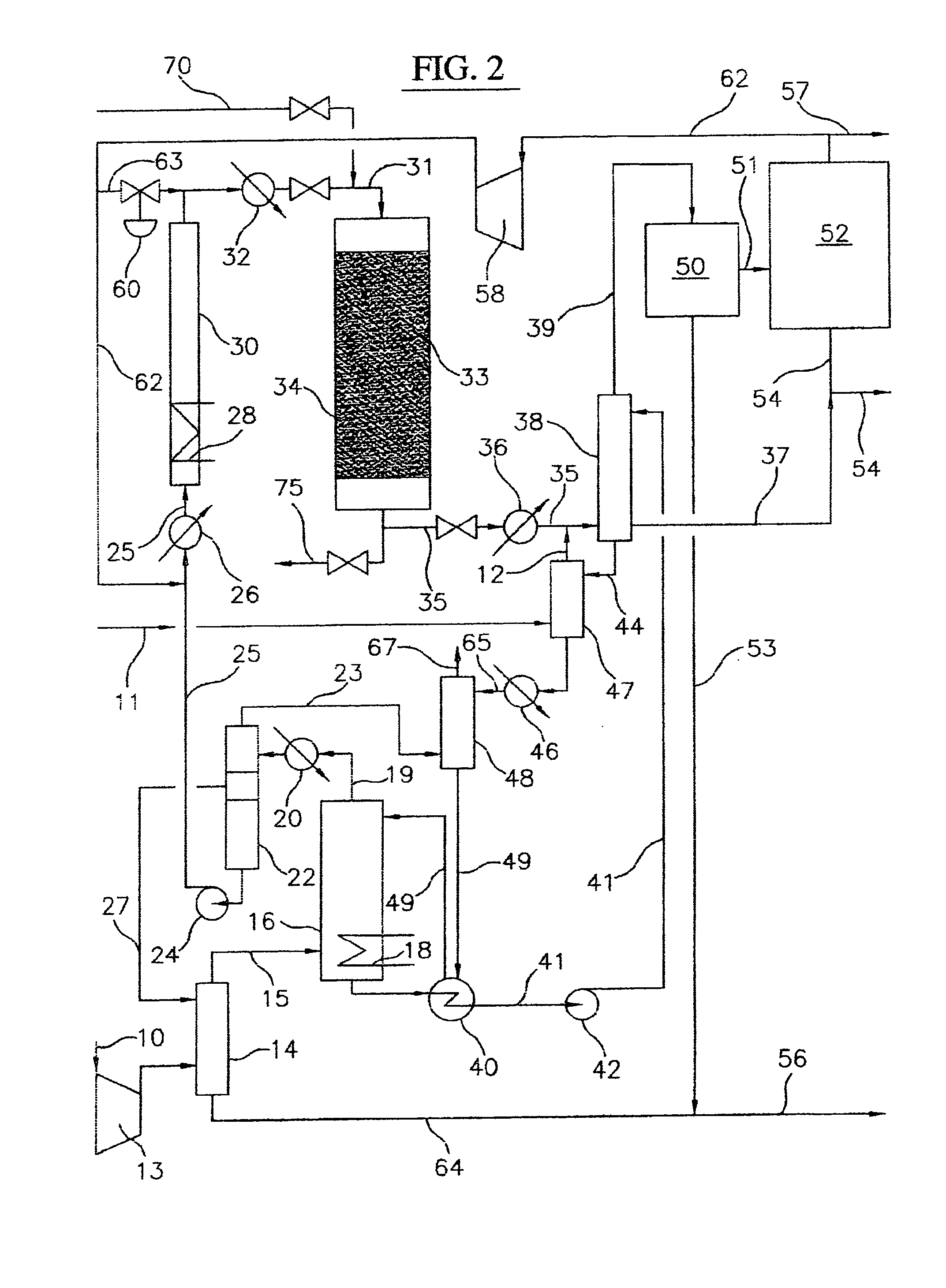
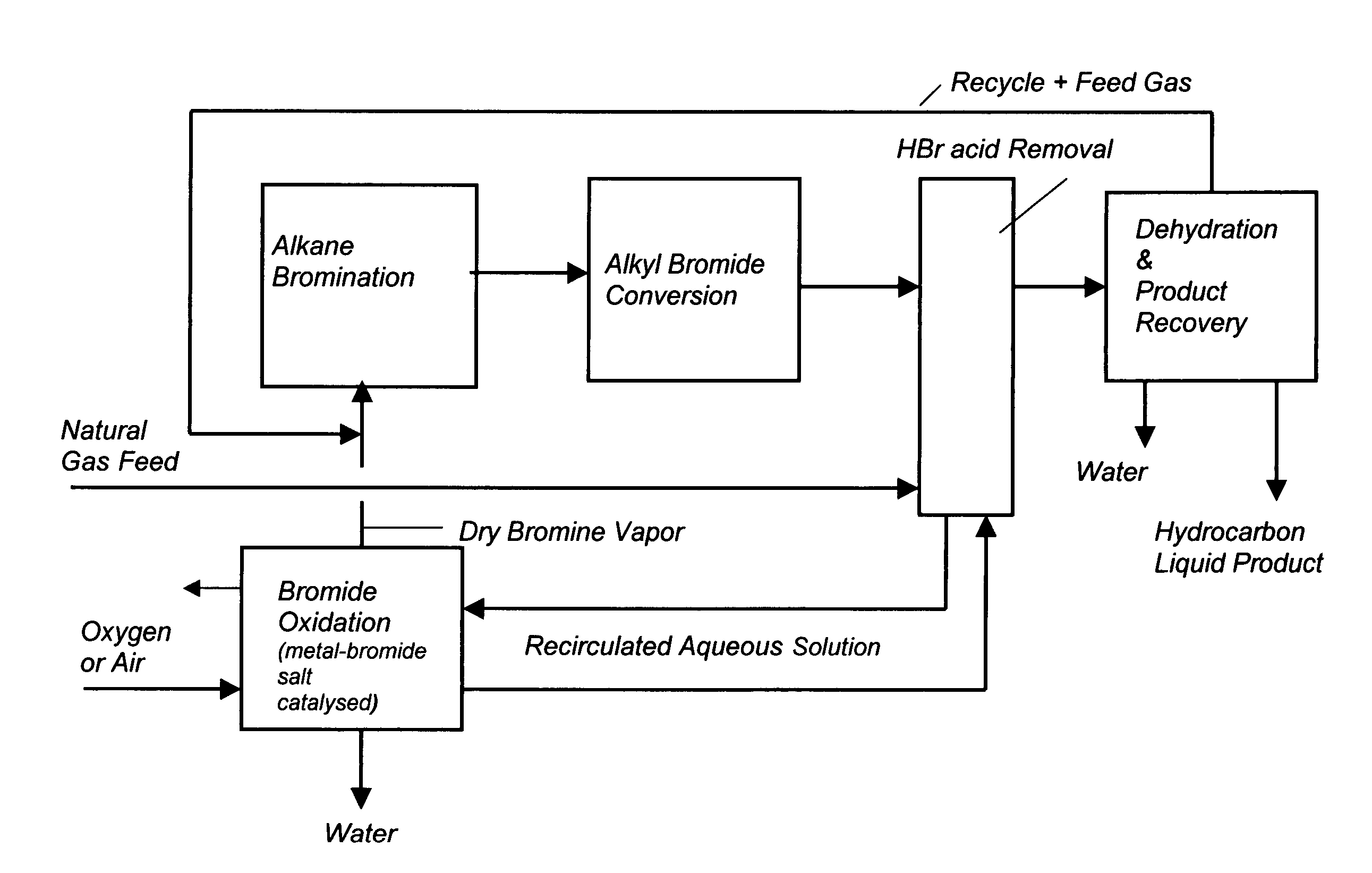
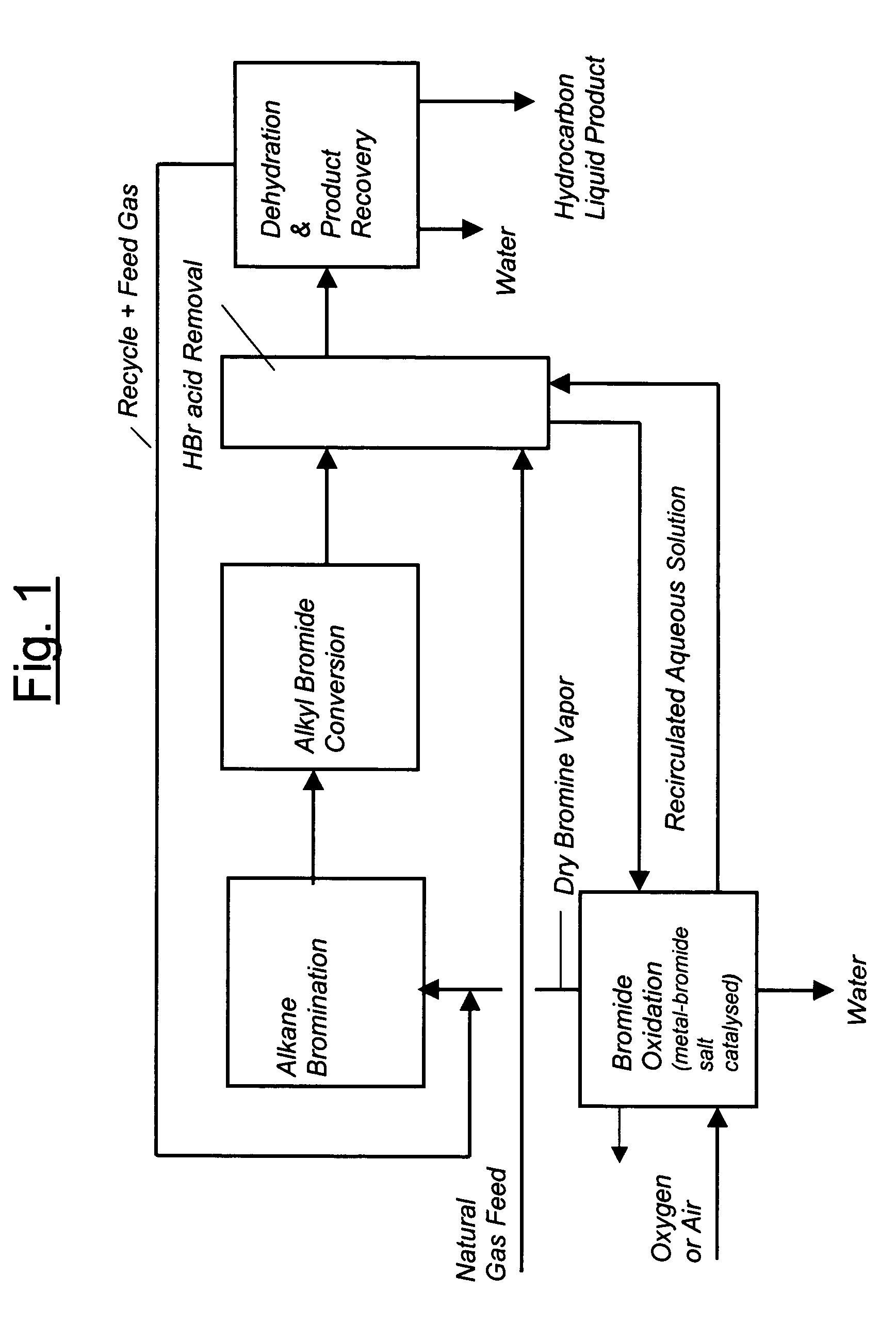
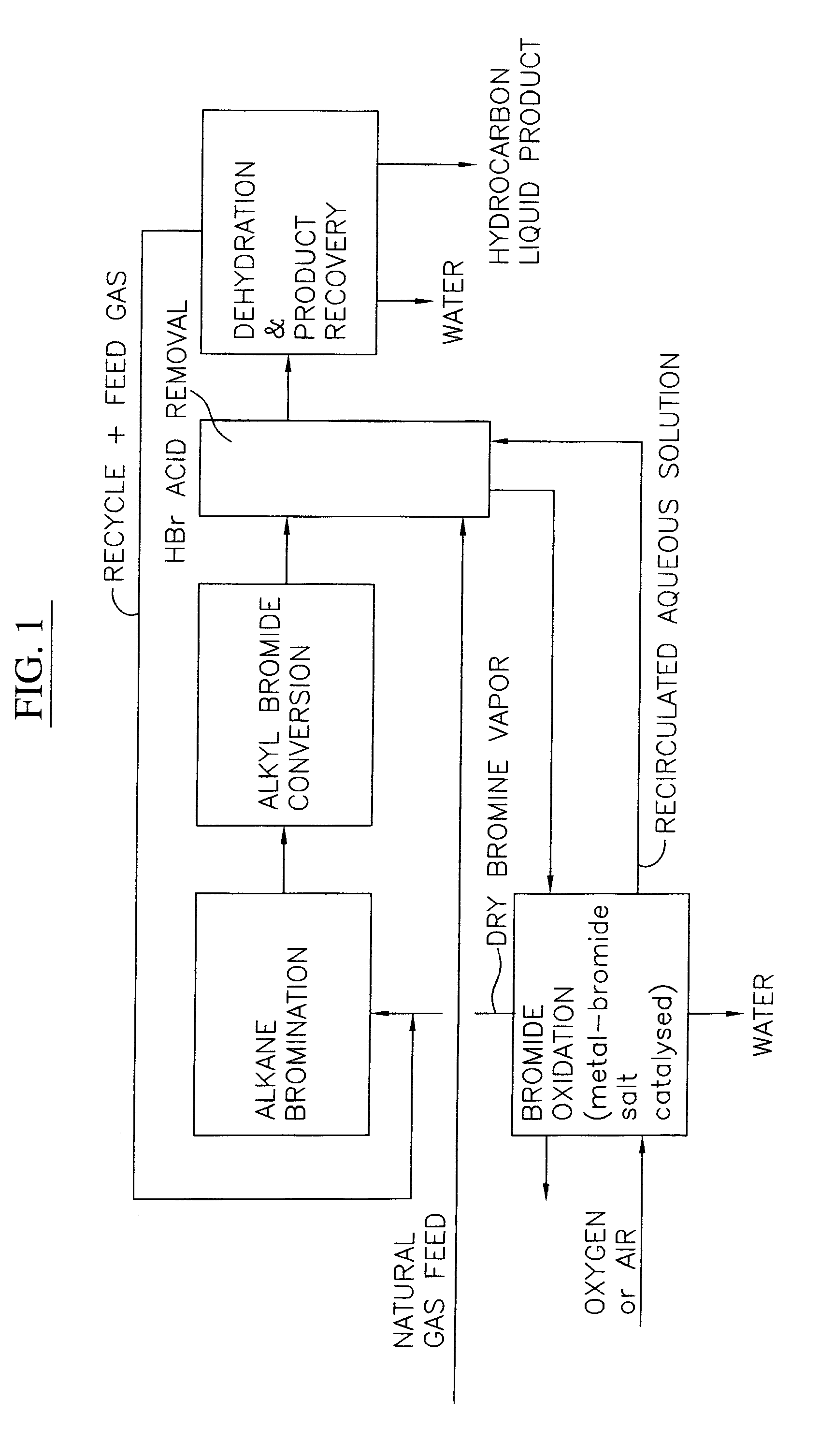
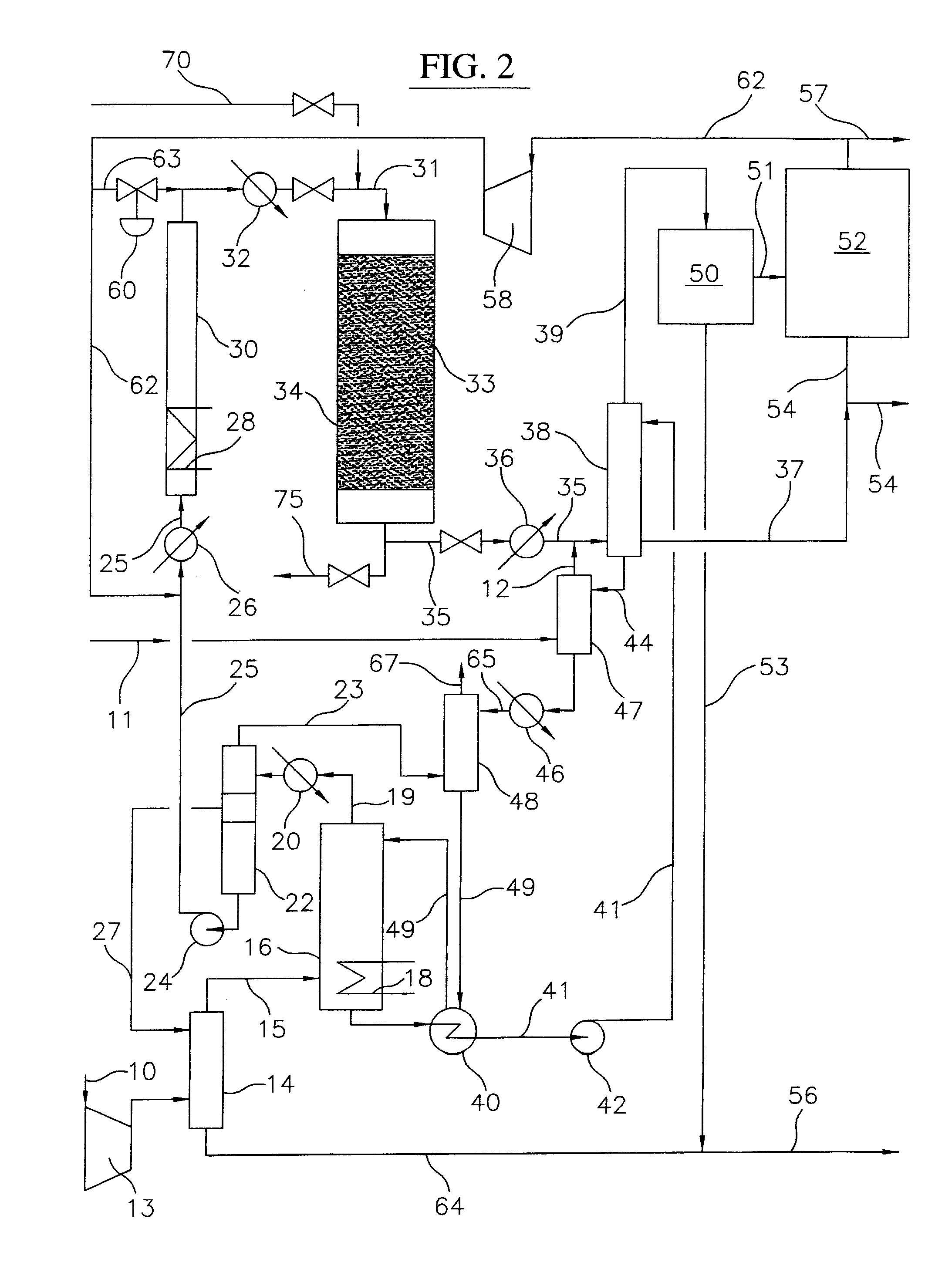
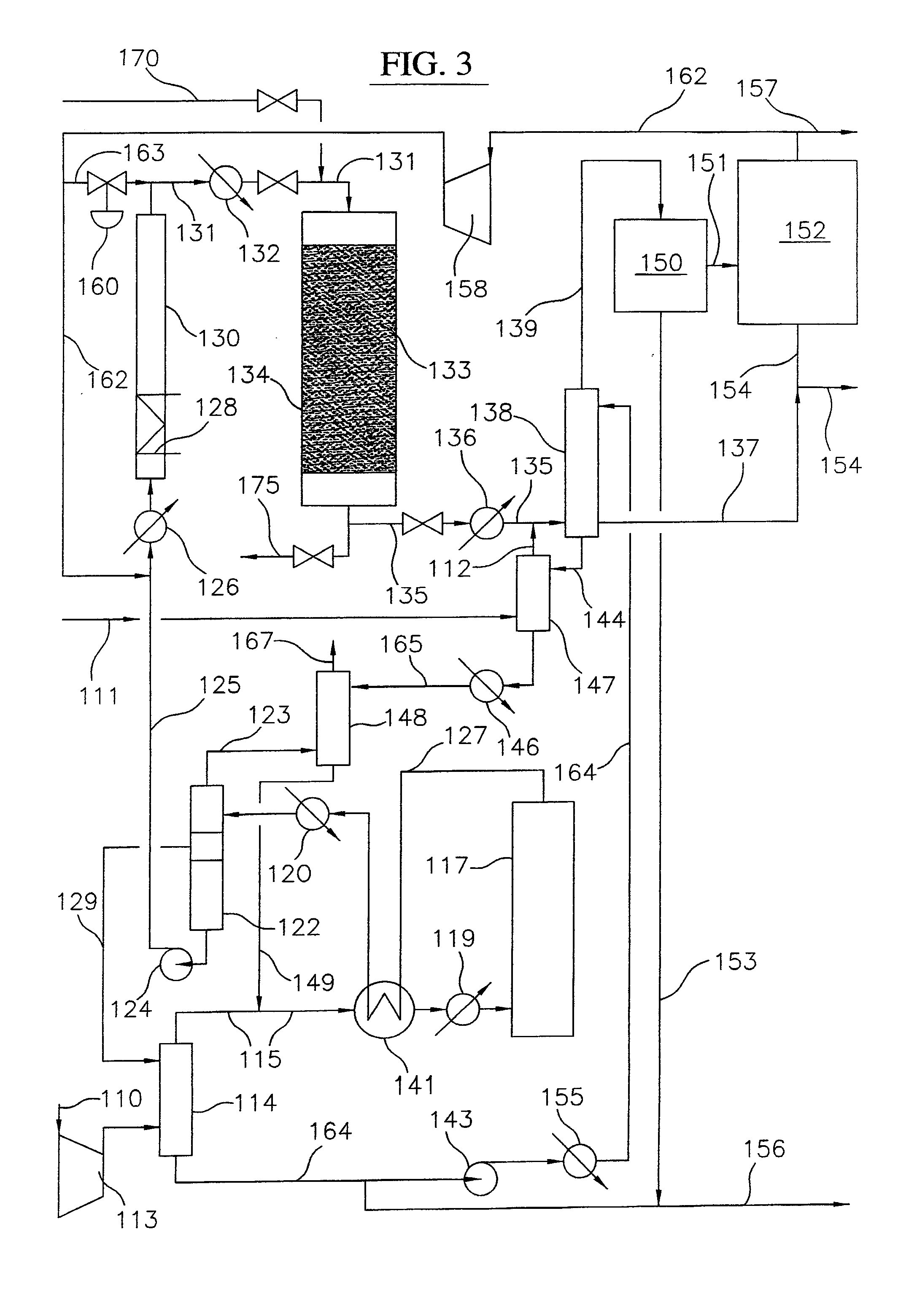
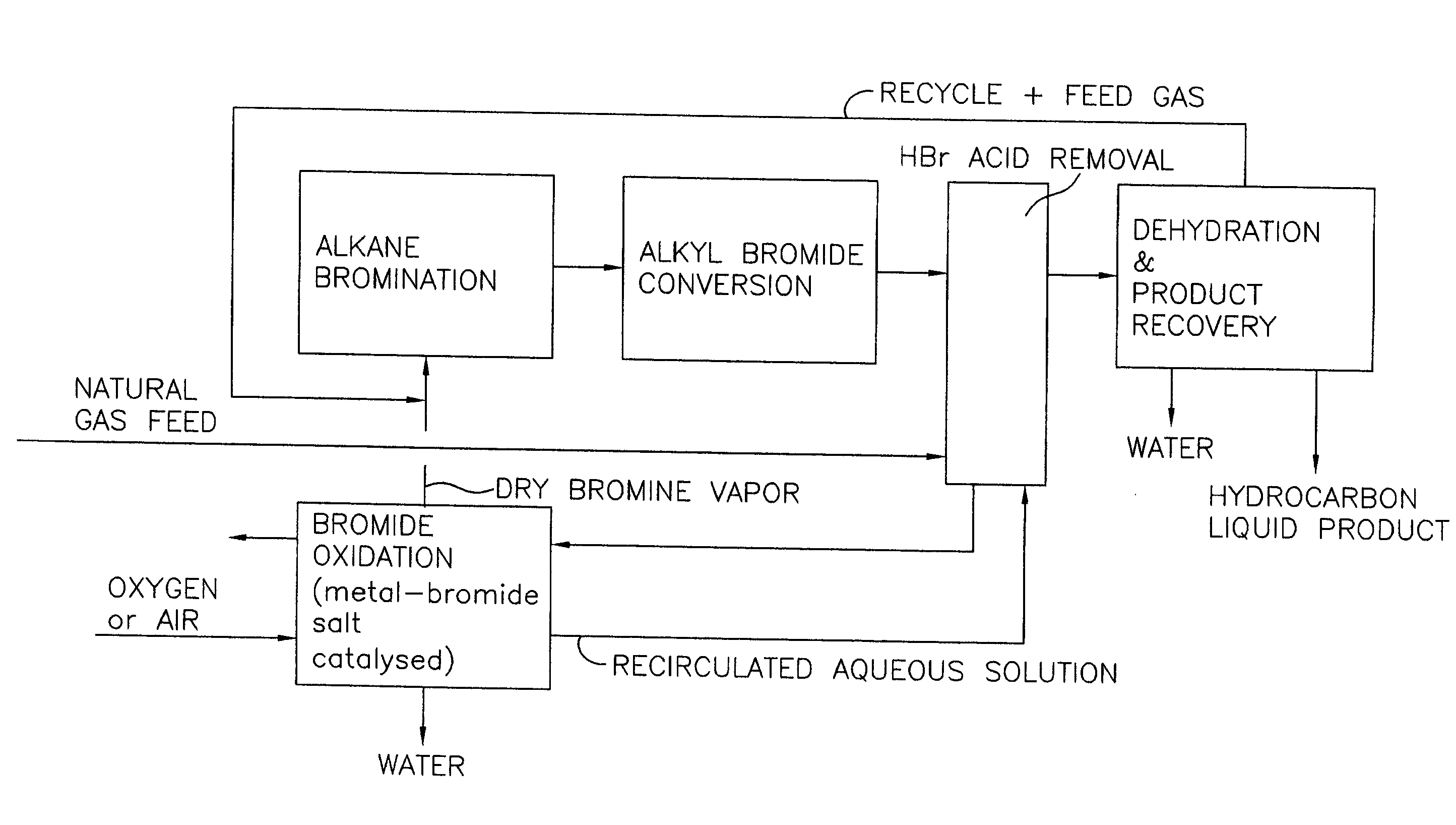
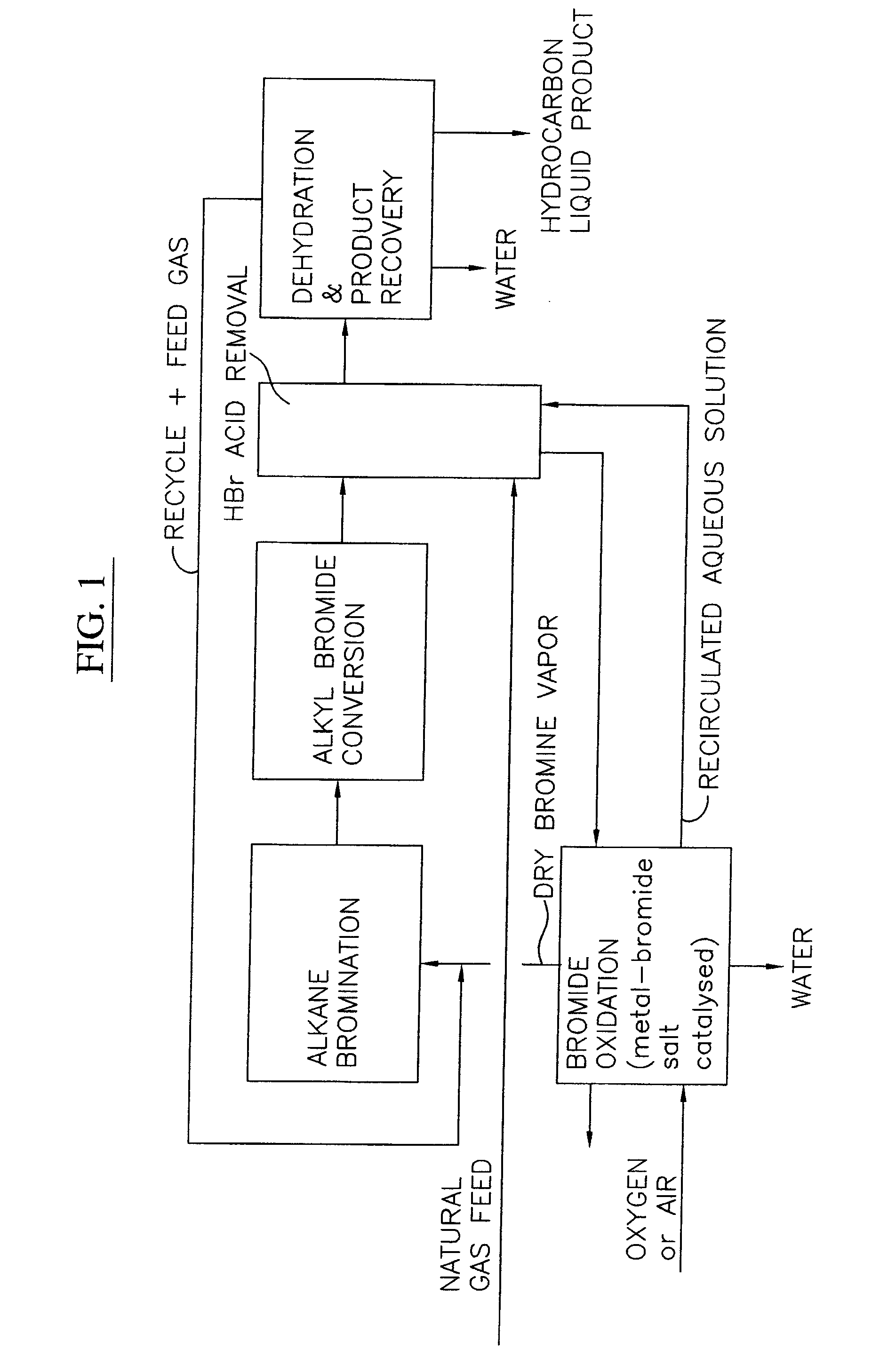
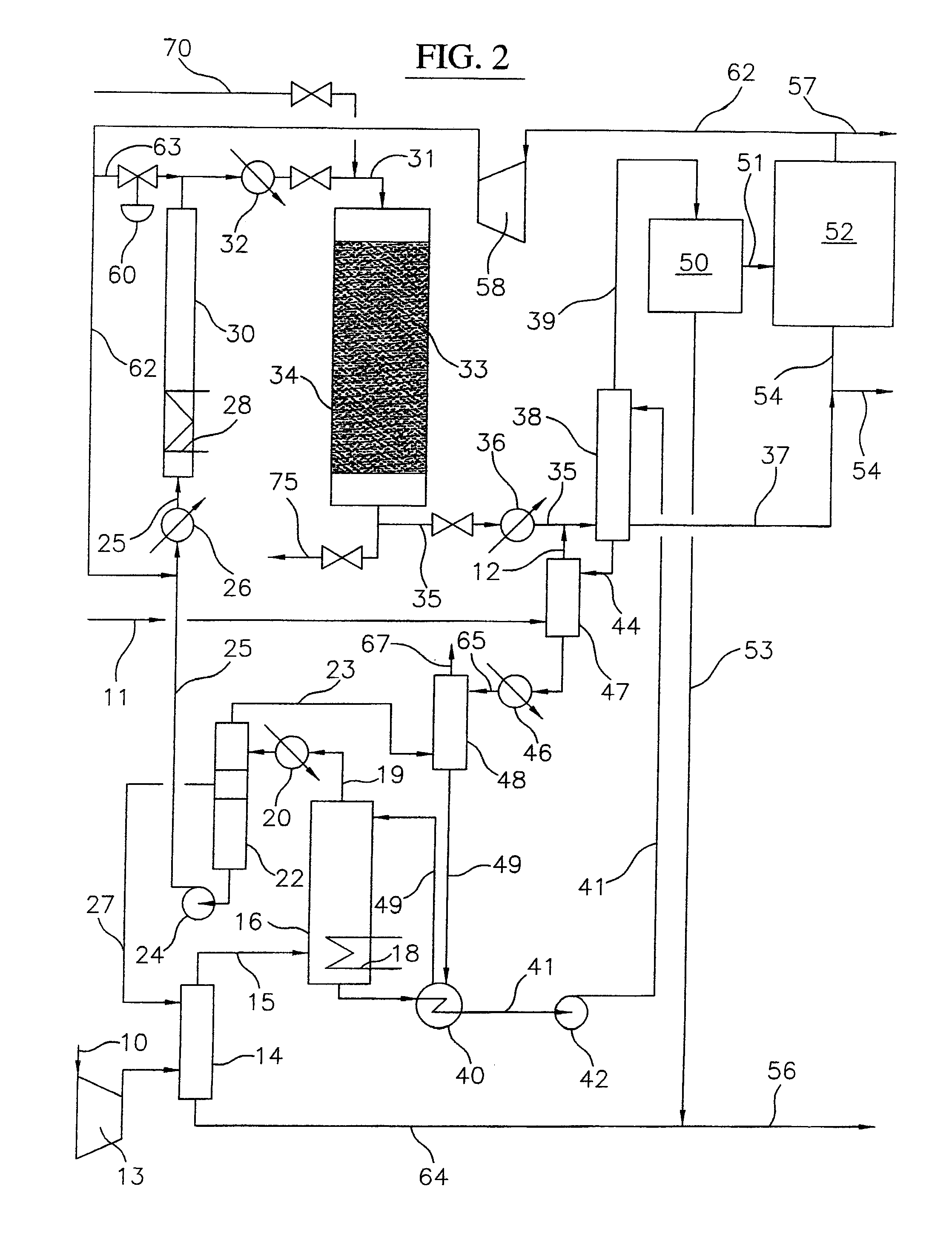
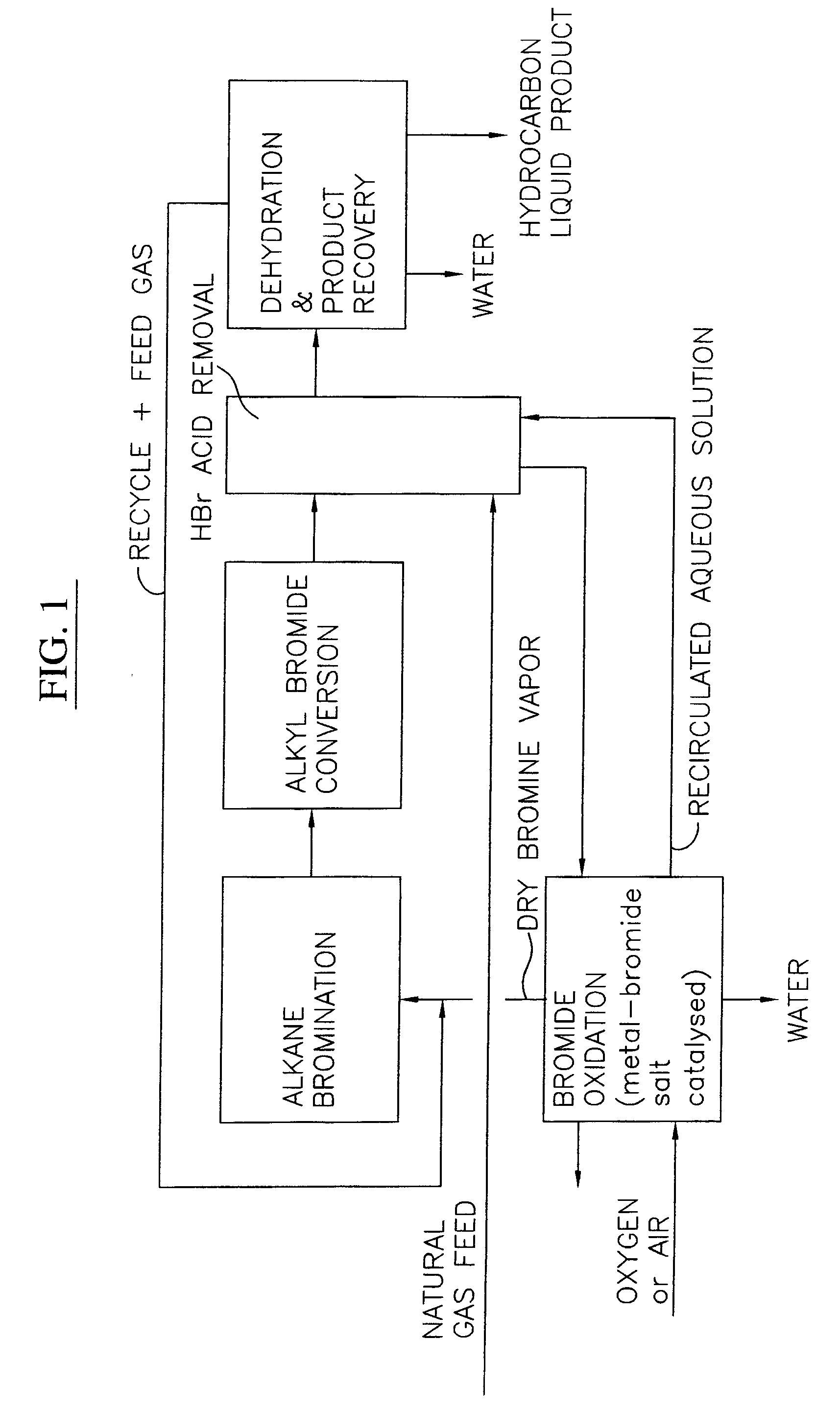
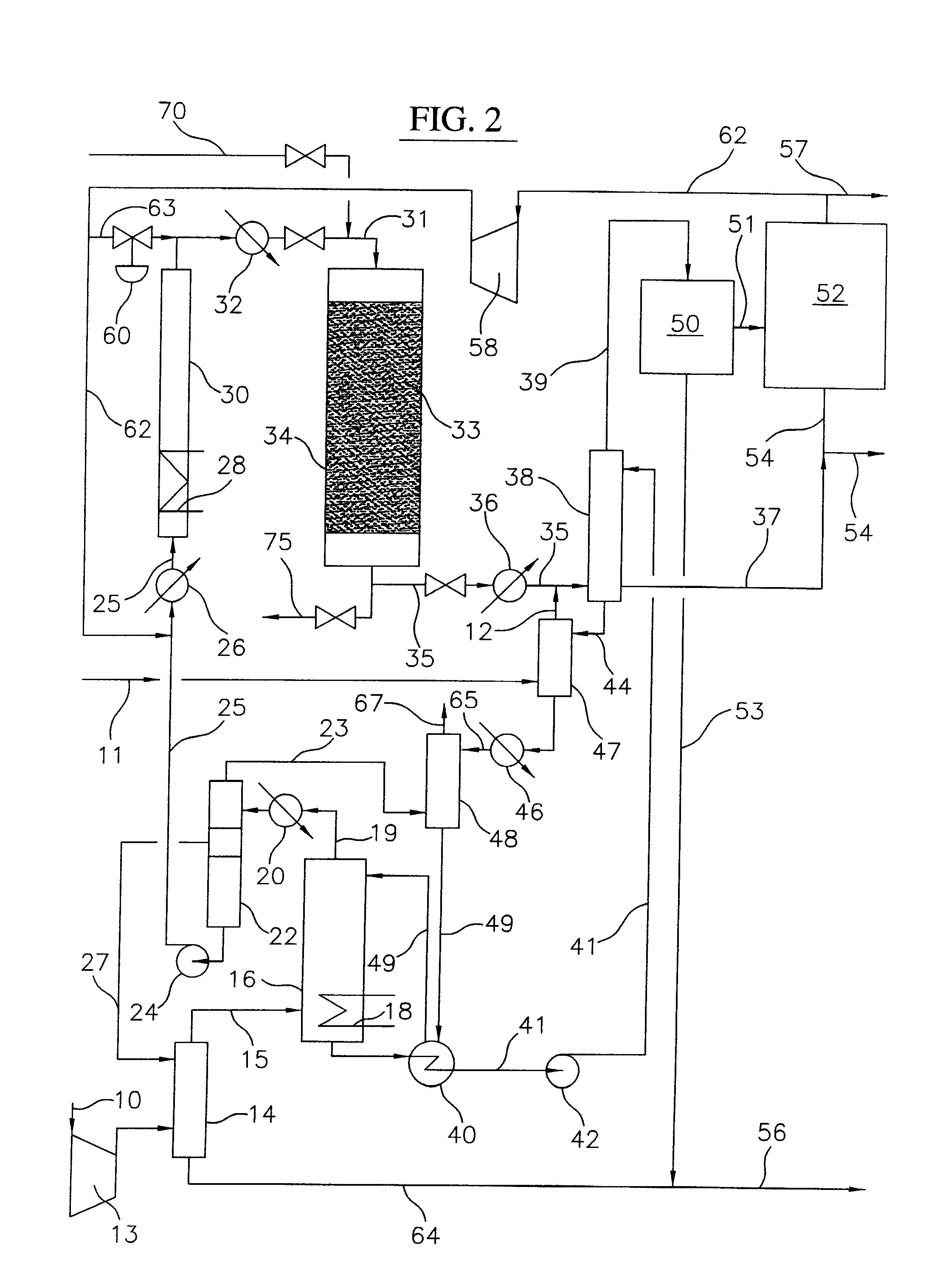
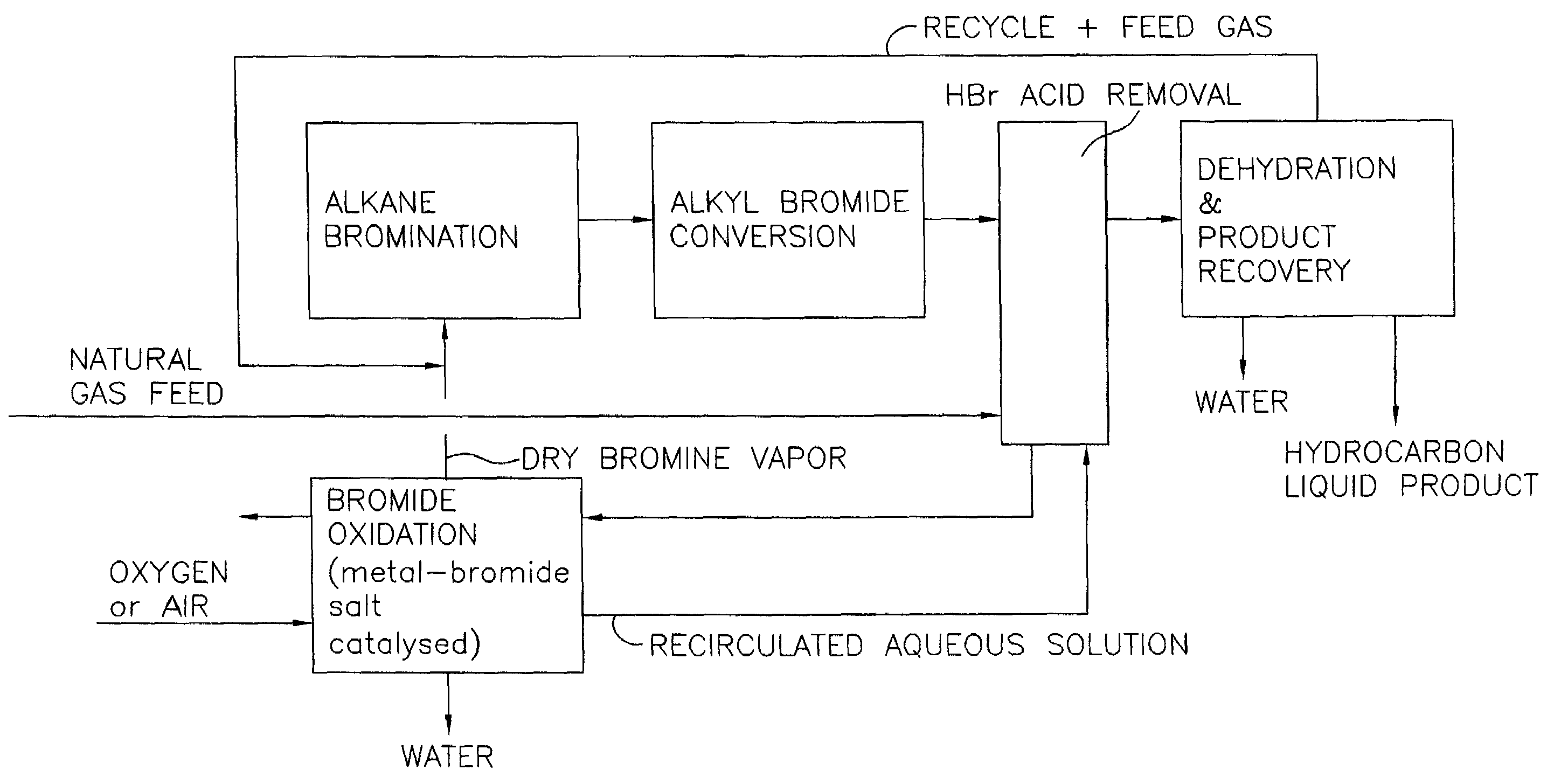
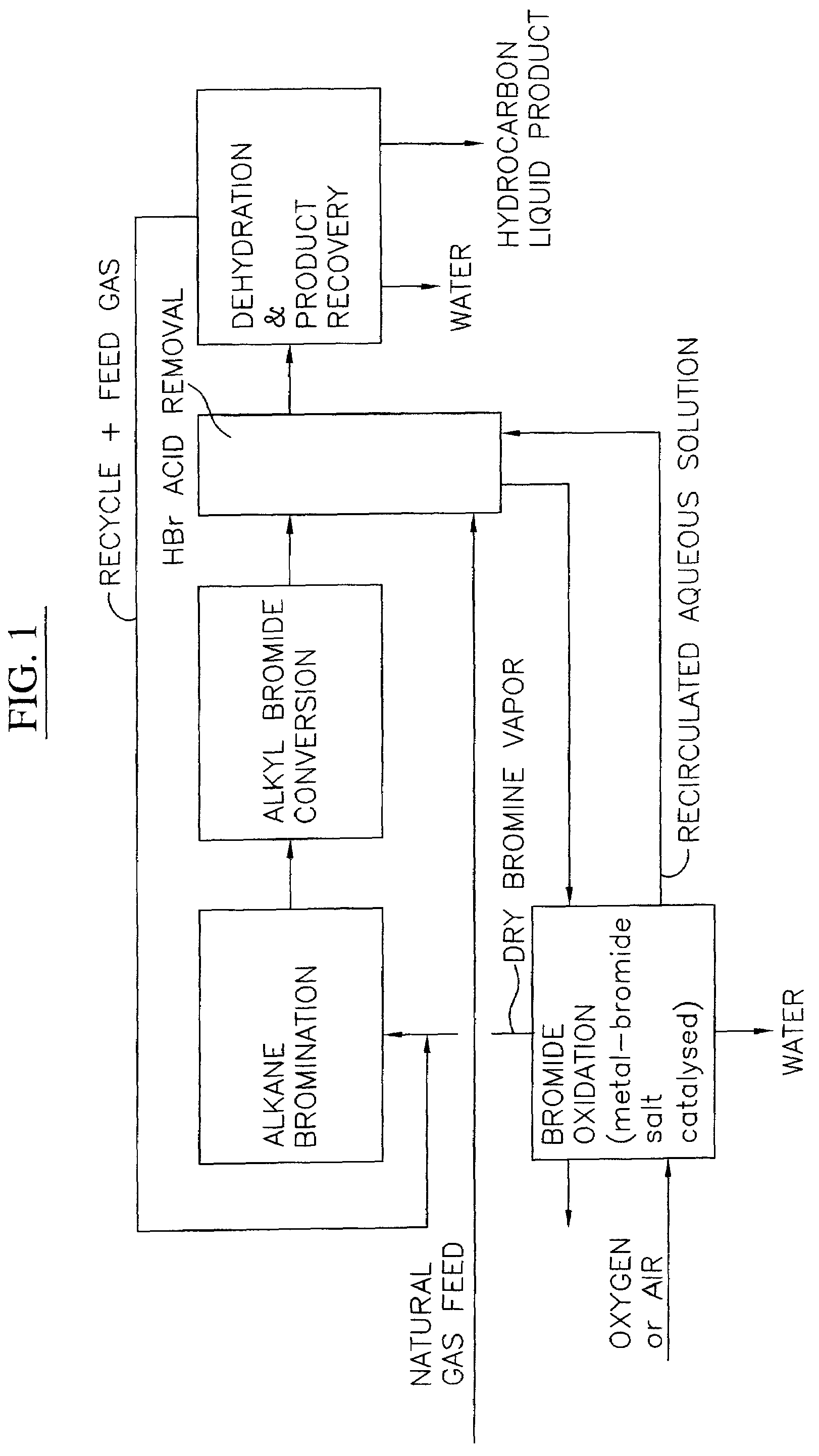
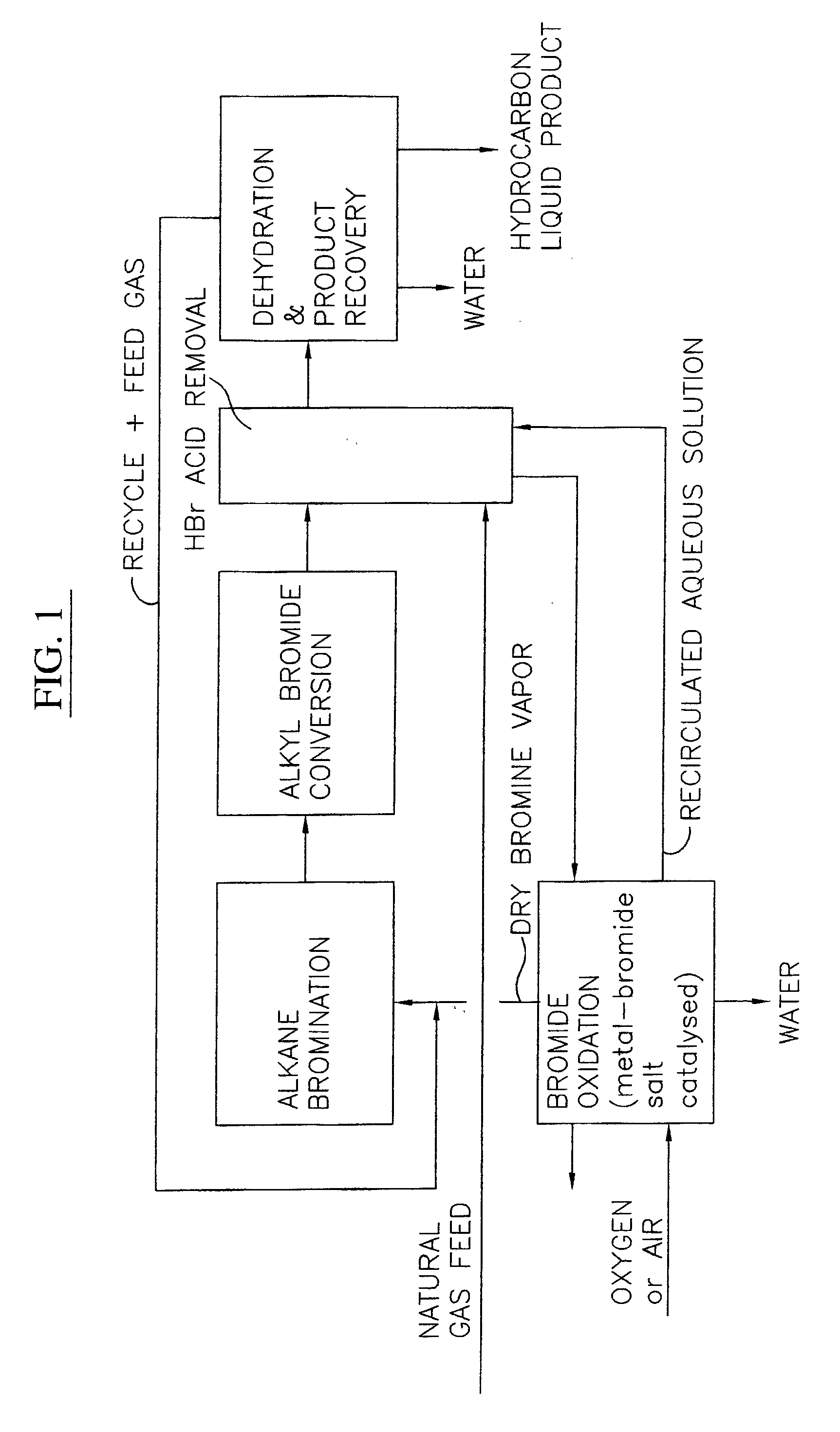
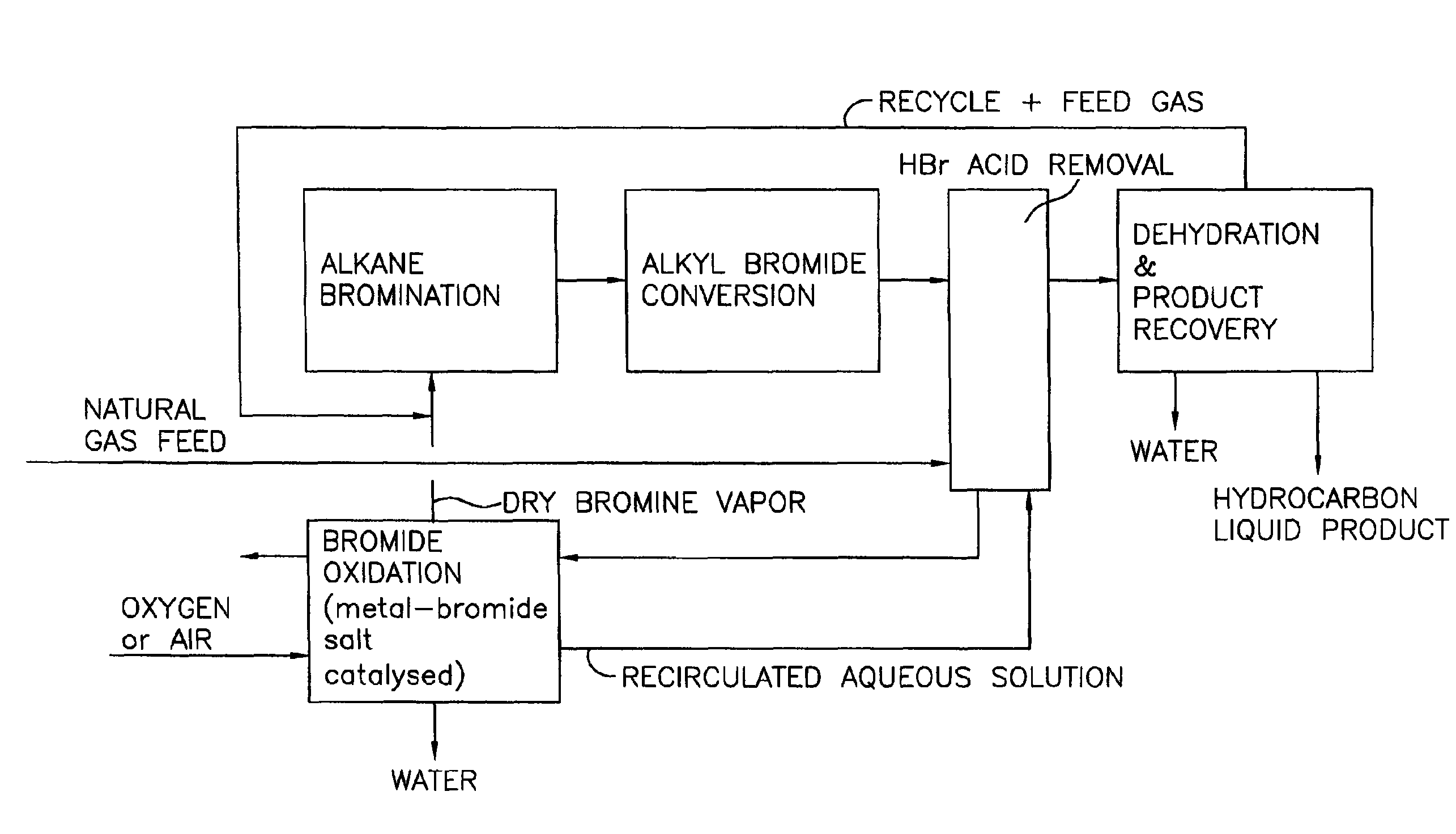
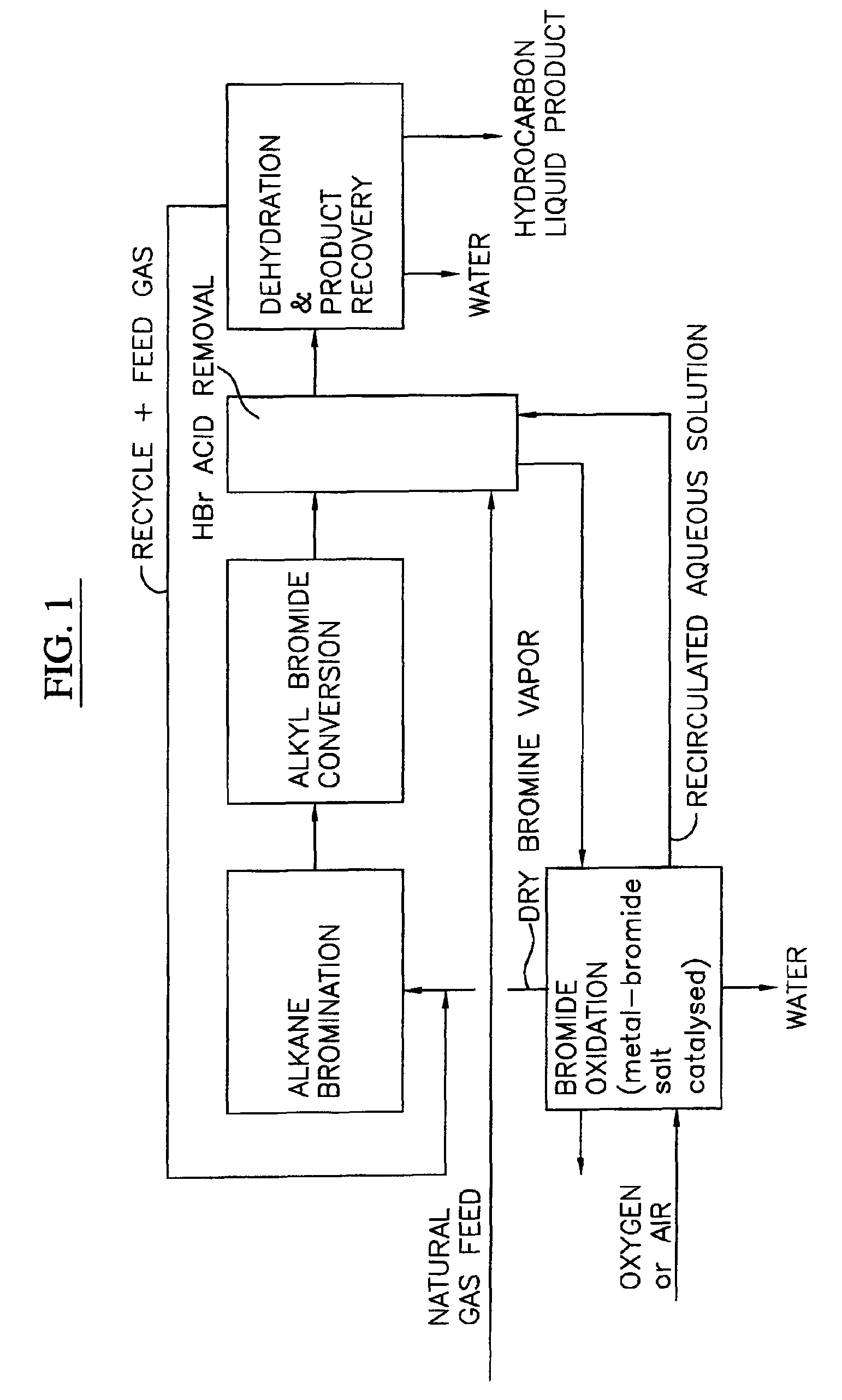
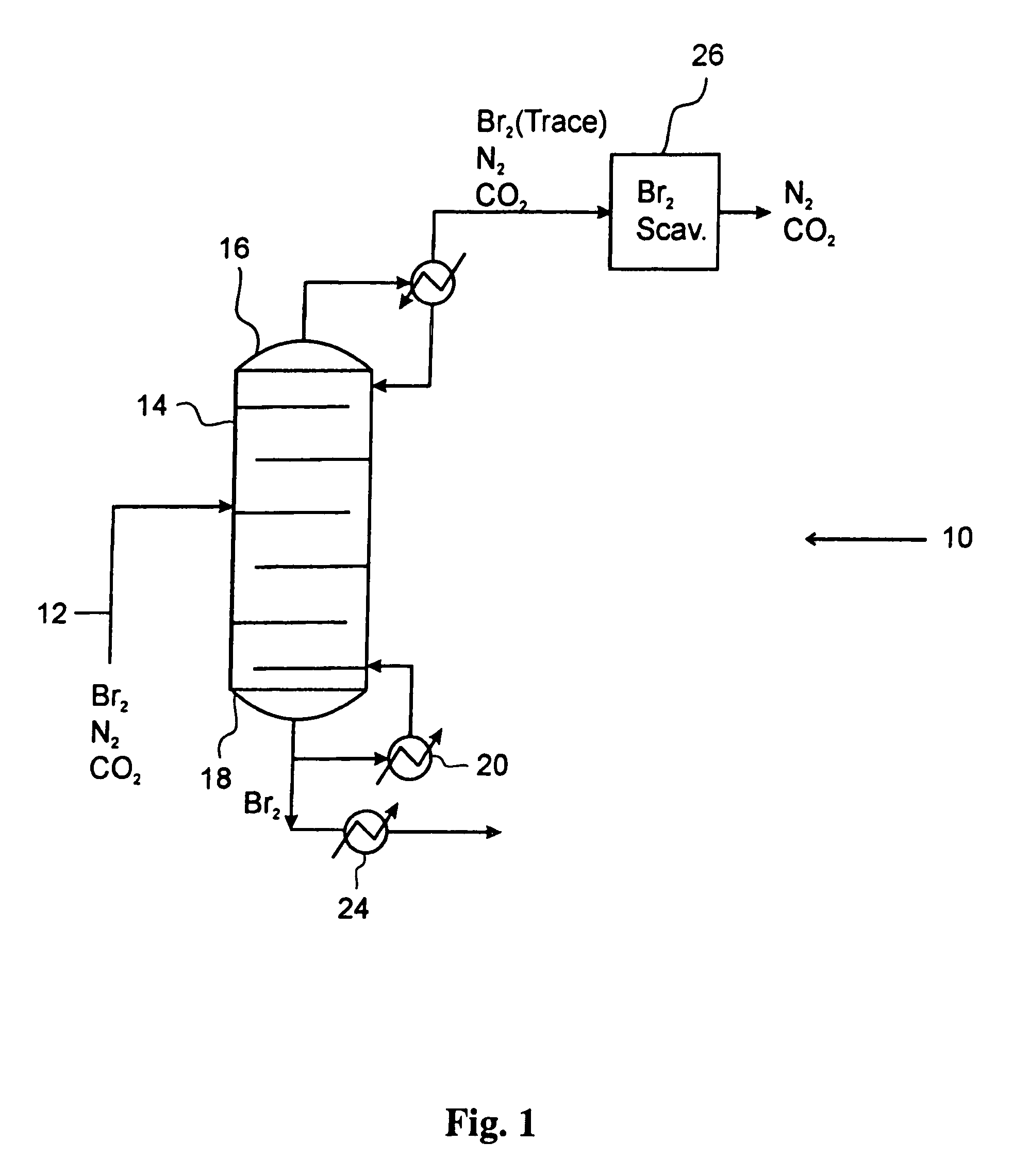
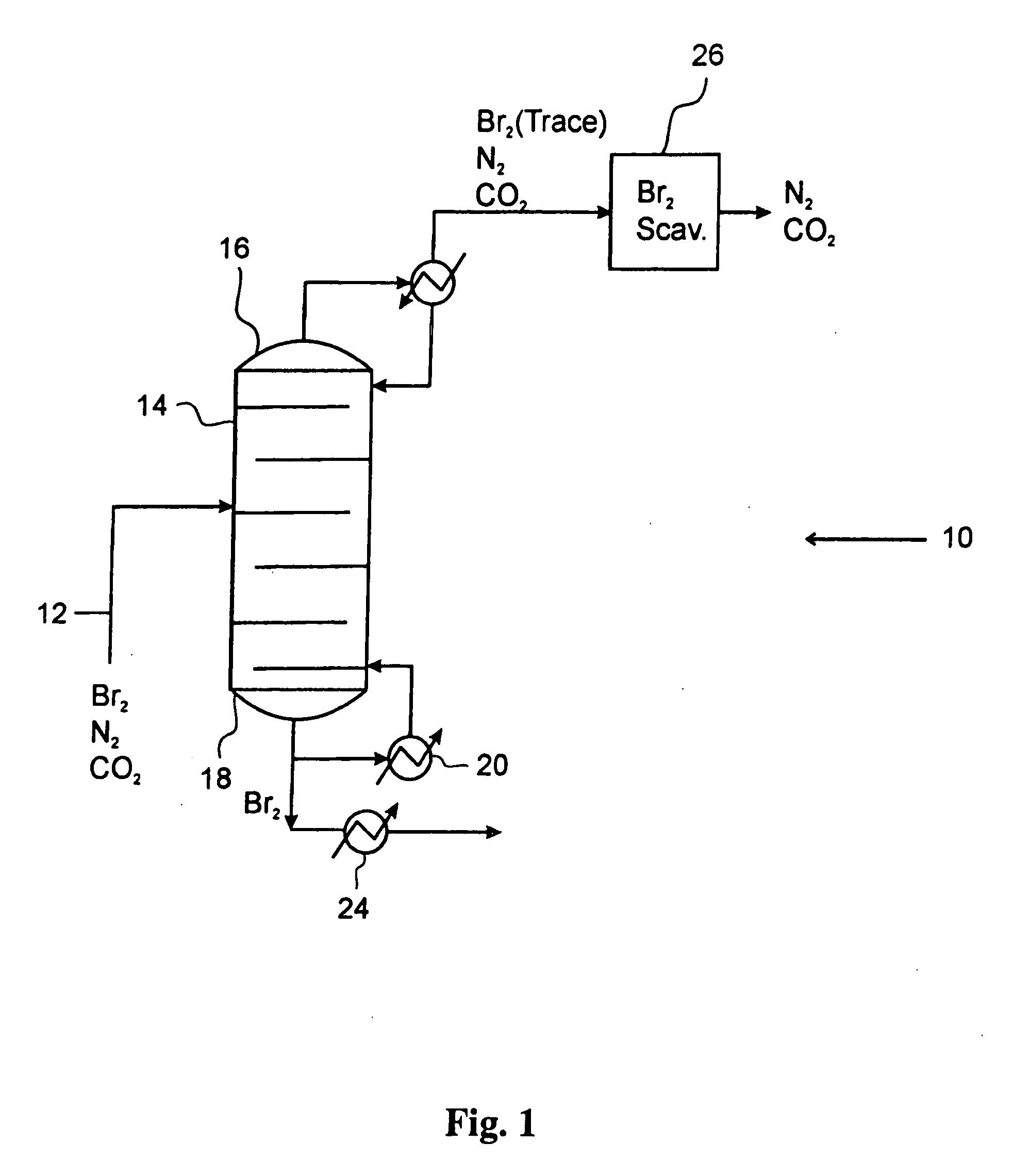
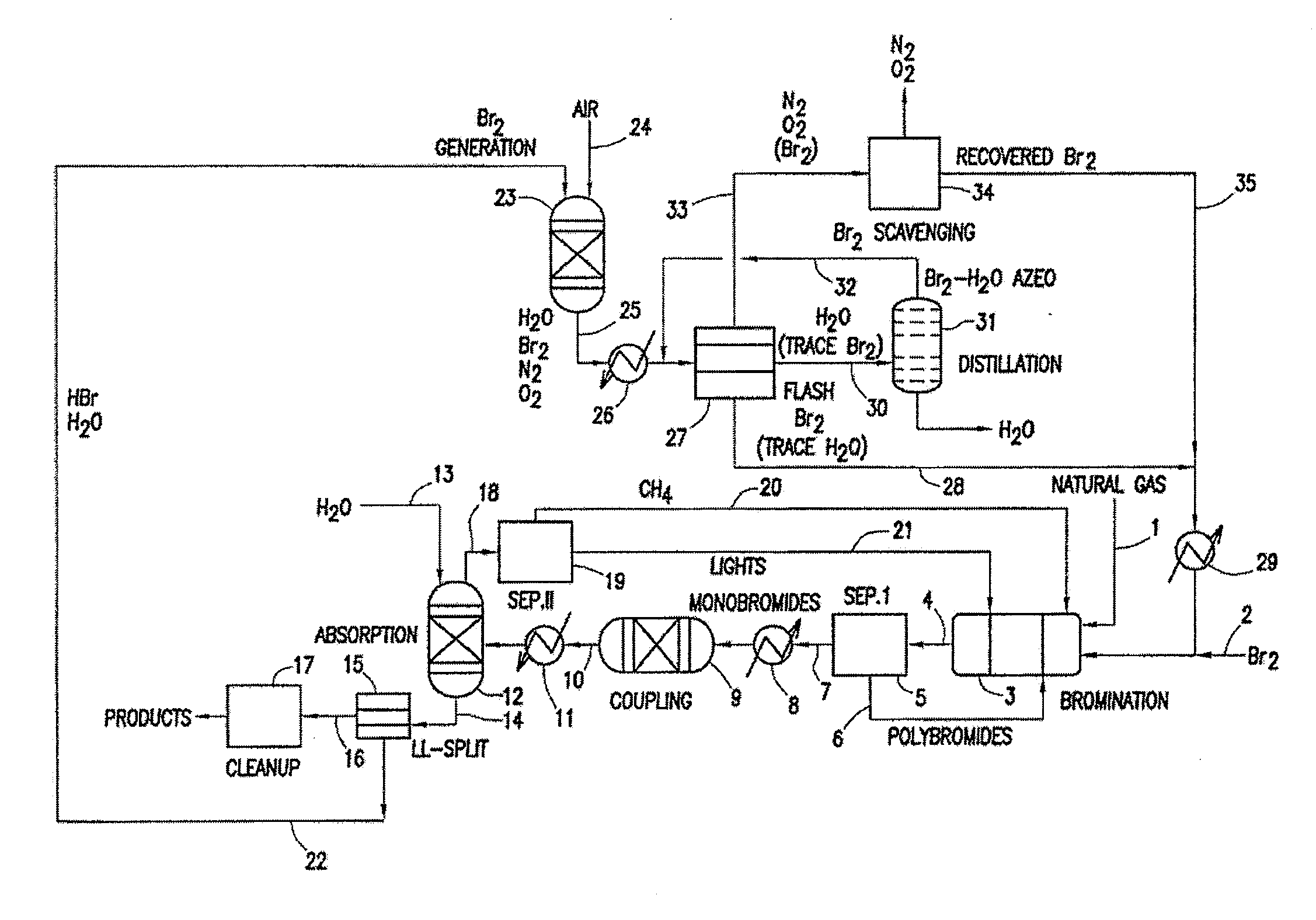
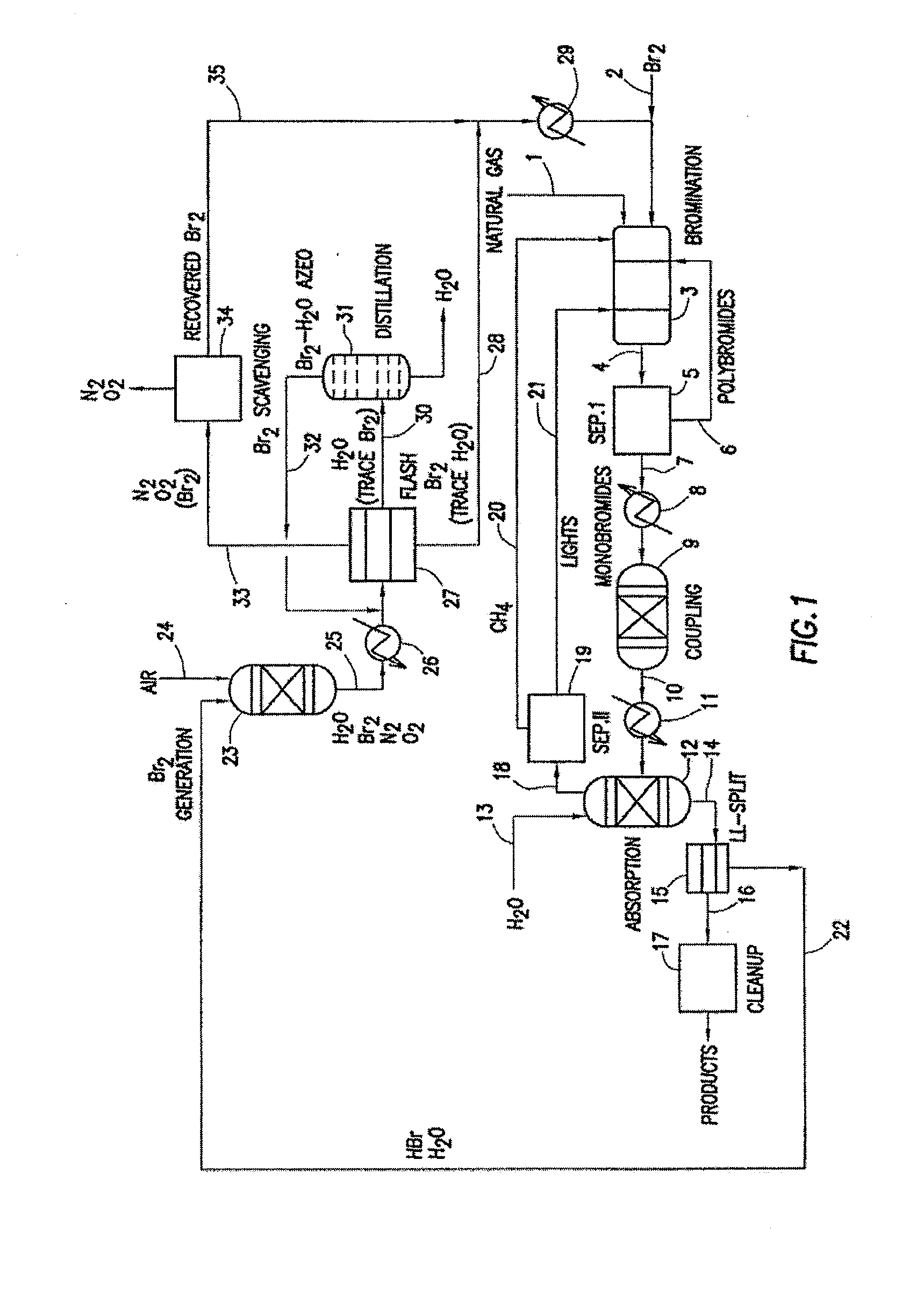
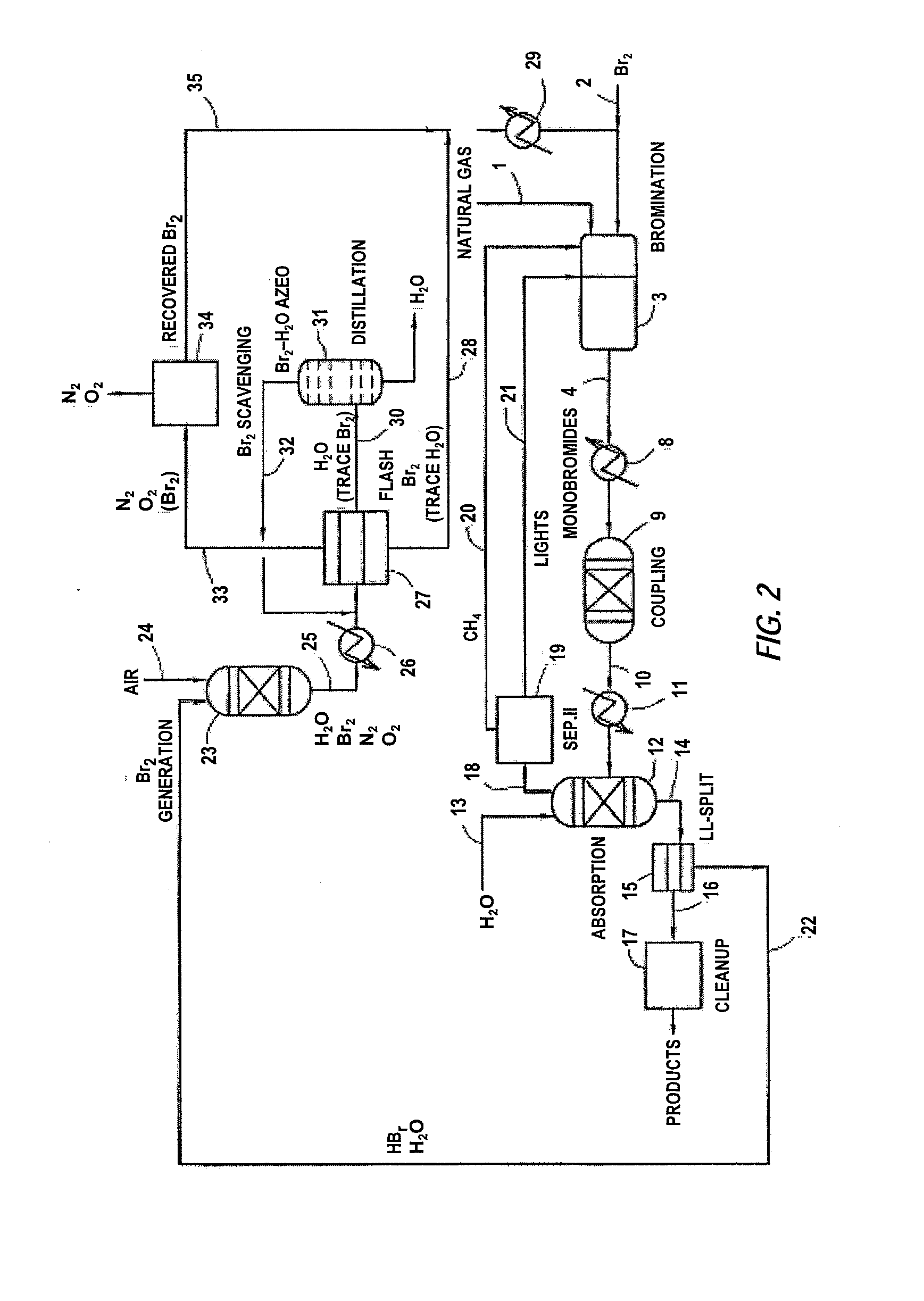
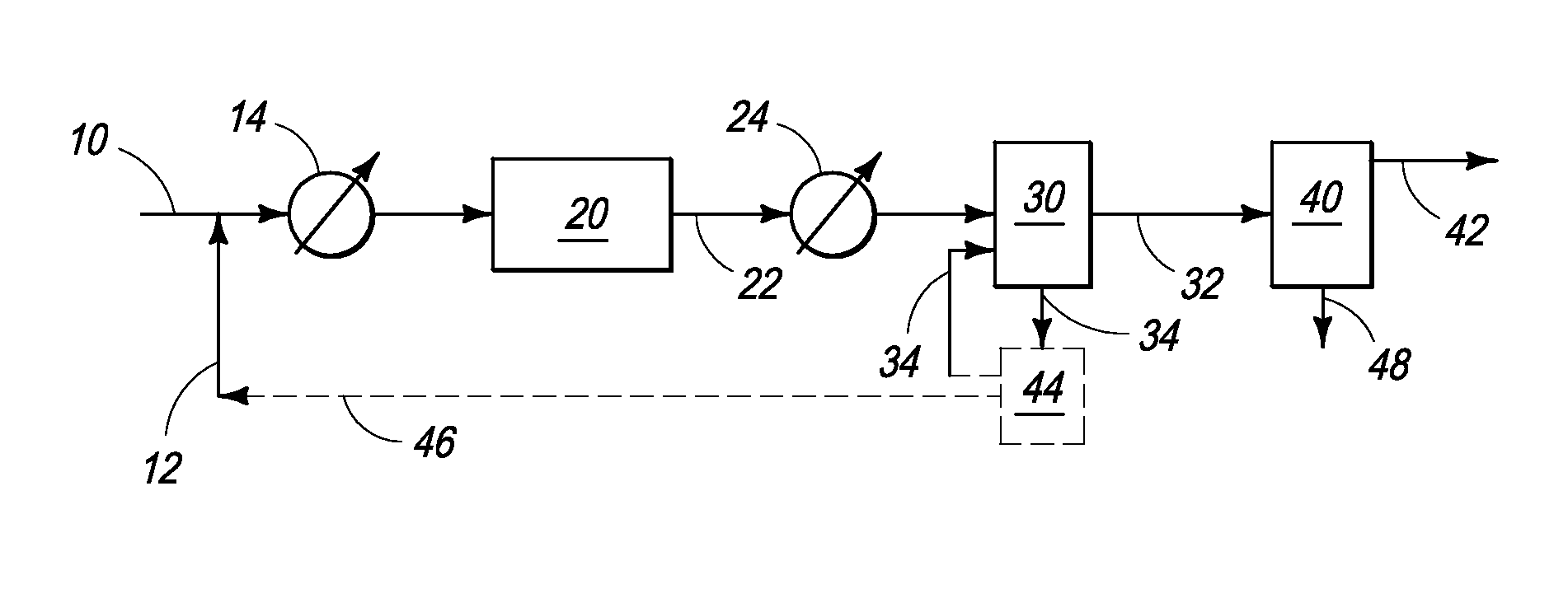
Process for converting gaseous alkanes to liquid hydrocarbons
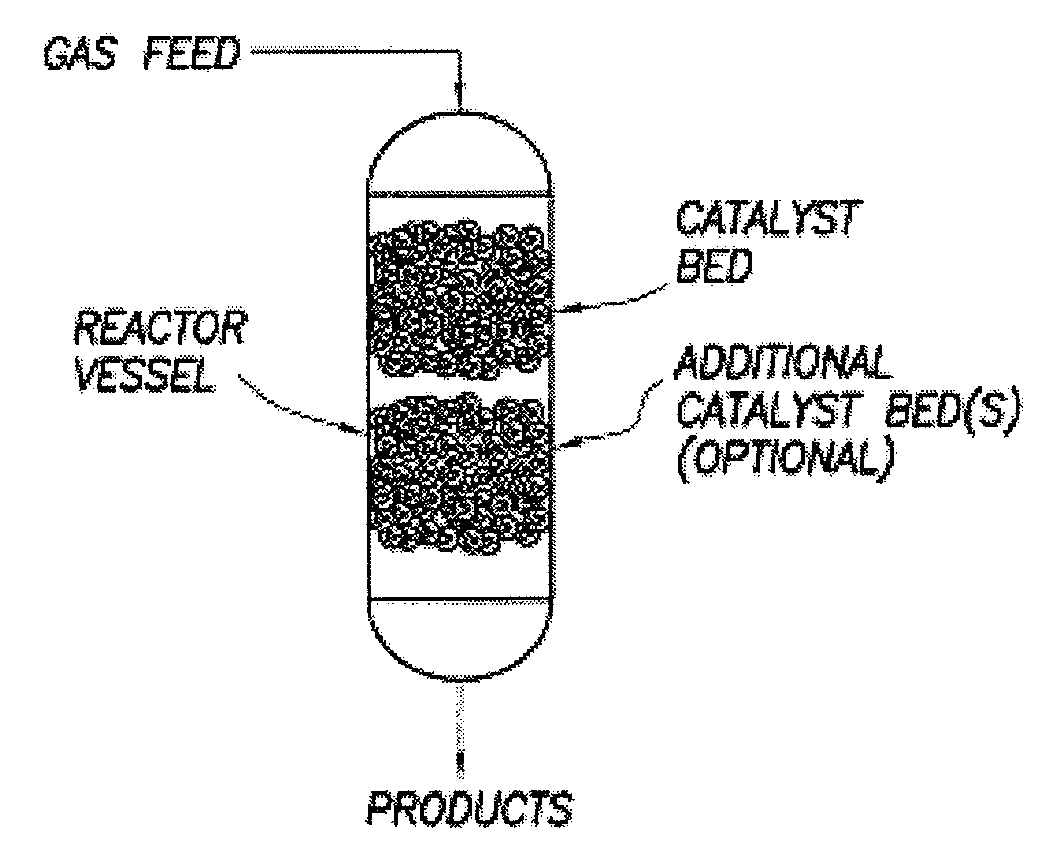
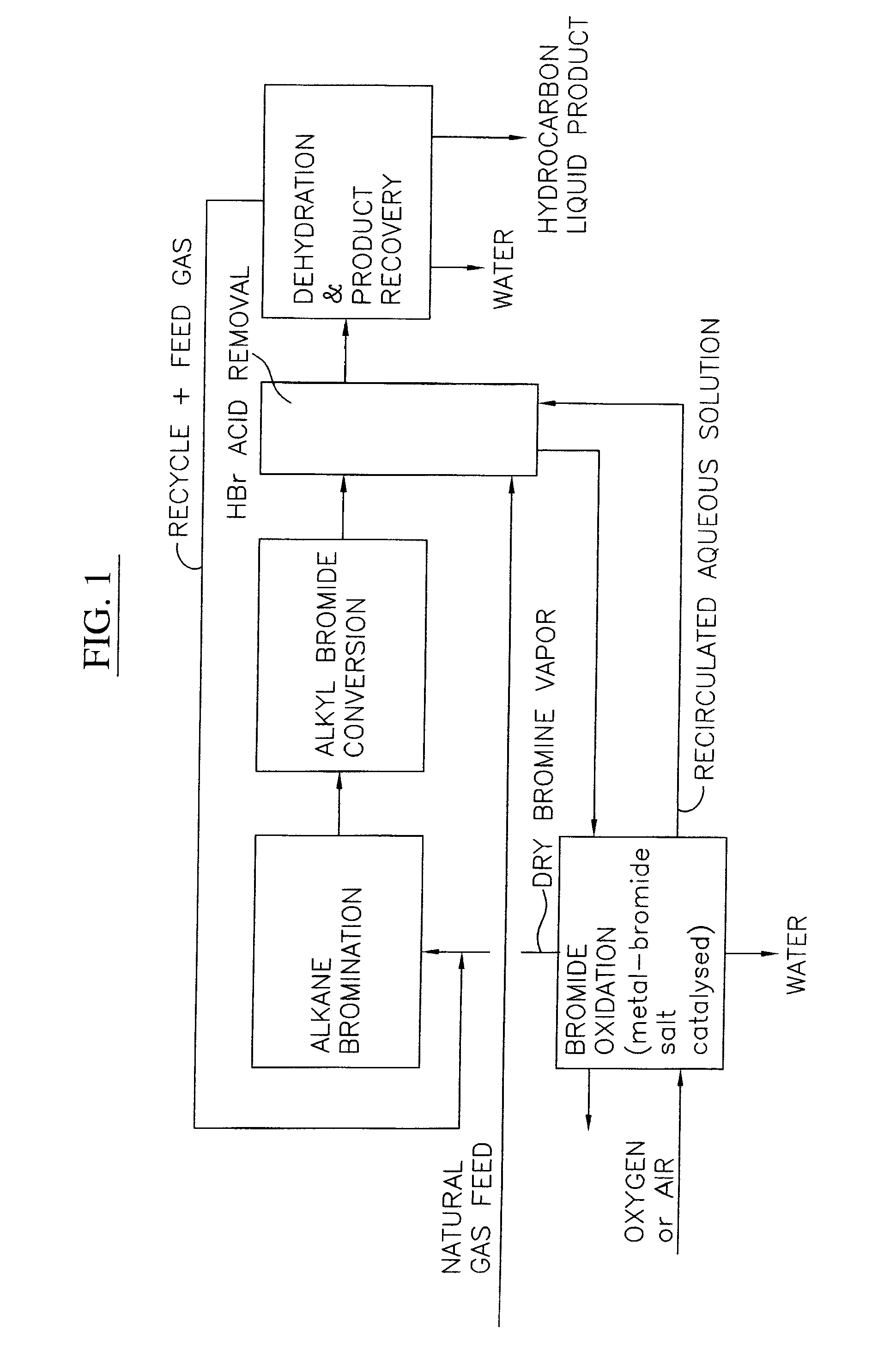
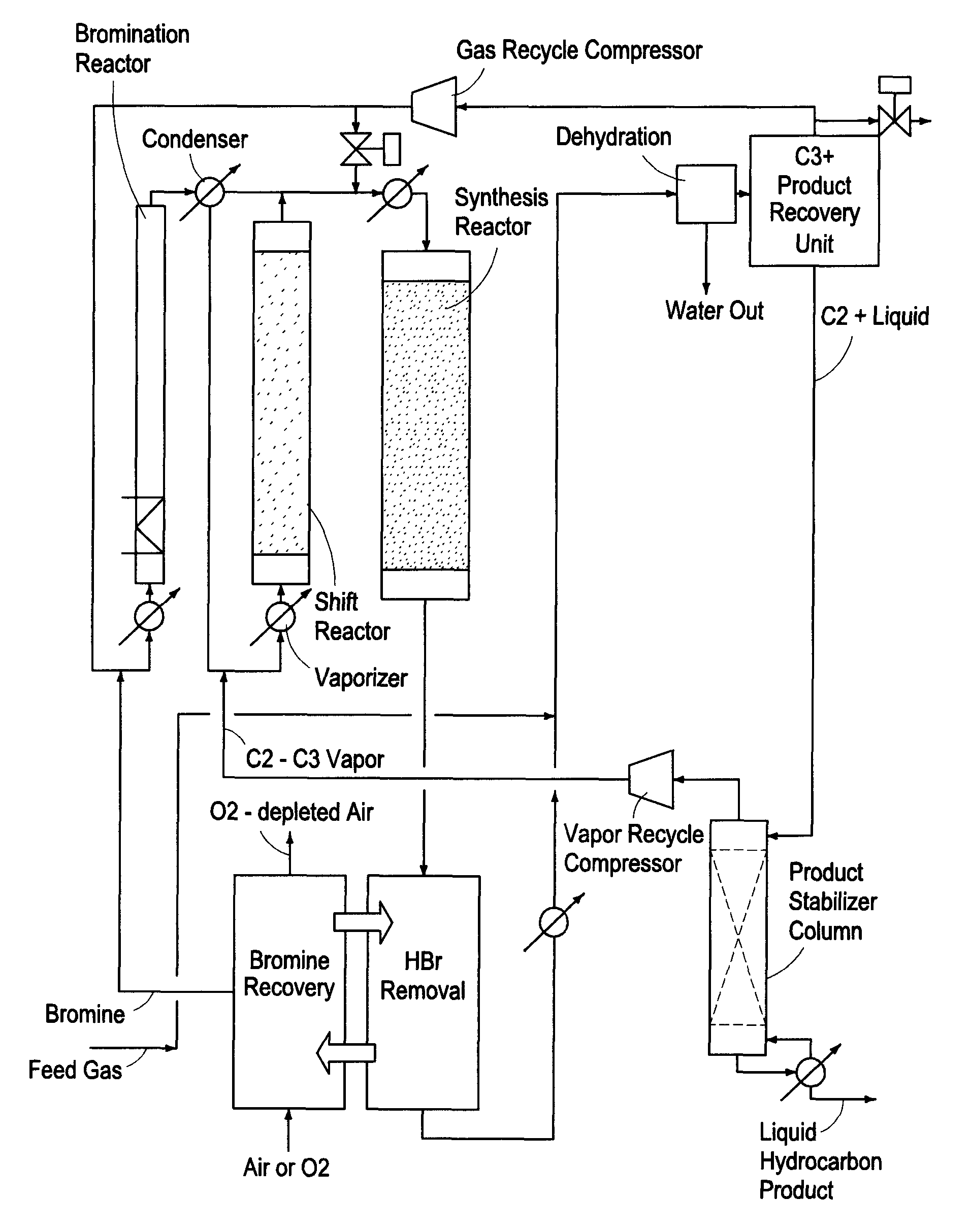
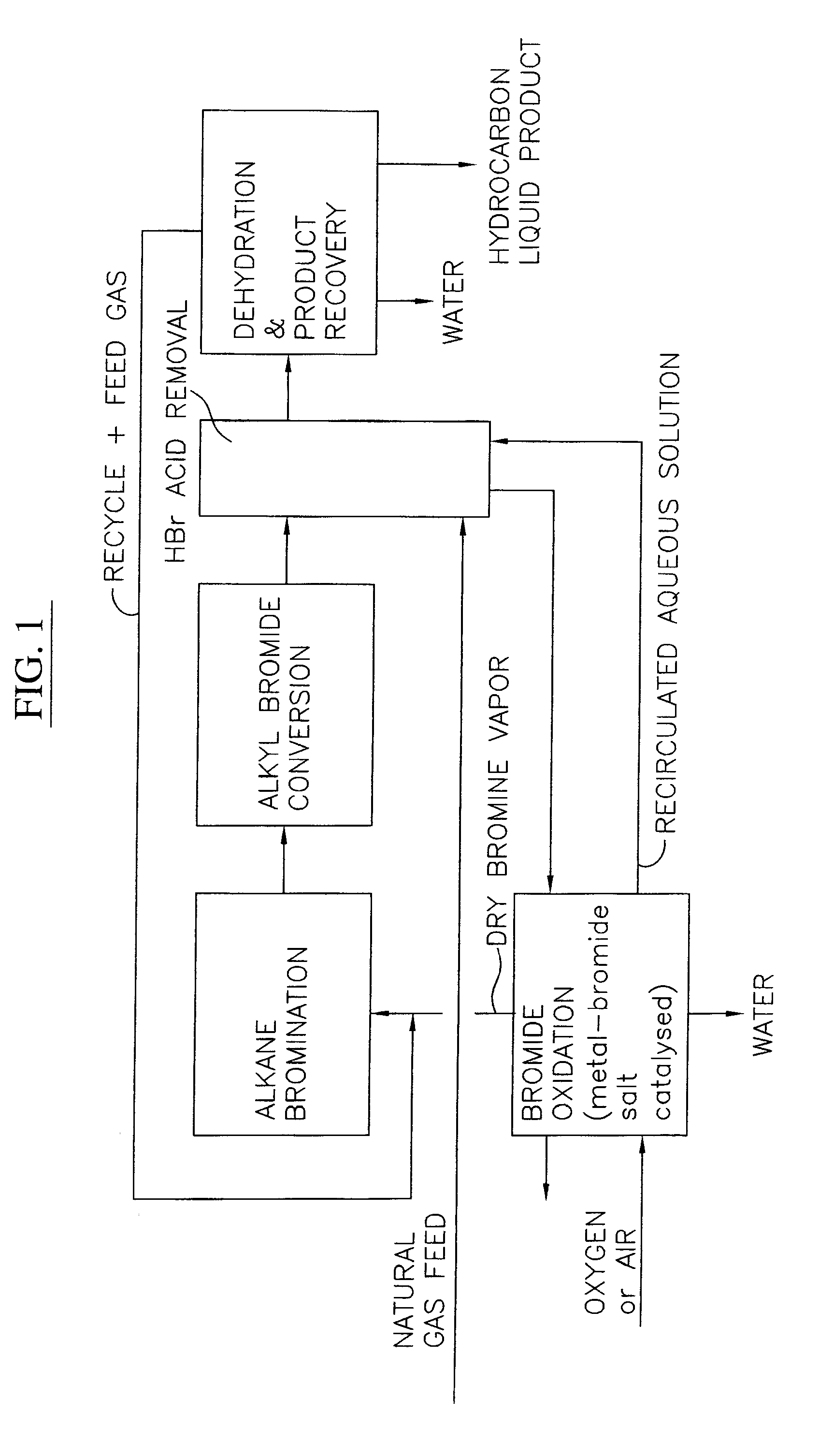
A process for converting gaseous alkanes to liquid hydrocarbons wherein a gaseous feed containing alkanes is reacted with a dry bromine vapor to form alkyl bromides and hydrobromic acid vapor. The mixture of alkyl bromides and hydrobromic acid are then reacted over a synthetic crystalline alumino-silicate catalyst, such as a ZSM-5 zeolite, at a temperature of from about 150° C. to about 400° C. so as to form higher molecular weight hydrocarbons and hydrobromic acid vapor. Hydrobromic acid vapor is removed from the higher molecular weight hydrocarbons. A portion of the propane and butane is removed from the higher molecular weight hydrocarbons and reacted with the mixture of alkyl bromides and hydrobromic acid over the synthetic crystalline alumino-silicate catalyst to form C5+ hydrocarbons.
Owner:SULZER MANAGEMENT AG
Process for converting gaseous alkanes to liquid hydrocarbons
Owner:SULZER MANAGEMENT AG
Process for converting gaseous alkanes to olefins and liquid hydrocarbons
InactiveUS20060100469A1High selectivityAvoid disadvantagesBromide preparationRefining with non-metalsAlkaneBromine
A process for converting gaseous alkanes to olefins and liquid hydrocarbons wherein a gaseous feed containing alkanes is reacted with a dry bromine vapor to form alkyl bromides and hydrobromic acid vapor. The mixture of alkyl bromides and hydrobromic acid are then reacted over a synthetic crystalline alumino-silicate catalyst, such as an X or Y type zeolite, at a temperature of from about 250° C. to about 500° C. so as to form olefins, higher molecular weight hydrocarbons and hydrobromic acid vapor. Various methods are disclosed to remove the hydrobromic acid vapor from the higher molecular weight hydrocarbons and to generate bromine from the hydrobromic acid for use in the process.
Owner:MARATHON GTF TECH
Process for converting gaseous alkanes to liquid hydrocarbons
A process for converting gaseous alkanes to liquid hydrocarbons wherein a gaseous feed containing alkanes is reacted with a dry bromine vapor to form alkyl bromides and hydrobromic acid vapor. The mixture of alkyl bromides and hydrobromic acid are then reacted over a synthetic crystalline alumino-silicate catalyst, such as a ZSM-5 zeolite, at a temperature of from about 150° C. to about 450° C. so as to form higher molecular weight hydrocarbons and hydrobromic acid vapor. Propane and butane which comprise a portion of the products may be recovered or recycled back through the process to form additional C5+ hydrocarbons. Various methods are disclosed to remove the hydrobromic acid vapor from the higher molecular weight hydrocarbons and to generate bromine from the hydrobromic acid for use in the process.
Owner:SULZER MANAGEMENT AG
Process for converting gaseous alkanes to liquid hydrocarbons
A process for converting gaseous alkanes to liquid hydrocarbons wherein a gaseous feed containing alkanes is reacted with a dry bromine vapor to form alkyl bromides and hydrobromic acid vapor. The mixture of alkyl bromides and hydrobromic acid are then reacted over a synthetic crystalline alumino-silicate catalyst, such as a ZSM-5 zeolite, at a temperature of from about 150° C. to about 400° C. so as to form higher molecular weight hydrocarbons and hydrobromic acid vapor. Hydrobromic acid vapor is removed from the higher molecular weight hydrocarbons. A portion of the propane and butane is removed from the higher molecular weight hydrocarbons and reacted with the mixture of alkyl bromides and hydrobromic acid over the synthetic crystalline alumino-silicate catalyst to form C5+ hydrocarbons.
Owner:SULZER MANAGEMENT AG
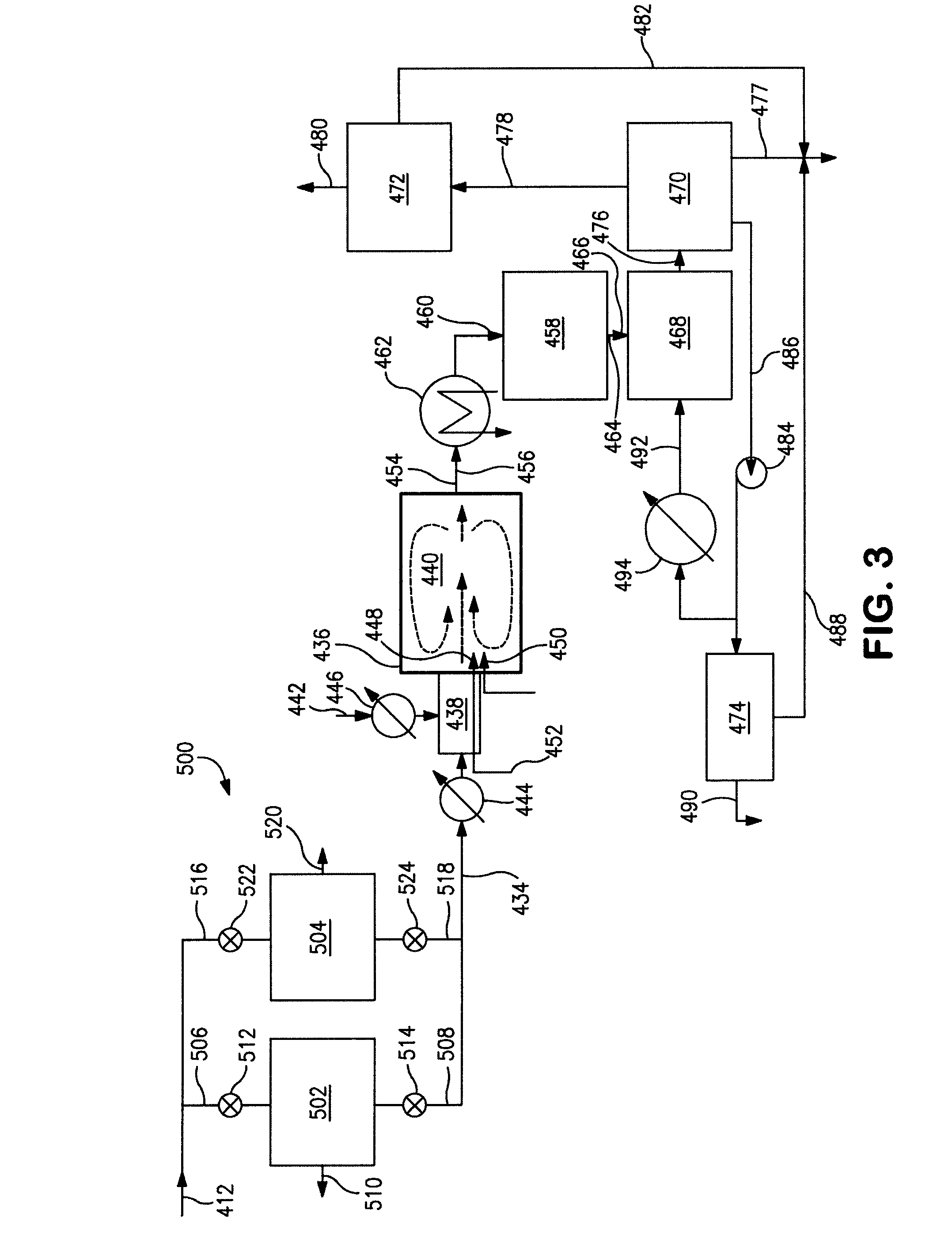
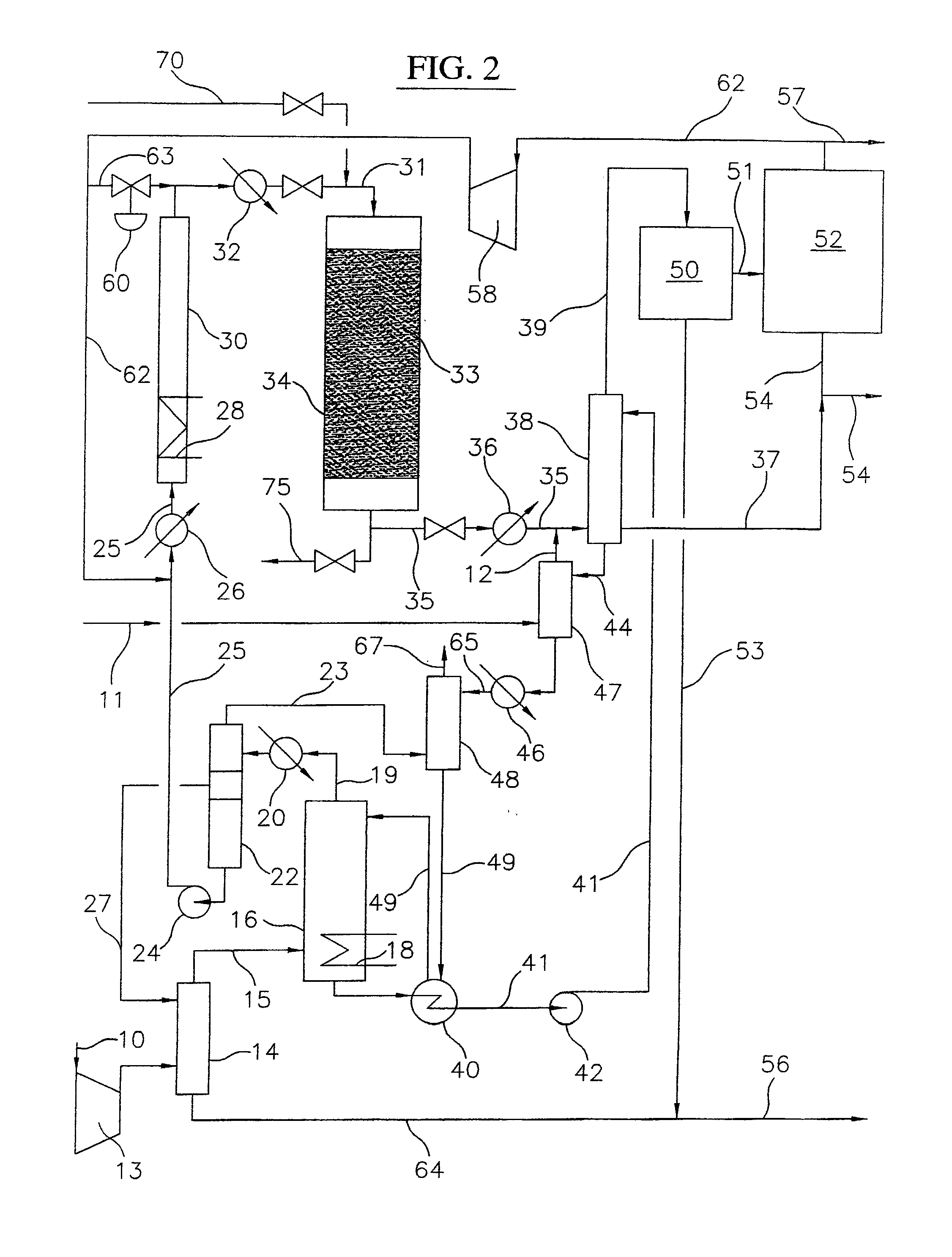
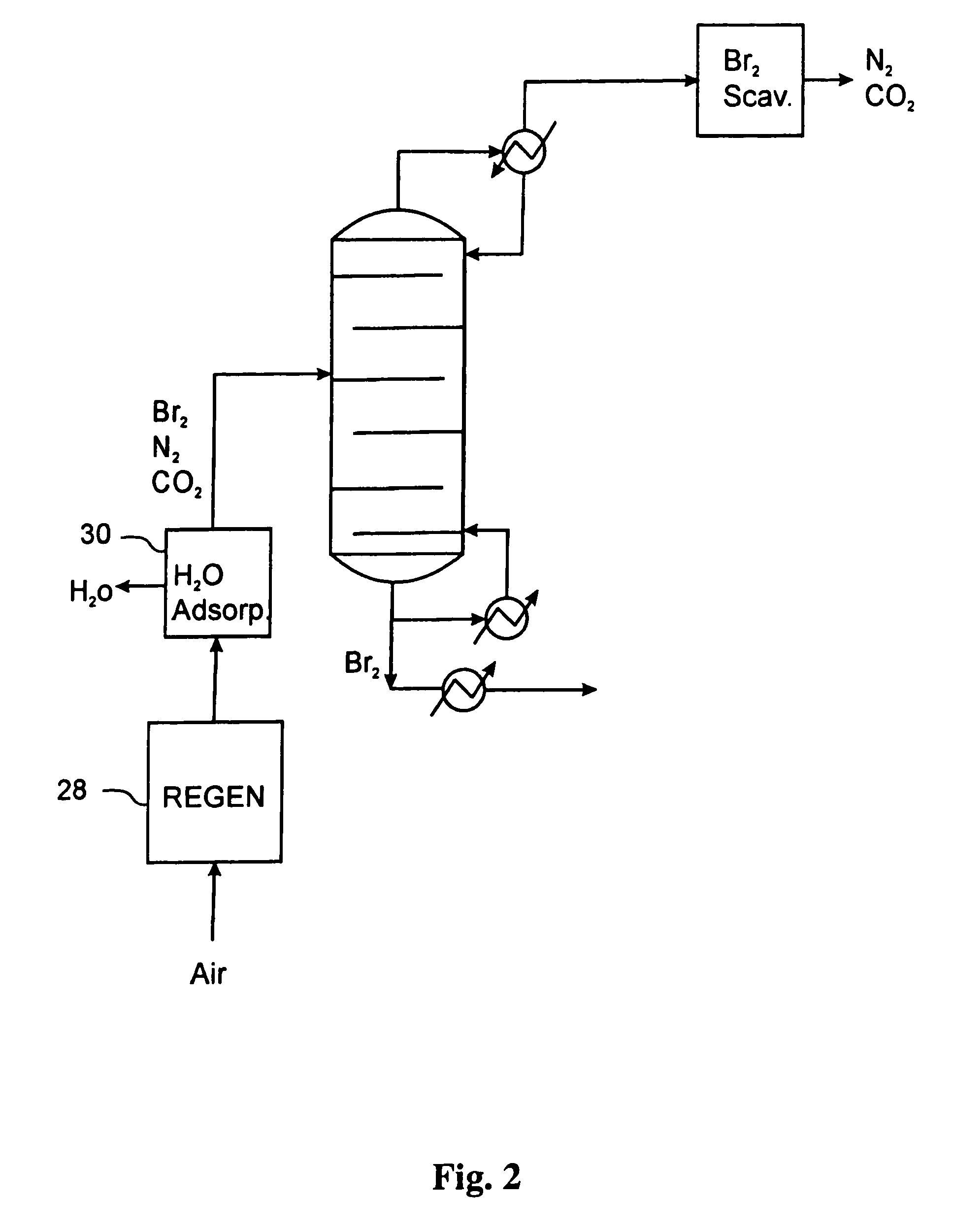
Processes for converting gaseous alkanes to liquid hydrocarbons
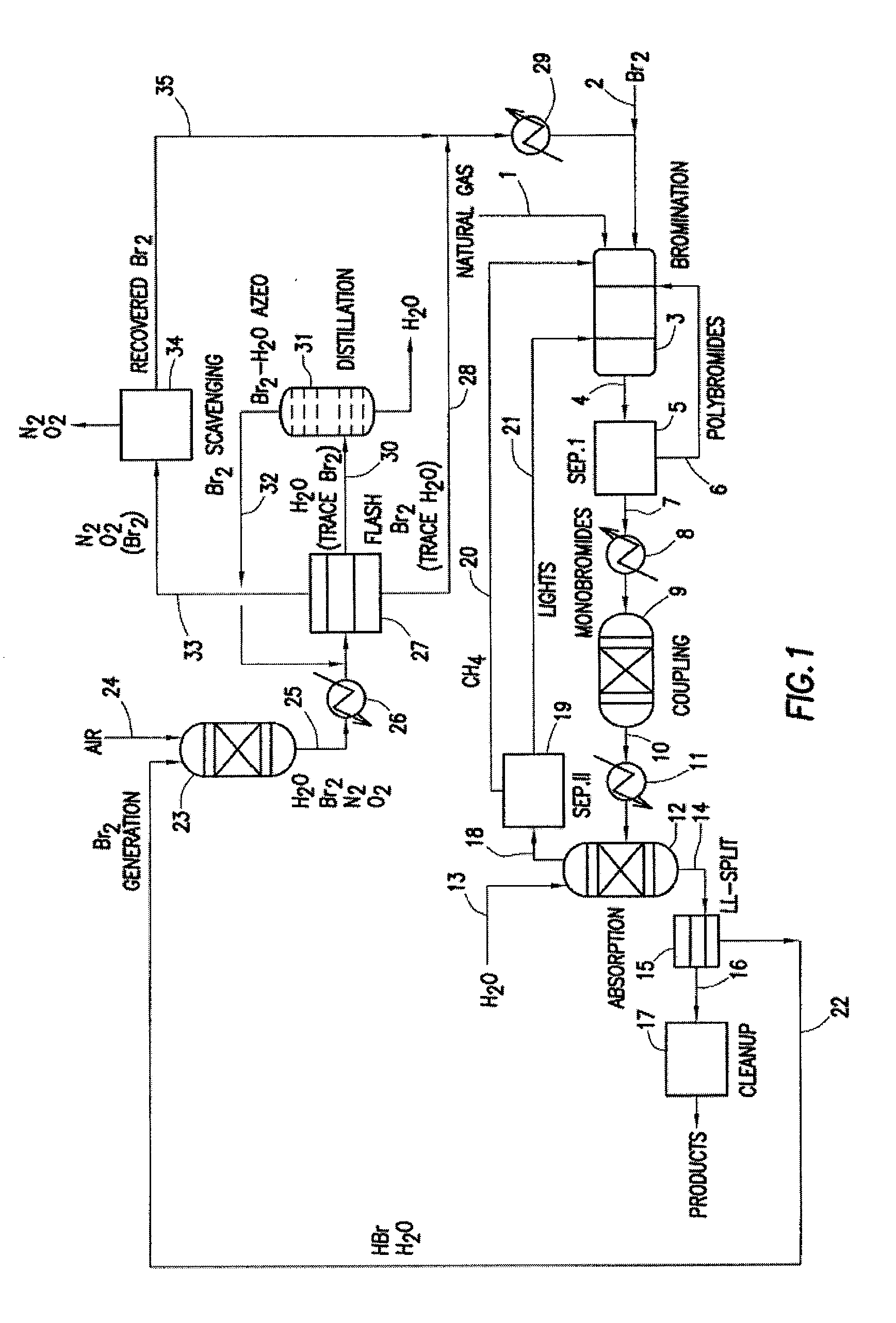
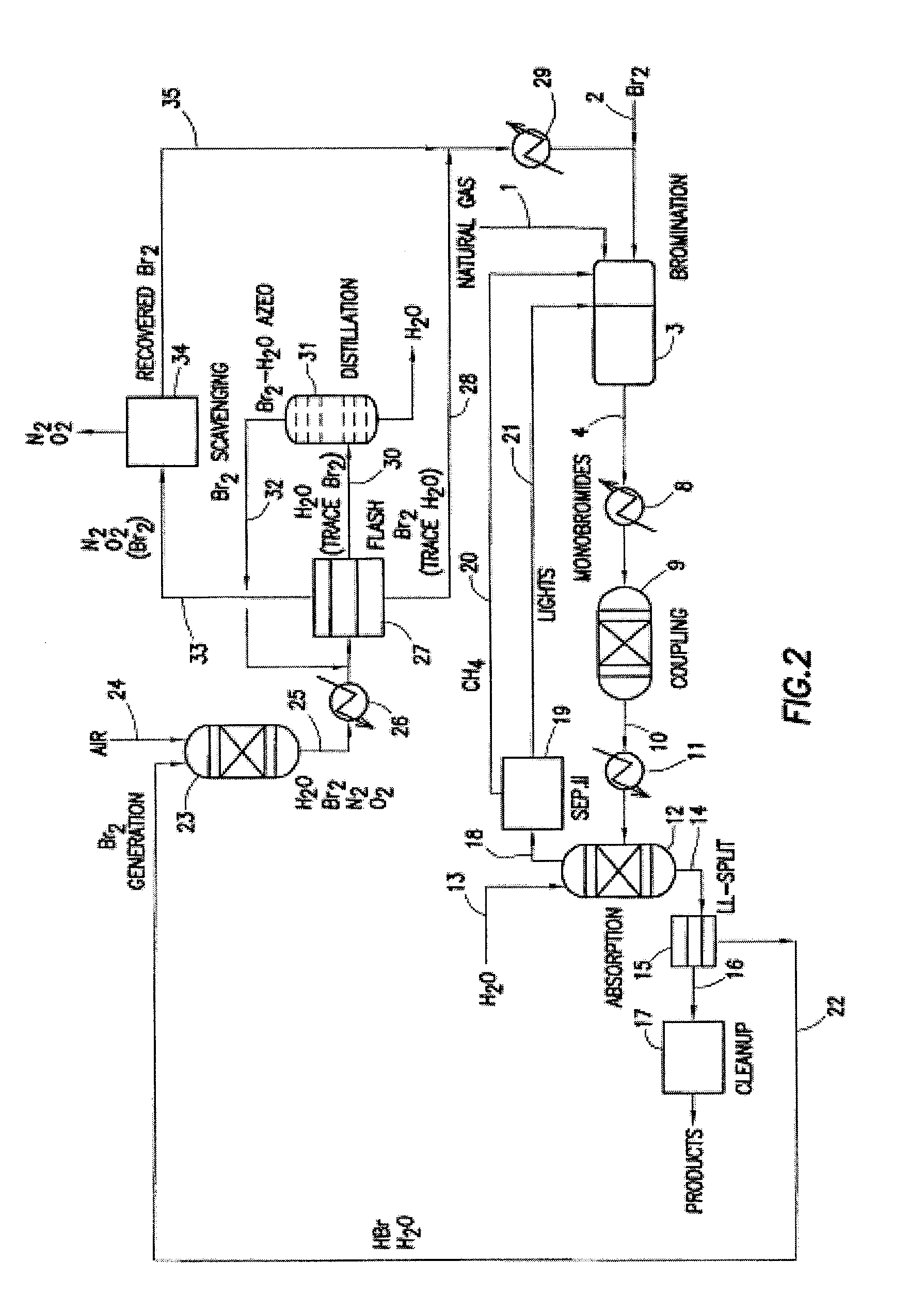
A process for converting gaseous alkanes to olefins, higher molecular weight hydrocarbons or mixtures thereof wherein a gaseous feed containing alkanes is thermally reacted with a dry bromine vapor to form alkyl bromides and hydrogen bromide. Poly-brominated alkanes present in the alkyl bromides are further reacted with methane over a suitable catalyst to form mono-brominated species. The mixture of alkyl bromides and hydrogen bromide is then reacted over a suitable catalyst at a temperature sufficient to form olefins, higher molecular weight hydrocarbons or mixtures thereof and hydrogen bromide. Various methods are disclosed to remove the hydrogen bromide from the higher molecular weight hydrocarbons, to generate bromine from the hydrogen bromide for use in the process, and to selectively form mono-brominated alkanes in the bromination step.
Owner:SULZER MANAGEMENT AG
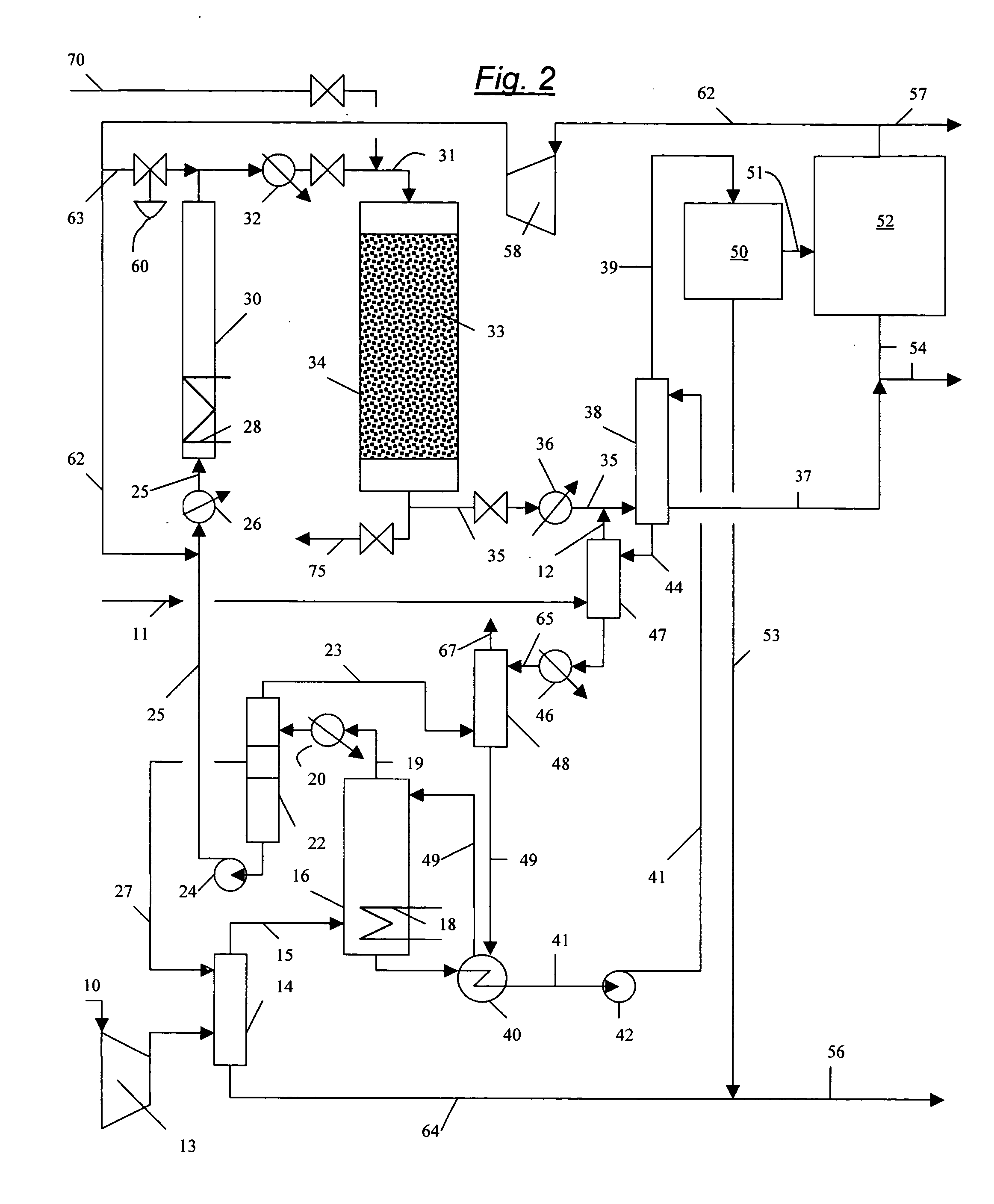
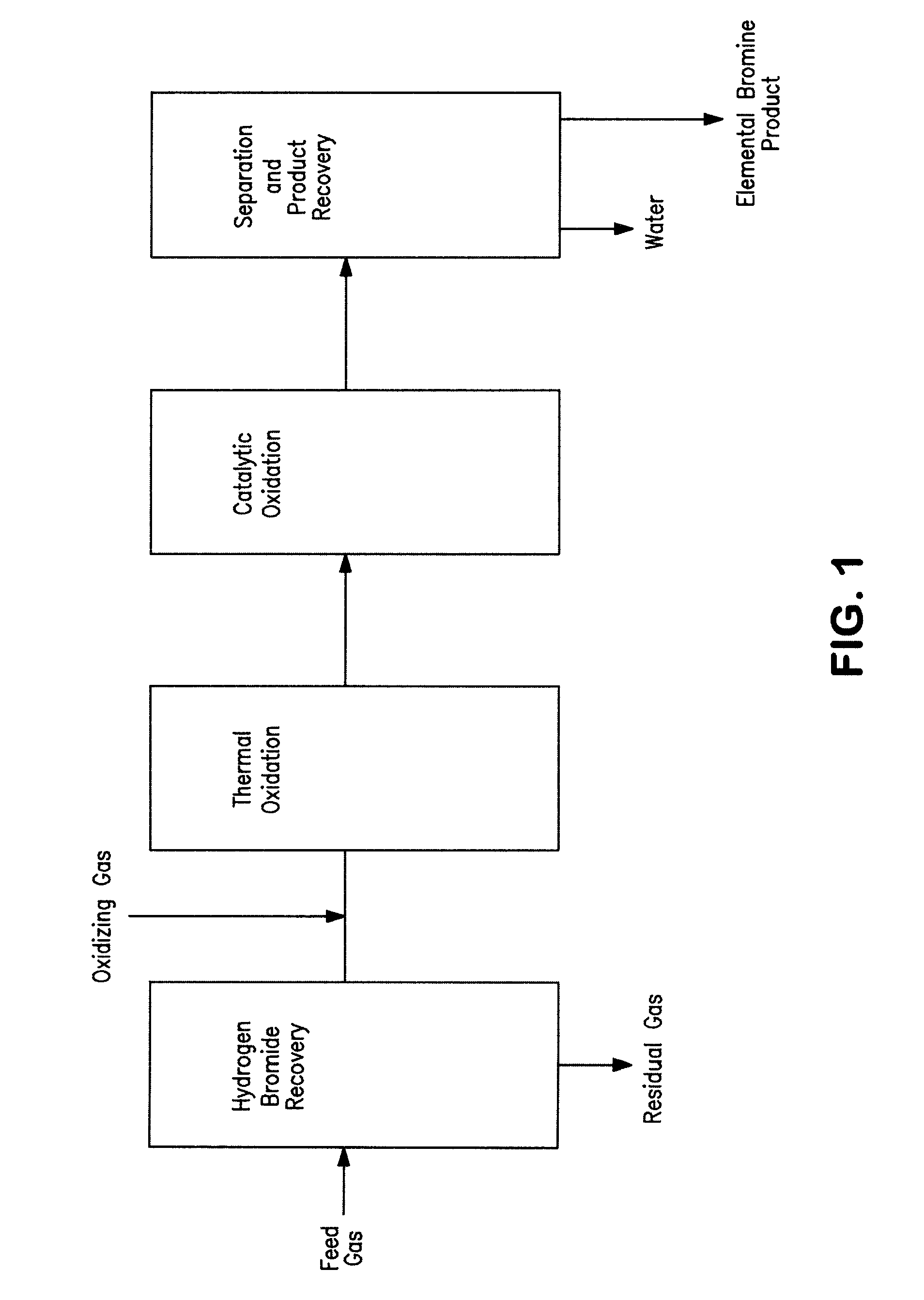
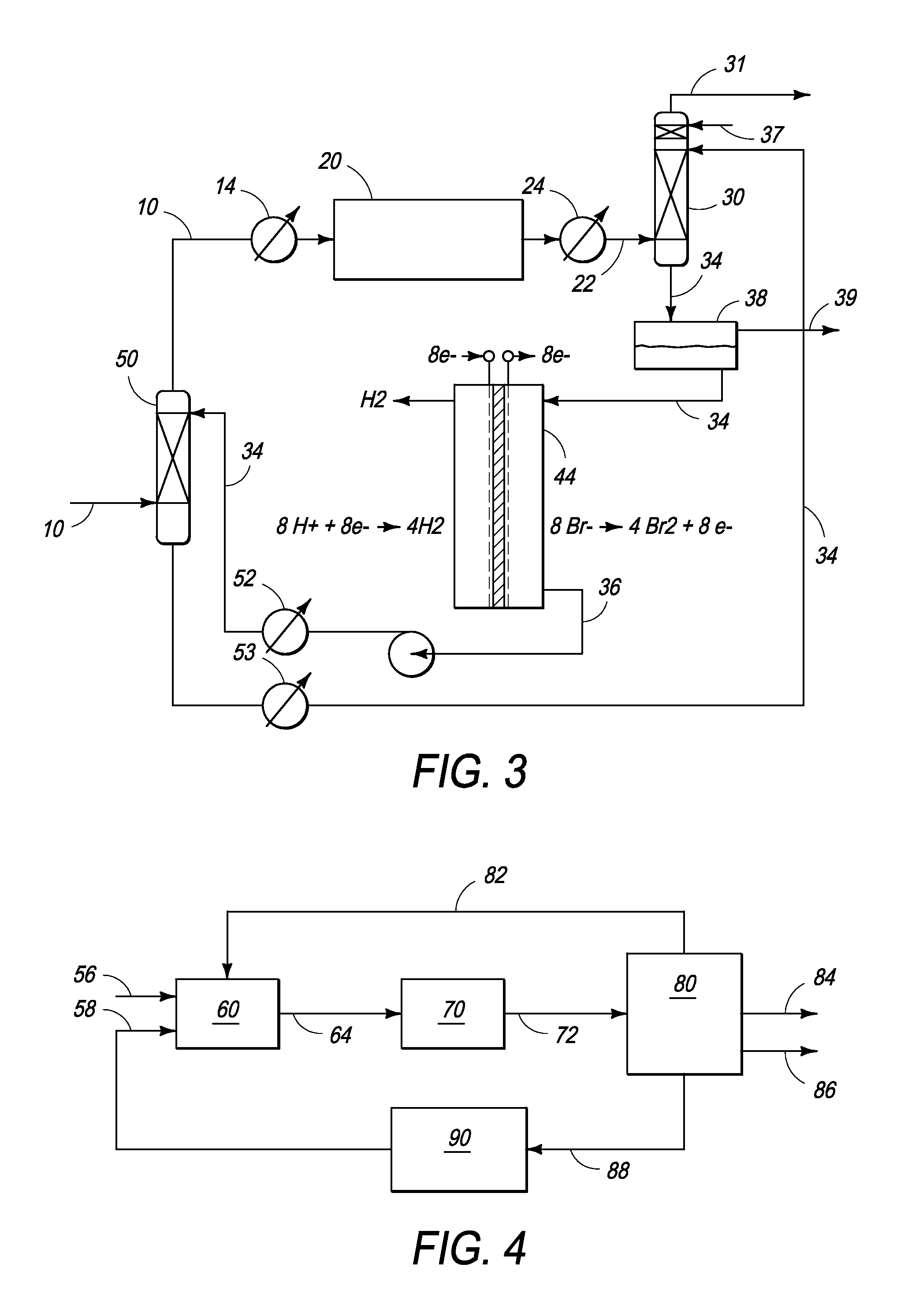
Conversion of hydrogen bromide to elemental bromine
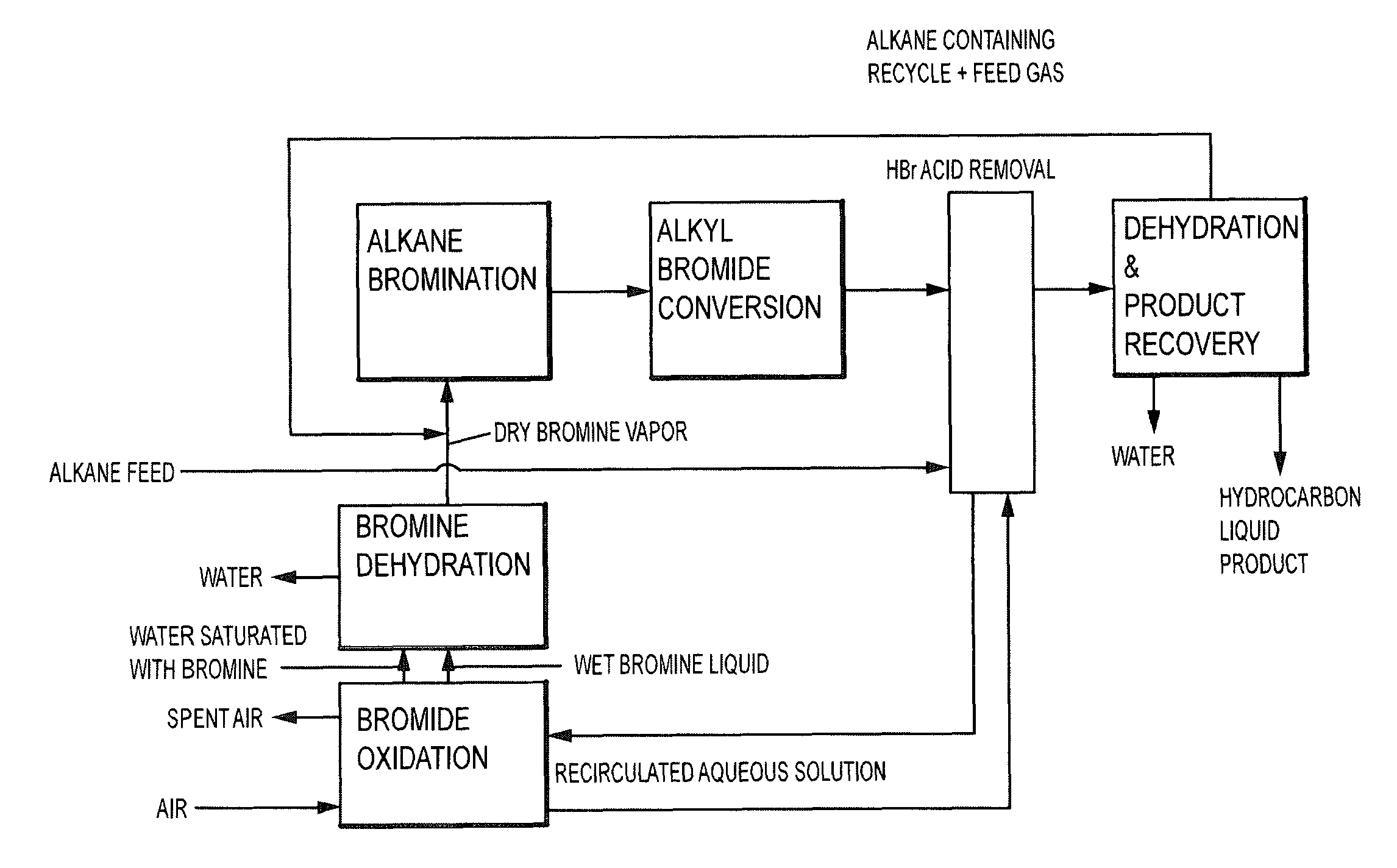
A method is provided for converting hydrogen bromide to elemental bromine. A portion of an initial hydrogen bromide-rich gas is thermally oxidized at a thermal oxidation temperature to produce a first fraction of elemental bromine and a remainder of the initial hydrogen bromide-rich gas. At least a portion of the remainder of the initial hydrogen bromide-rich gas is catalytically oxidized at a lower catalytic oxidation temperature to produce a second fraction of elemental bromine.
Owner:GTC TECHNOLOGY US LLC
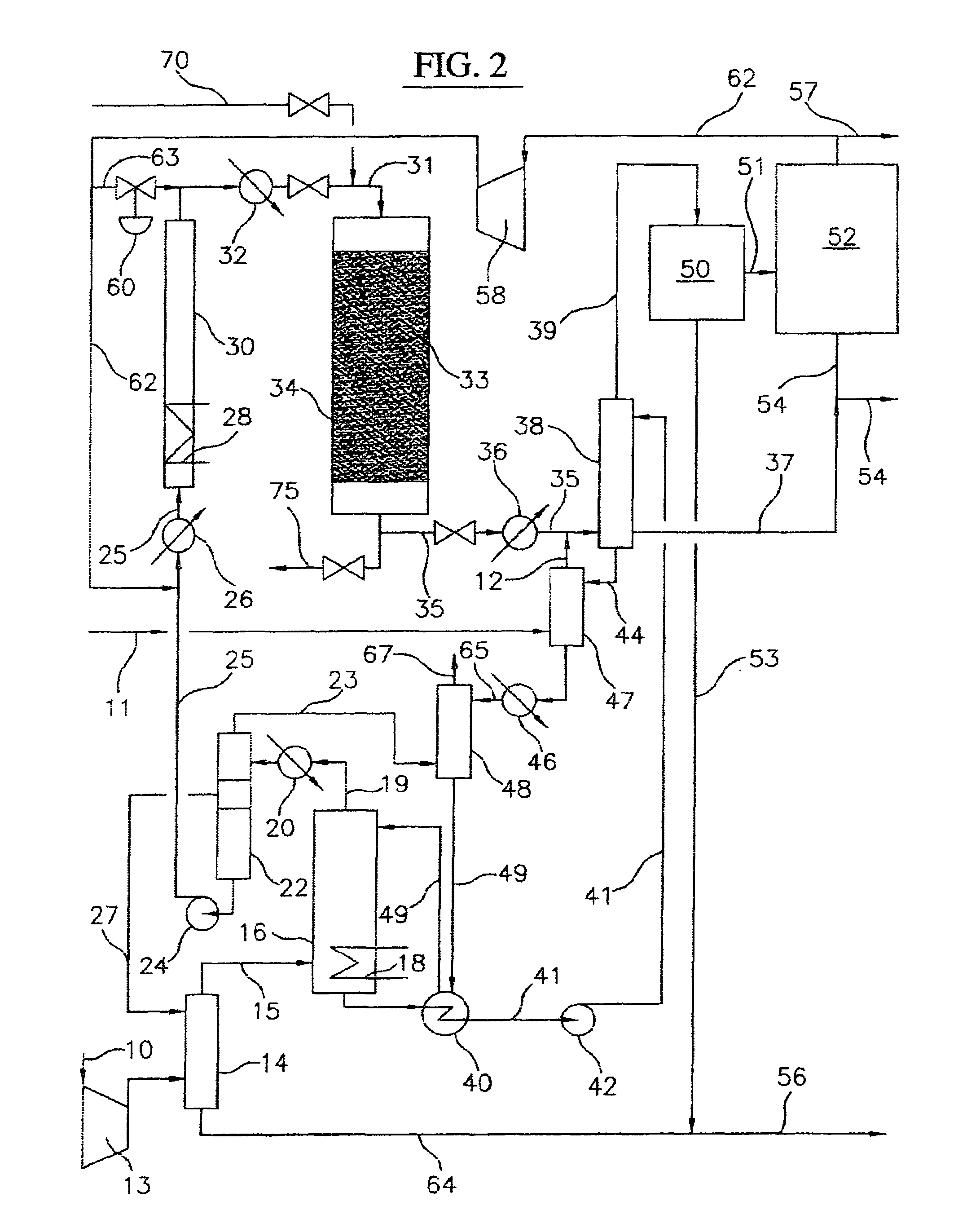
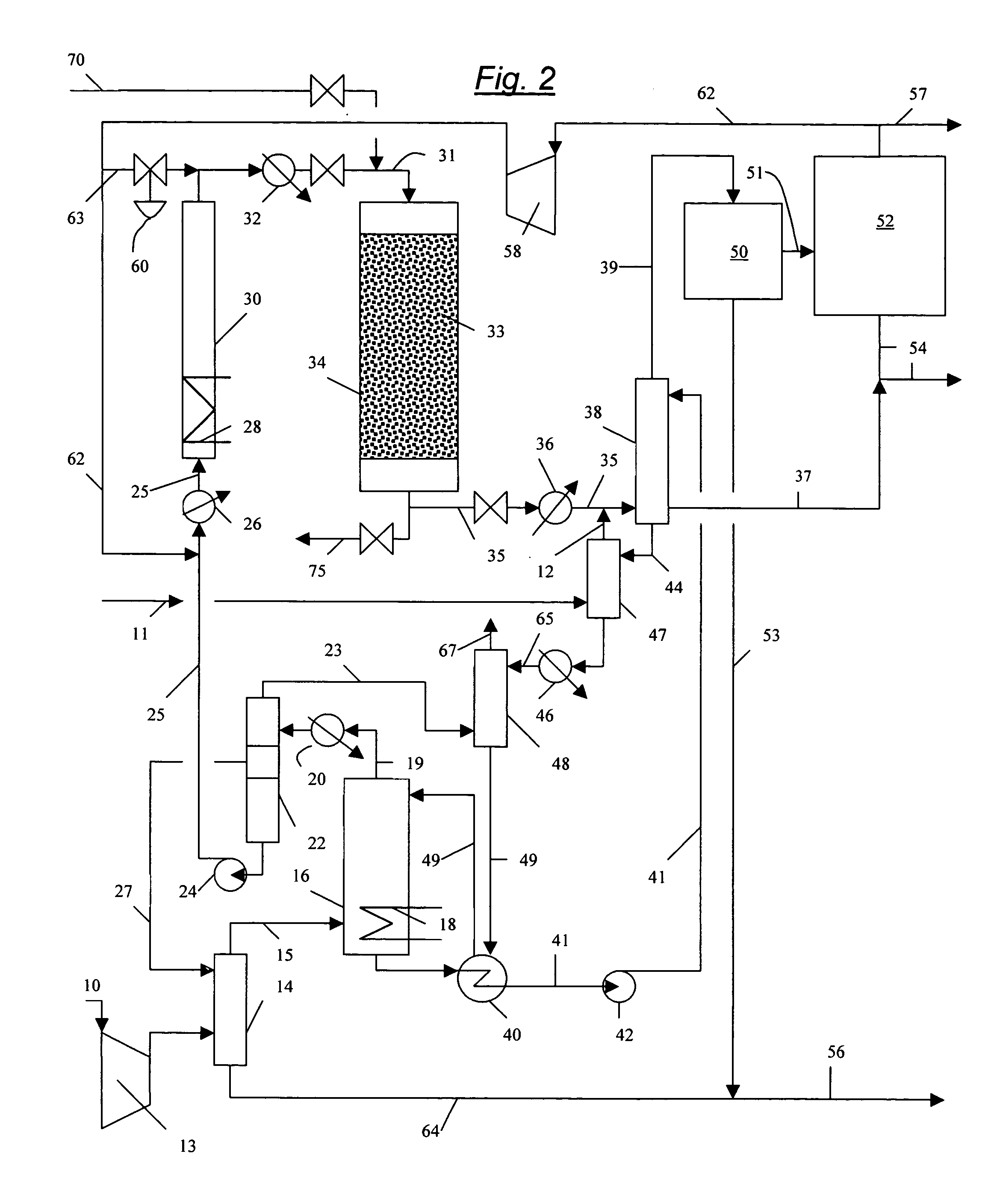
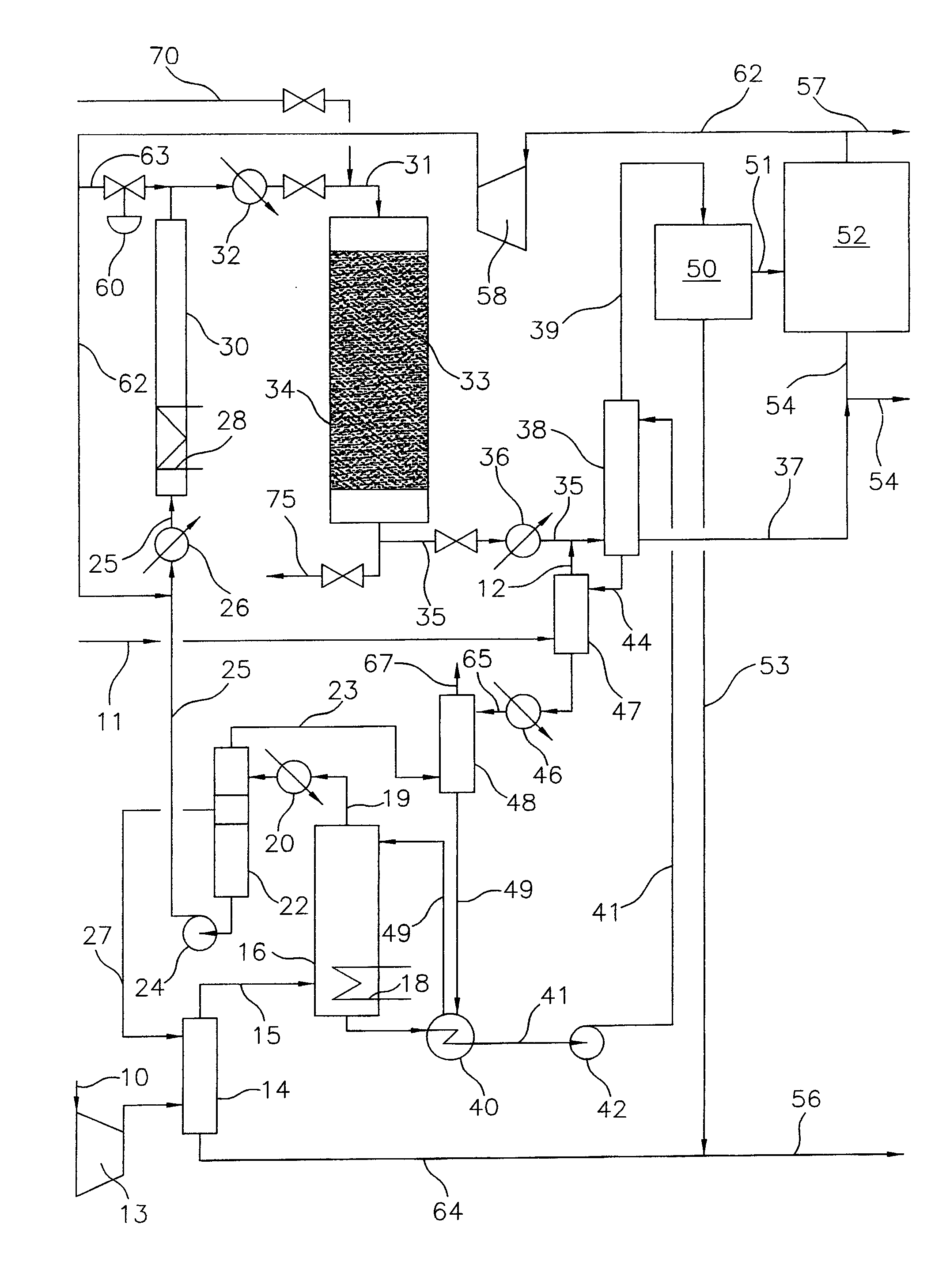
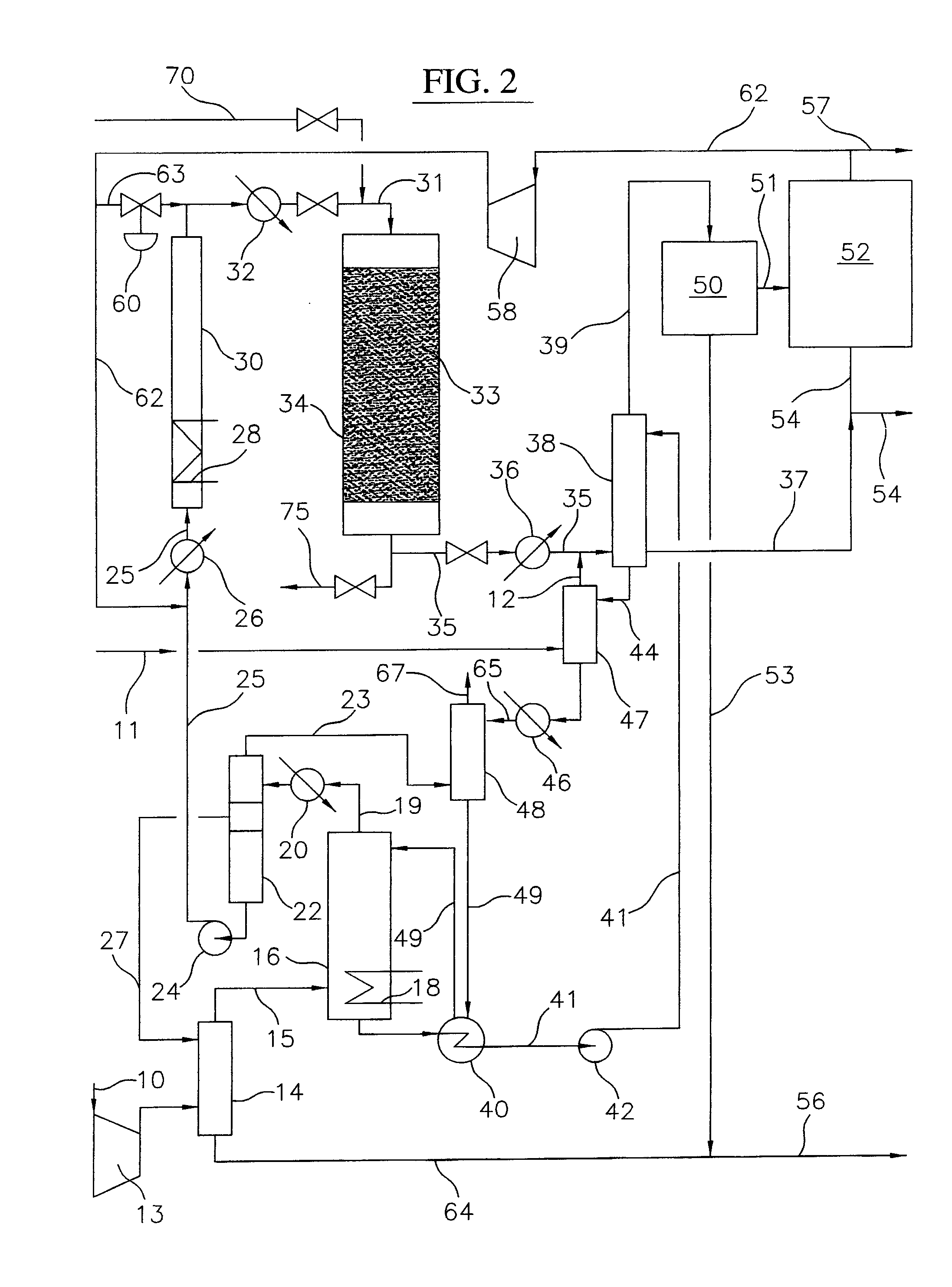
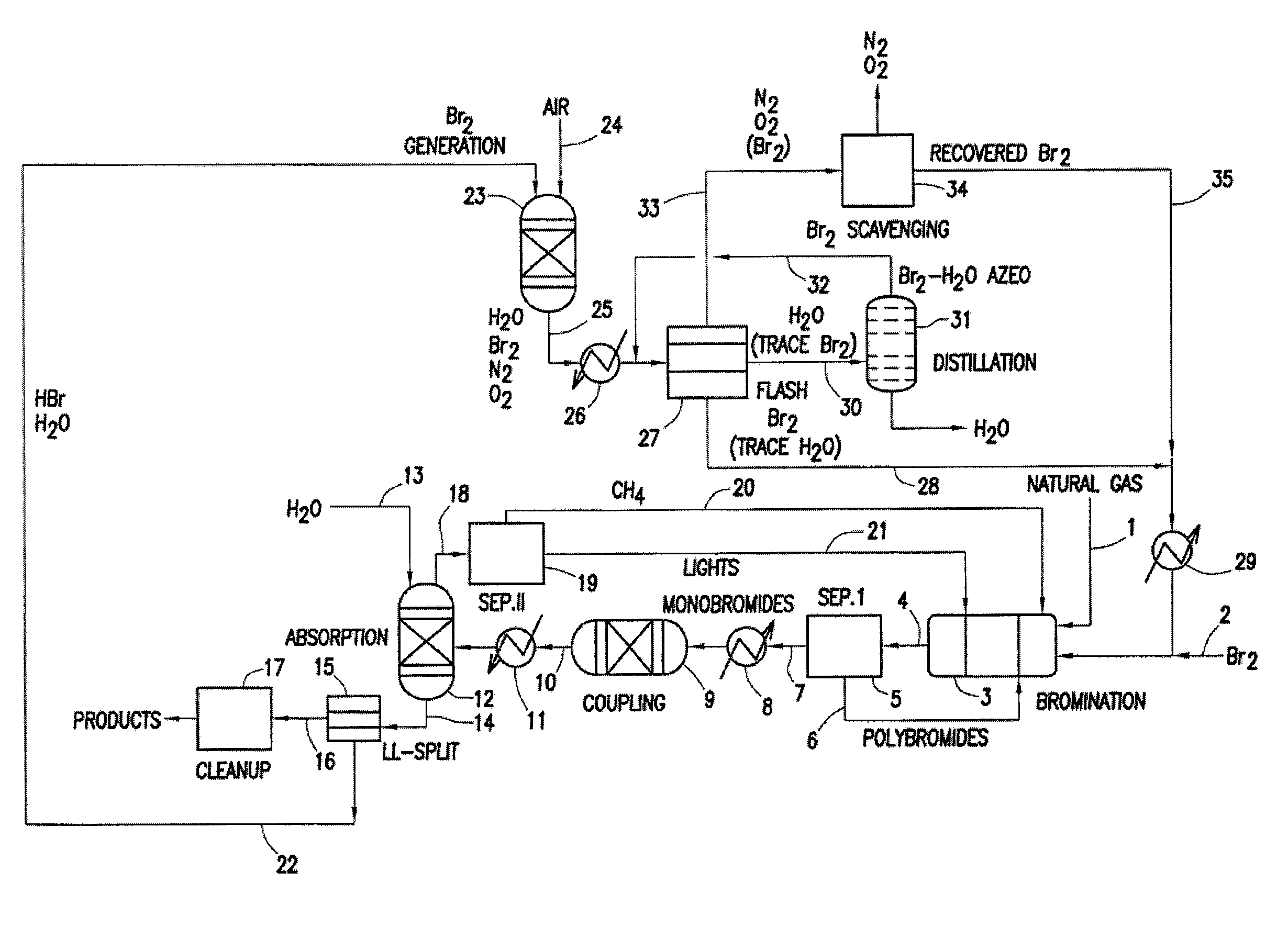
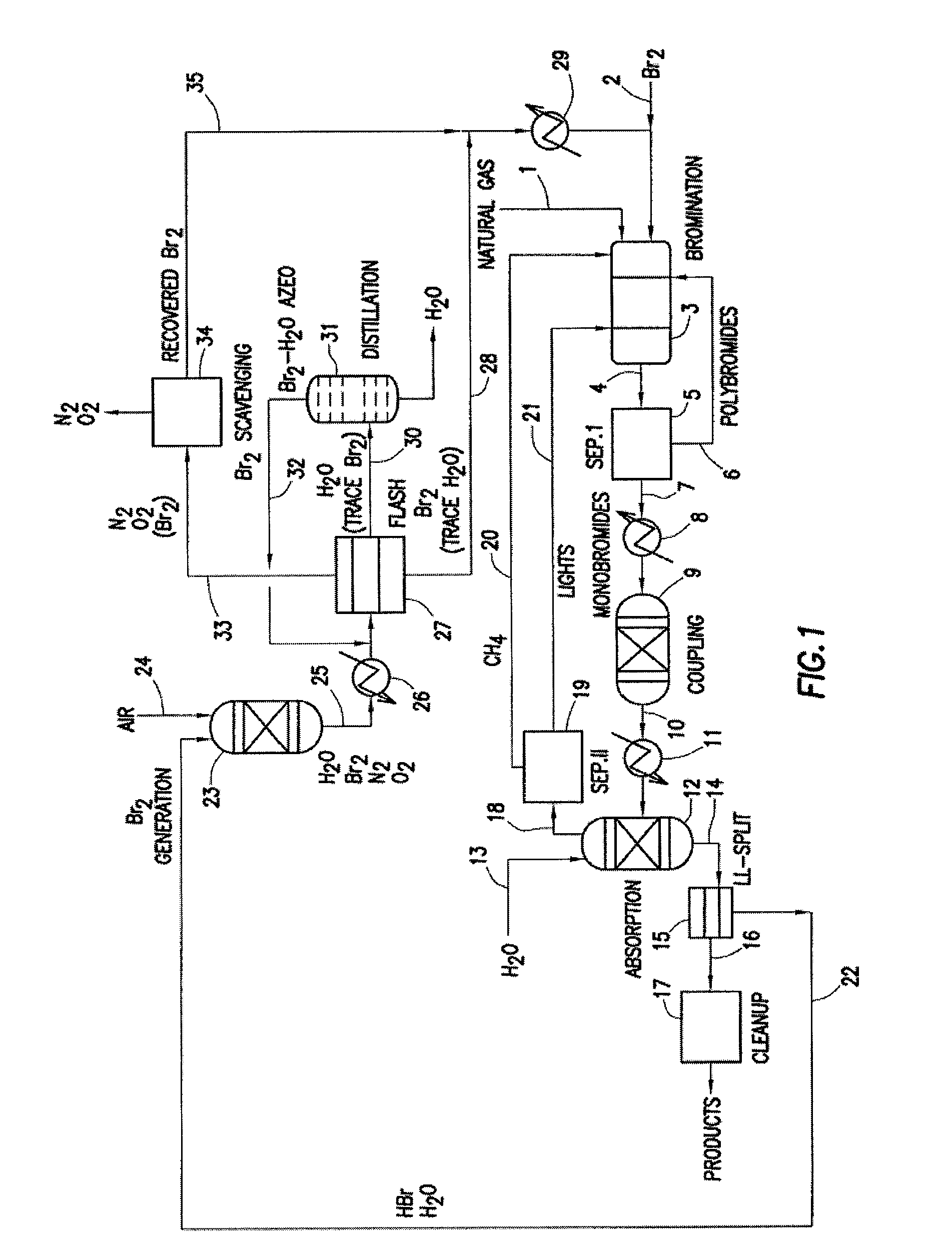
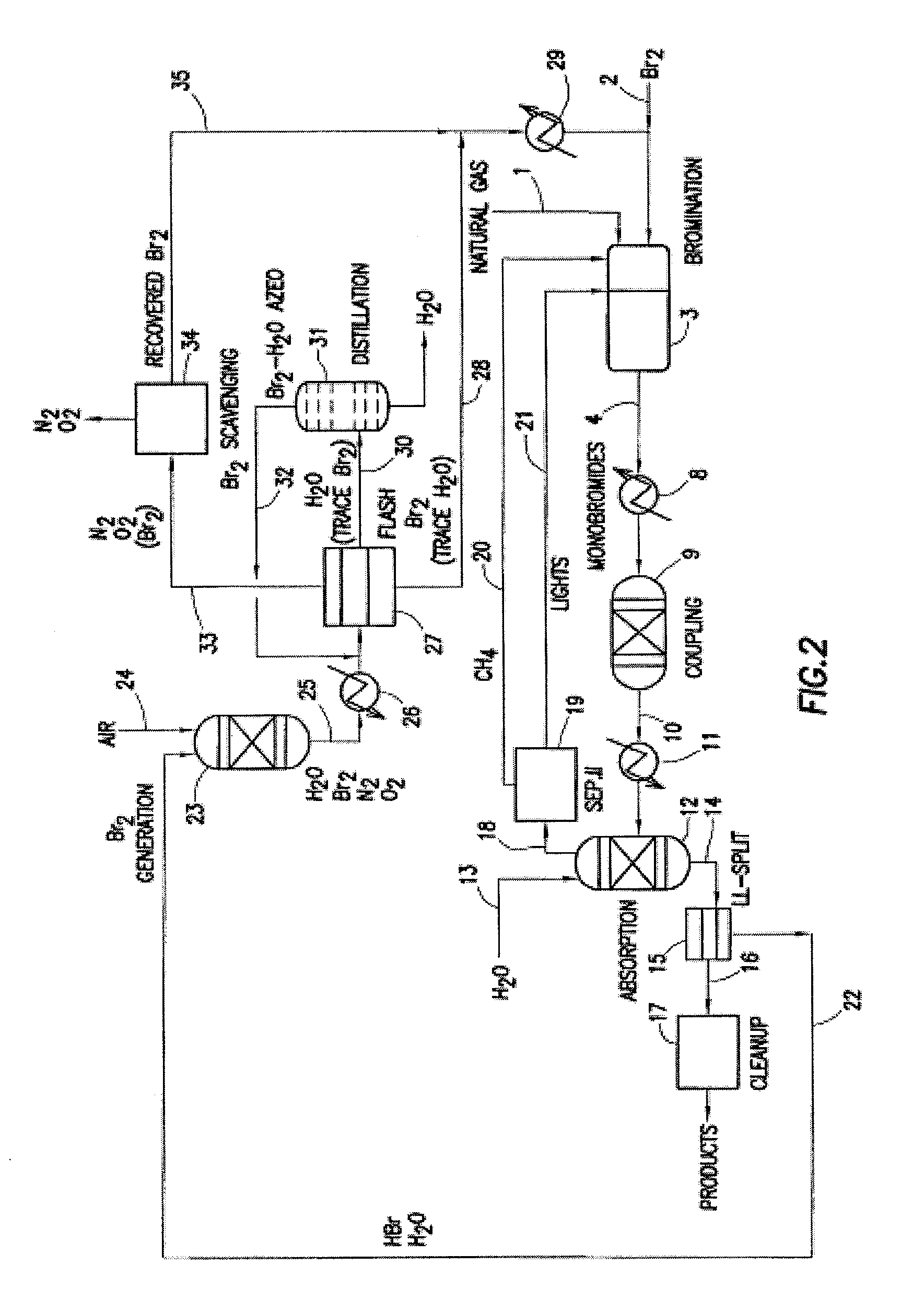
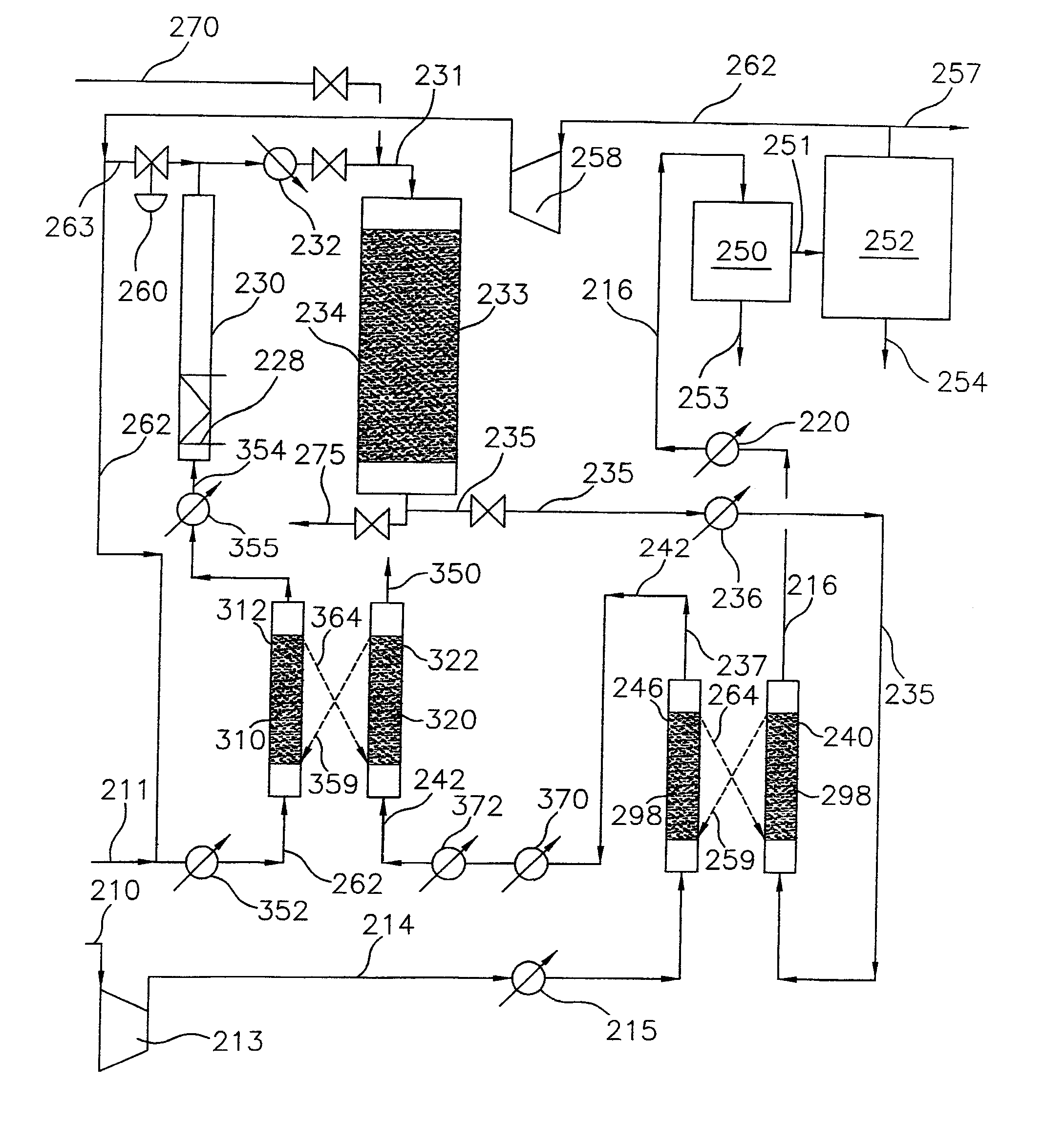
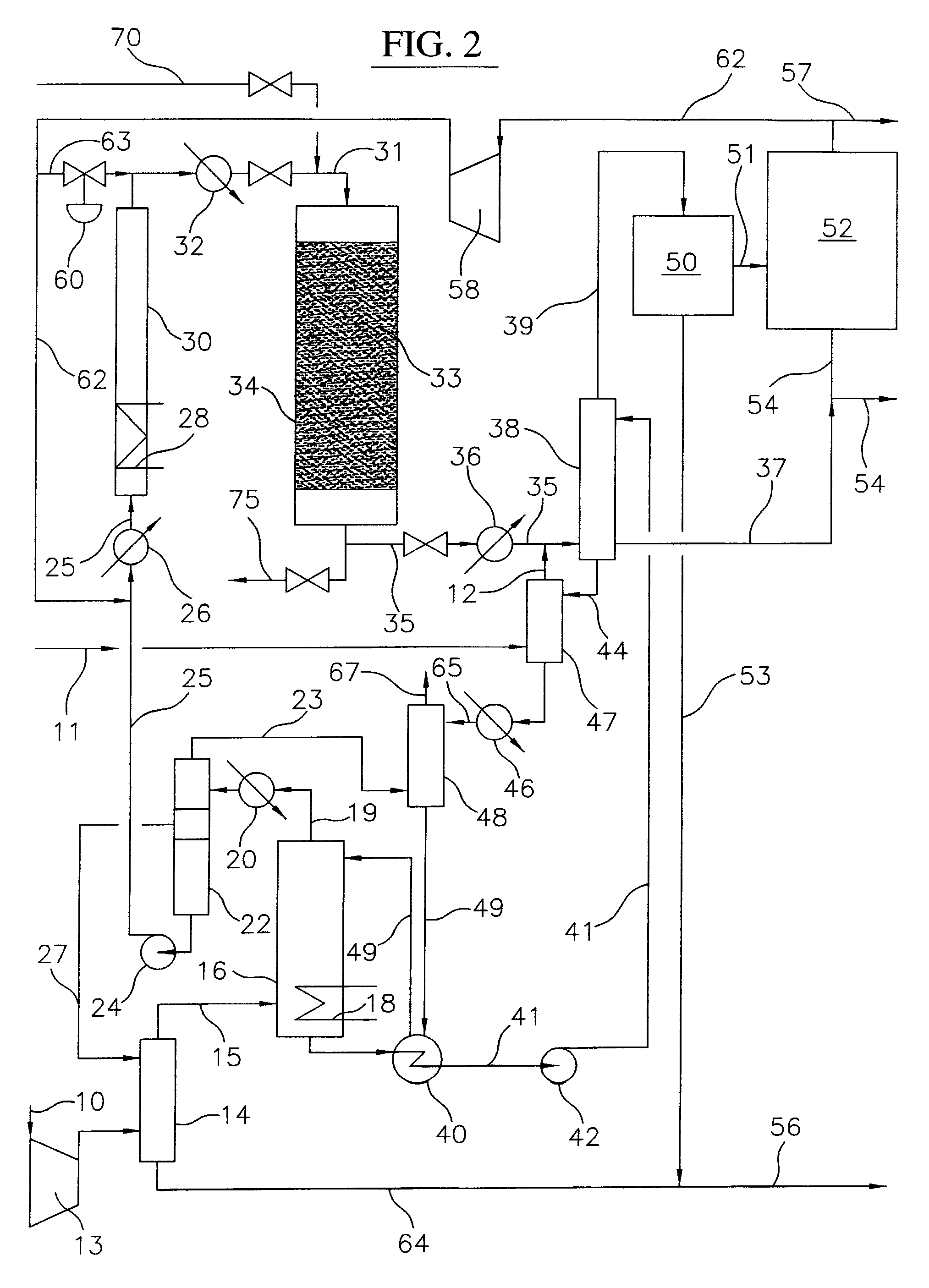
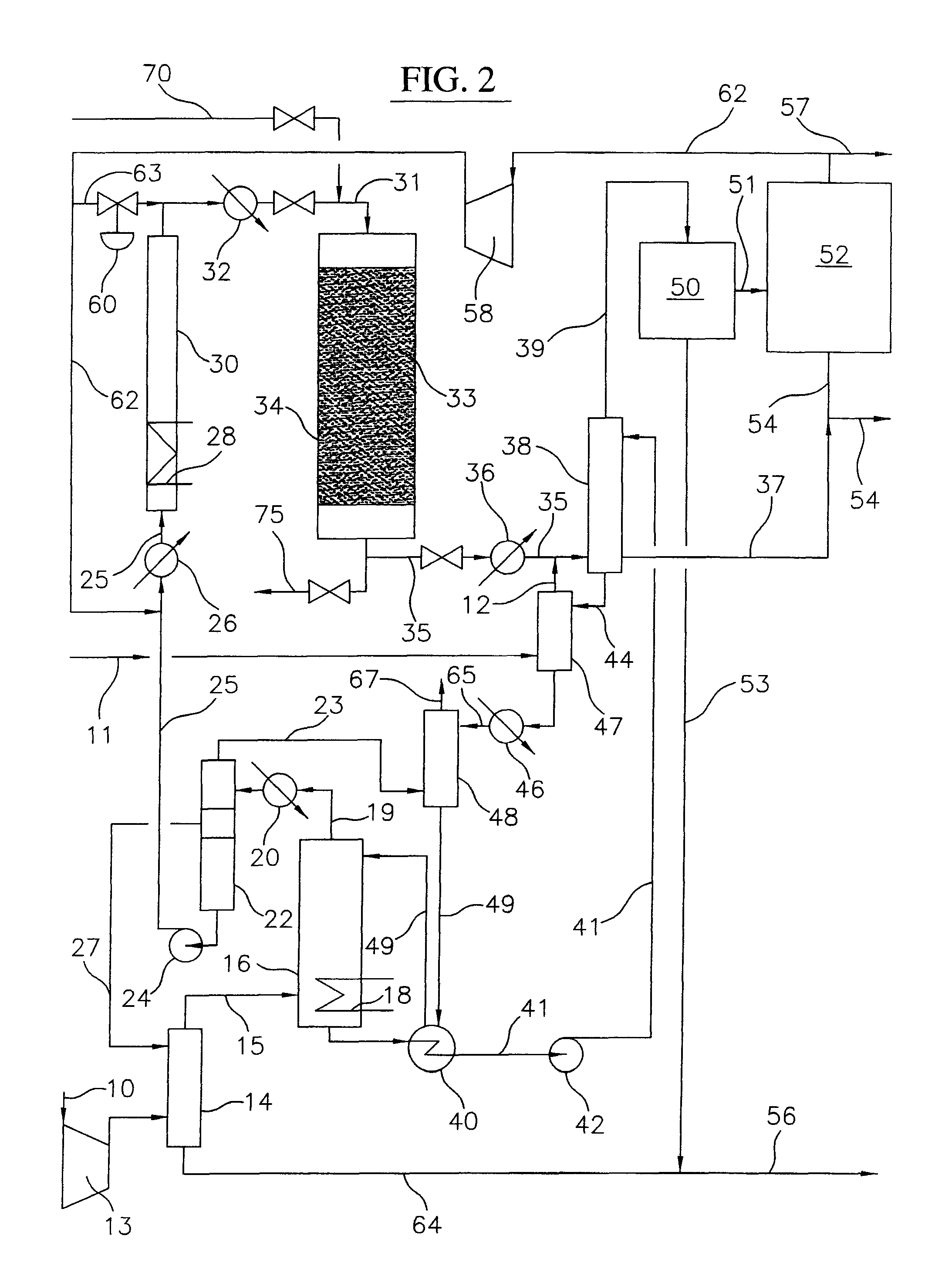
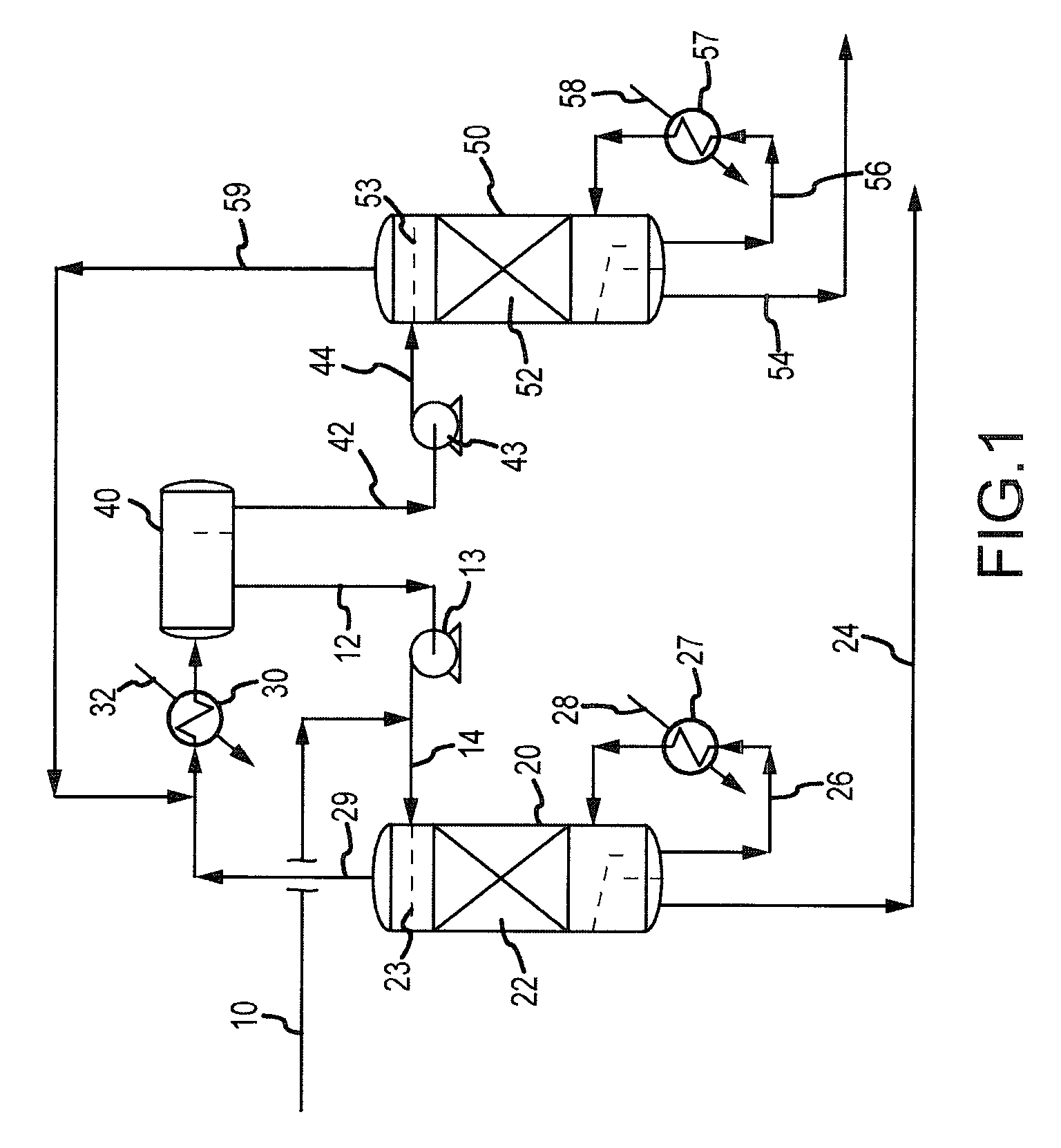
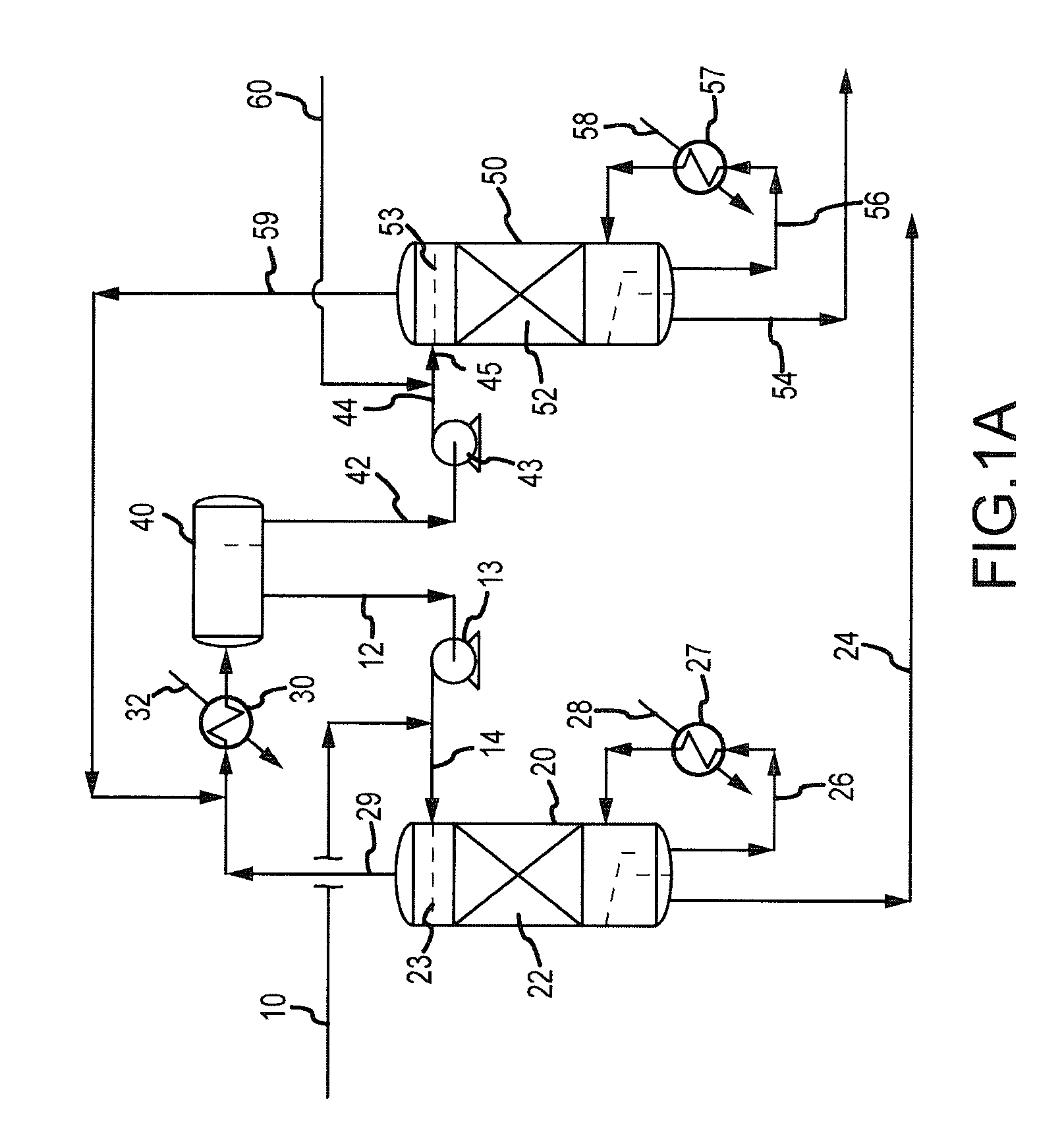
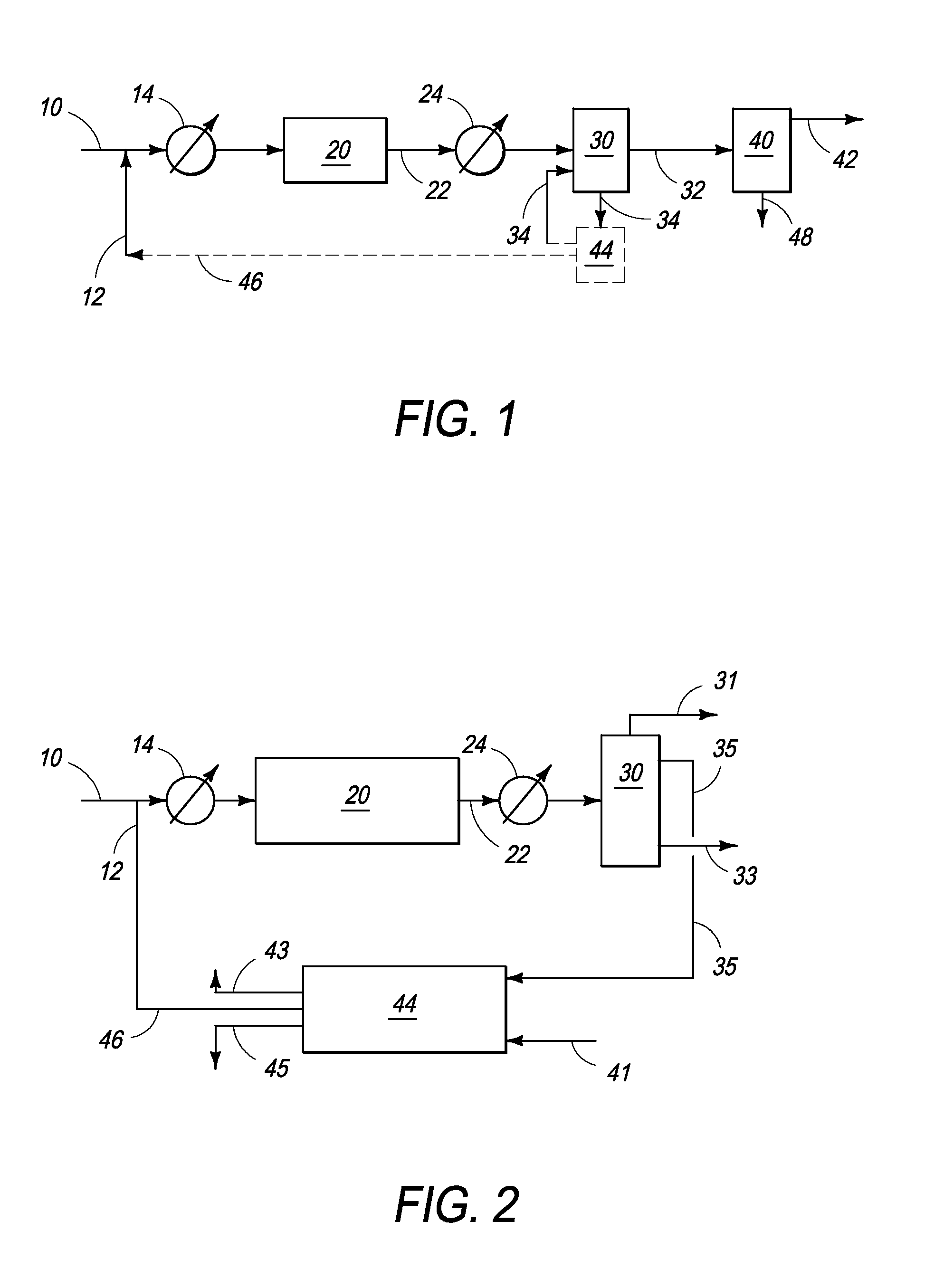
Continuous Process for Converting Natural Gas to Liquid Hydrocarbons
A method comprising: providing an alkyl halide stream; contacting at least some of the alkyl halides with a coupling catalyst to form a product stream comprising higher hydrocarbons and hydrogen halide; contacting the product stream with a solid reactant to remove at least a portion of the hydrogen halide from the product stream; and reacting the solid reactant with a source of oxygen to generate a corresponding halogen.
Owner:REACTION 35 LLC
Processes for converting gaseous alkanes to liquid hydrocarbons
A process for converting gaseous alkanes to olefins, higher molecular weight hydrocarbons or mixtures thereof wherein a gaseous feed containing alkanes is reacted with a dry bromine vapor to form alkyl bromides and hydrobromic acid vapor. The mixture of alkyl bromides and hydrobromic acid is then reacted over a suitable catalyst at a temperature sufficient to form olefins, higher molecular weight hydrocarbons or mixtures thereof and hydrobromic acid vapor. Various methods are disclosed to remove the hydrobromic acid vapor from the higher molecular weight hydrocarbons, to generate bromine from the hydrobromic acid for use in the process, and to selectively form monobrominated alkanes in the bromination step.
Owner:SULZER MANAGEMENT AG
Process for converting gaseous alkanes to liquid hydrocarbons
Embodiments disclose a process for converting gaseous alkanes to higher molecular weight hydrocarbons, olefins or mixtures thereofs wherein a gaseous feed containing alkanes may be reacted with a dry bromine vapor to form alkyl bromides and hydrobromic acid vapor. The mixture of alkyl bromides and hydrobromic acid then may be reacted over a synthetic crystalline alumino-silicate catalyst, such as a ZSM-5 or an X or Y type zeolite, at a temperature of from about 250° C. to about 500° C. so as to form hydrobromic acid vapor and higher molecular weight hydrocarbons, olefins or mixtures thereof. Various methods are disclosed to remove the hydrobromic acid vapor from the higher molecular weight hydrocarbons, olefins or mixtures thereof and to generate bromine from the hydrobromic acid for use in the process.
Owner:MARATHON GTF TECH
Process for converting gaseous alkanes to olefins and liquid hydrocarbons
ActiveUS20080200740A1High selectivityAvoid disadvantagesHydrogen bromideBromide preparationAlkaneBromine
A process for converting gaseous alkanes to olefins and higher molecular weight hydrocarbons wherein a gaseous feed containing alkanes is reacted with a dry bromine vapor to form alkyl bromides and hydrobromic acid vapor. The mixture of alkyl bromides and hydrobromic acid are then reacted over a synthetic crystalline alumino-silicate catalyst, such as an X or Y type zeolite, at a temperature of from about 250° C. to about 500° C. so as to form olefins, higher molecular weight hydrocarbons and hydrobromic acid vapor. Various methods are disclosed to remove the hydrobromic acid vapor from the higher molecular weight hydrocarbons and to generate bromine from the hydrobromic acid for use in the process.
Owner:SULZER MANAGEMENT AG
Processes for converting gaseous alkanes to liquid hydrocarbons
A process for converting gaseous alkanes to olefins, higher molecular weight hydrocarbons or mixtures thereof wherein a gaseous feed containing alkanes is reacted with a dry bromine vapor to form alkyl bromides and hydrobromic acid vapor. The mixture of alkyl bromides and hydrobromic acid is then reacted over a suitable catalyst at a temperature sufficient to form olefins, higher molecular weight hydrocarbons or mixtures thereof and hydrobromic acid vapor. Various methods are disclosed to remove the hydrobromic acid vapor from the higher molecular weight hydrocarbons, to generate bromine from the hydrobromic acid for use in the process, and to selectively form monobrominated alkanes in the bromination step.
Owner:SULZER MANAGEMENT AG
Stable oxidizing bromine formulations, method of manufacture and uses thereof for biofouling control
Stable biocide formulations containing oxidizing bromine are provided for biofouling control in industrial water systems. The formulations contain at least one stable oxidizing bromine compound that is prepared from at least one oxidizing chemical reagent, at least one bromine source and at least one bromine or halogen stabilizer. The resulting products are a mixture of stable oxidizing bromine compounds that can be used as a biocide in an industrial water system.
Owner:NALCO CHEM CO
Process for converting gaseous alkanes to liquid hydrocarbons
Embodiments disclose a process for converting gaseous alkanes to higher molecular weight hydrocarbons, olefins or mixtures thereofs wherein a gaseous feed containing alkanes may be reacted with a dry bromine vapor to form alkyl bromides and hydrobromic acid vapor. The mixture of alkyl bromides and hydrobromic acid then may be reacted over a synthetic crystalline alumino-silicate catalyst, such as a ZSM-5 or an X or Y type zeolite, at a temperature of from about 250° C. to about 500° C. so as to form hydrobromic acid vapor and higher molecular weight hydrocarbons, olefins or mixtures thereof. Various methods are disclosed to remove the hydrobromic acid vapor from the higher molecular weight hydrocarbons, olefins or mixtures thereof and to generate bromine from the hydrobromic acid for use in the process.
Owner:MARATHON GTF TECH
Processes for oxidation of bromides to produce bromine and catalysts useful therein
Owner:ALBEMARLE CORP
Process for converting gaseous alkanes to olefins and liquid hydrocarbons
ActiveUS8008535B2High selectivityAvoid disadvantagesBromide preparationHydrogen bromideAlkaneAlkyl bromide
A process for converting gaseous alkanes to olefins and higher molecular weight hydrocarbons wherein a gaseous feed containing alkanes is reacted with a dry bromine vapor to form alkyl bromides and hydrobromic acid vapor. The mixture of alkyl bromides and hydrobromic acid are then reacted over a synthetic crystalline alumino-silicate catalyst, such as an X or Y type zeolite, at a temperature of from about 250° C. to about 500° C. so as to form olefins, higher molecular weight hydrocarbons and hydrobromic acid vapor. Various methods are disclosed to remove the hydrobromic acid vapor from the olefins and higher molecular weight hydrocarbons and to generate bromine from the hydrobromic acid for use in the process.
Owner:SULZER MANAGEMENT AG
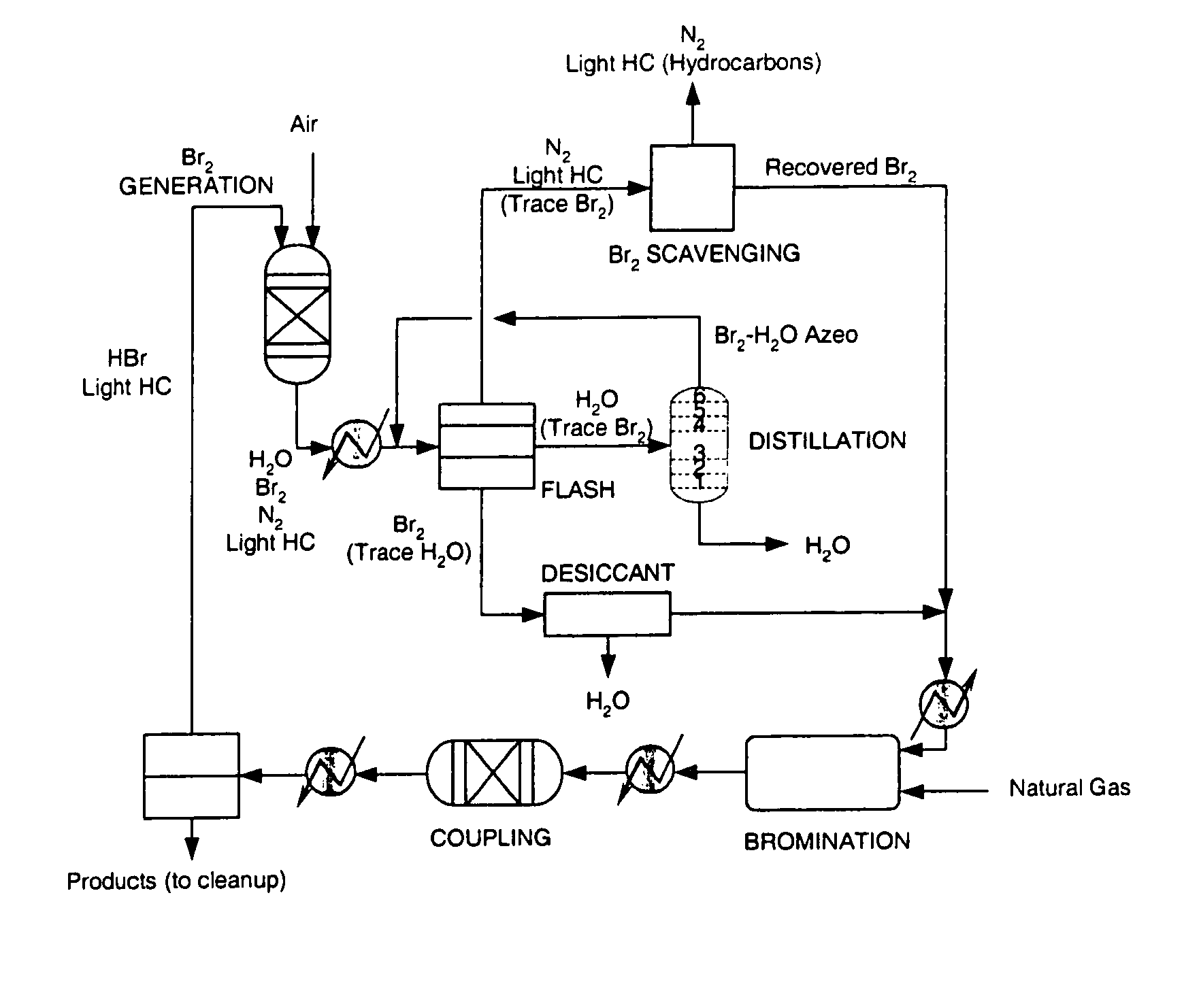
Separation of light gases from halogens
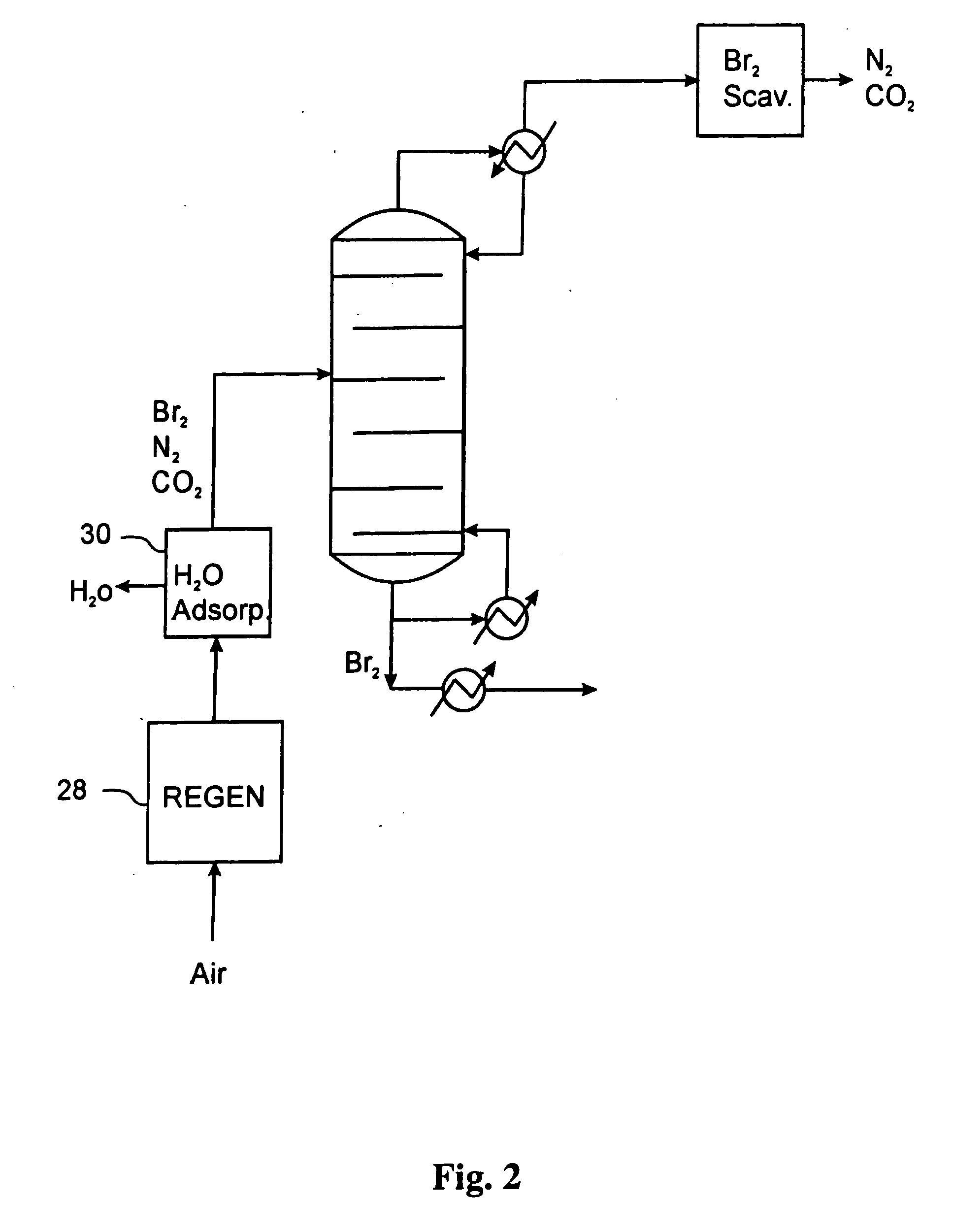
A process is provided for separating one or more light gases from bromine or chlorine using one or more physical separations and contact with a chemical scrubber to recover additional halogen. In one aspect, the process comprises (a) providing a feed of halogen containing one or more light gases to a distillation column or flash vaporizer; (b) operating the distillation column or flash vaporizer to separate the feed into (i) a first liquid containing a major amount of halogen and no more than a minor amount of light gas(es), and (ii) a first vapor containing a major amount of light gas(es) and no more than a minor amount of halogen; and (c) providing the vapor to a chemical scrubber to recover halogen from the vapor.
Owner:REACTION 35 LLC
Separation of light gases from halogens
A process is provided for separating one or more light gases from bromine or chlorine using one or more physical separations and contact with a chemical scrubber to recover additional halogen. In one aspect, the process comprises (a) providing a feed of halogen containing one or more light gases to a distillation column or flash vaporizer; (b) operating the distillation column or flash vaporizer to separate the feed into (i) a first liquid containing a major amount of halogen and no more than a minor amount of light gas(es), and (ii) a first vapor containing a major amount of light gas(es) and no more than a minor amount of halogen; and (c) providing the vapor to a chemical scrubber to recover halogen from the vapor.
Owner:REACTION 35 LLC
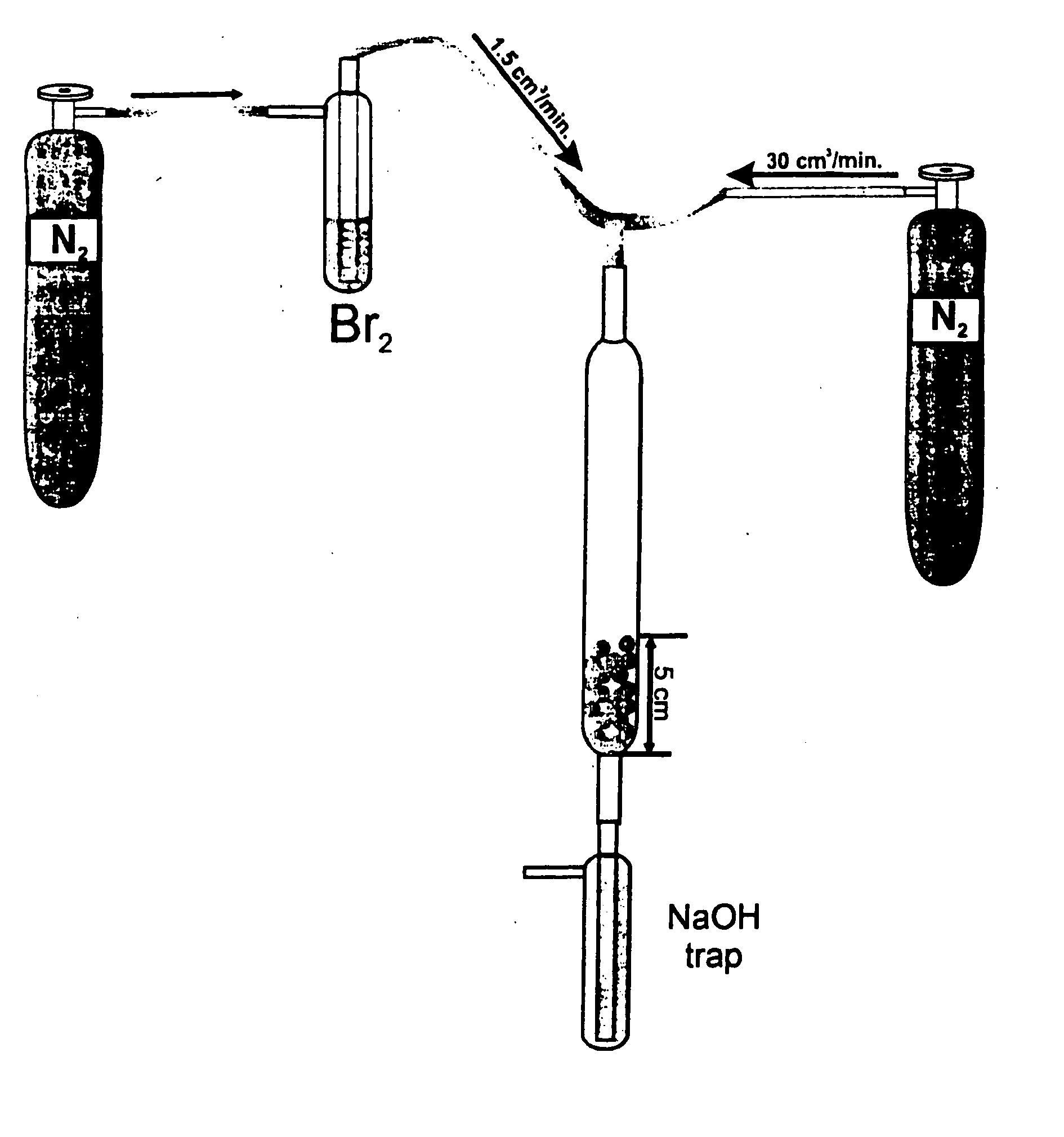
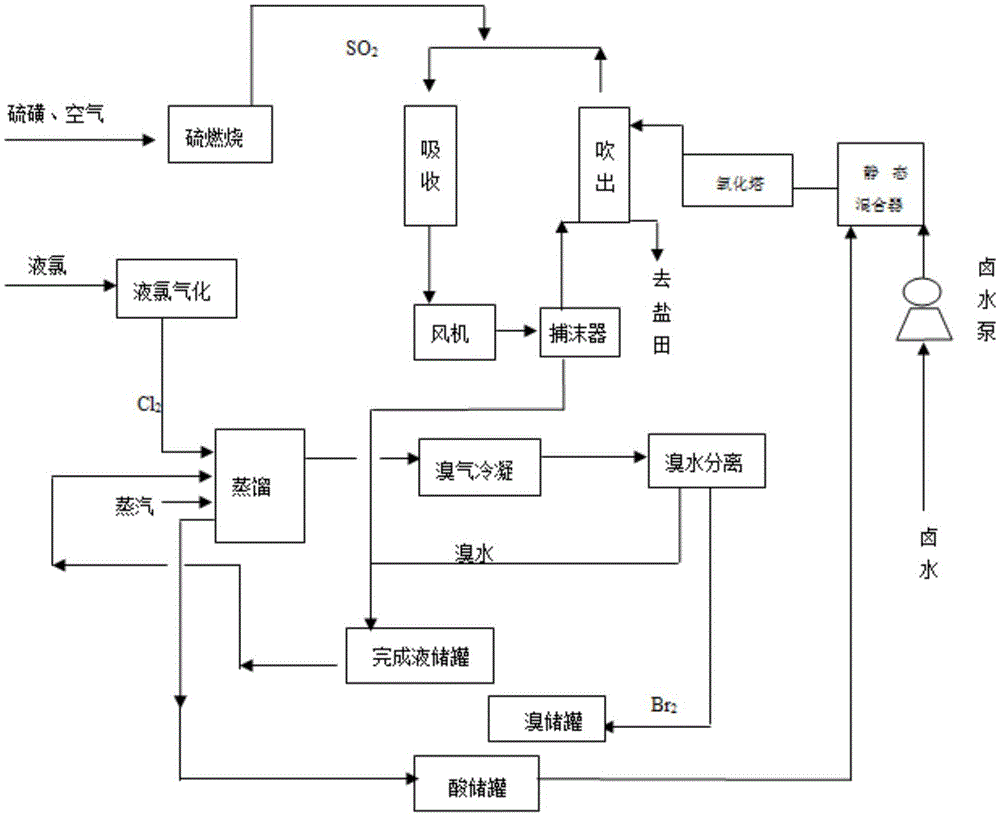
Method for extracting bromine from brine
The invention discloses a method for extracting bromine from brine. The method comprises the following steps: (1) introducing chlorine gas into acidified brine and reacting to prepare oxidized brine; (2) downwards spraying the oxidized brine from the top of a stripping tower, simultaneously blowing air up through a fan from the lower part of the stripping tower so that the air comes into full contact with the oxidized brine to resolve free bromine and the free bromine is diffused into the air, and stripping the bromine-containing air from the upper part of the stripping tower; (3) feeding the bromine-containing air into an absorption tower, spraying by a spraying solution, then forming an absorption solution containing hydrobromic acid and enabling the absorption solution to flow out of the bottom of the absorption tower; and (4) feeding the absorption solution into a distillation tower, introducing the chlorine gas to react with hydrobromic acid in the absorption solution to generate hydrogen chloride and the free bromine, wherein the hydrogen chloride generates hydrochloric acid when meeting water and hydrochloric acid is discharged from the bottom of the distillation tower, the free bromine is evaporated to form bromine vapor, and the bromine vapor is cooled to become a final bromine product. The method has the advantages of simplicity and easiness in operation of process steps and high extraction rate of bromine.
Owner:新疆罗能化工科技有限公司
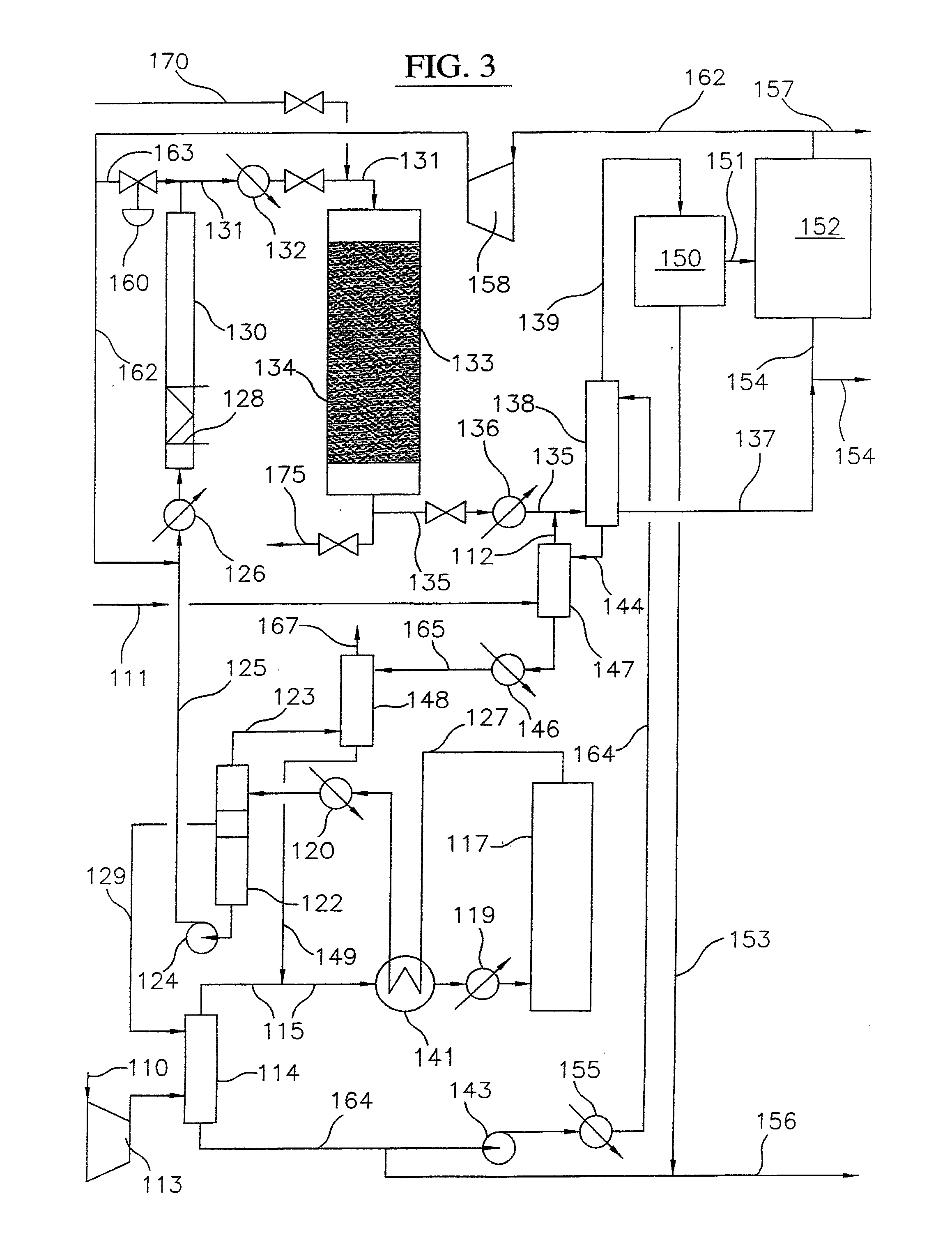
Processes for converting gaseous alkanes to liquid hydrocarbons
A process for converting gaseous alkanes to olefins, higher molecular weight hydrocarbons or mixtures thereof wherein a gaseous feed containing alkanes is thermally reacted with a dry bromine vapor to form alkyl bromides and hydrogen bromide. Poly-brominated alkanes present in the alkyl bromides are further reacted with methane over a suitable catalyst to form mono-brominated species. The mixture of alkyl bromides and hydrogen bromide is then reacted over a suitable catalyst at a temperature sufficient to form olefins, higher molecular weight hydrocarbons or mixtures thereof and hydrogen bromide. Various methods are disclosed to remove the hydrogen bromide from the higher molecular weight hydrocarbons, to generate bromine from the hydrogen bromide for use in the process, and to selectively form mono-brominated alkanes in the bromination step.
Owner:SULZER MANAGEMENT AG
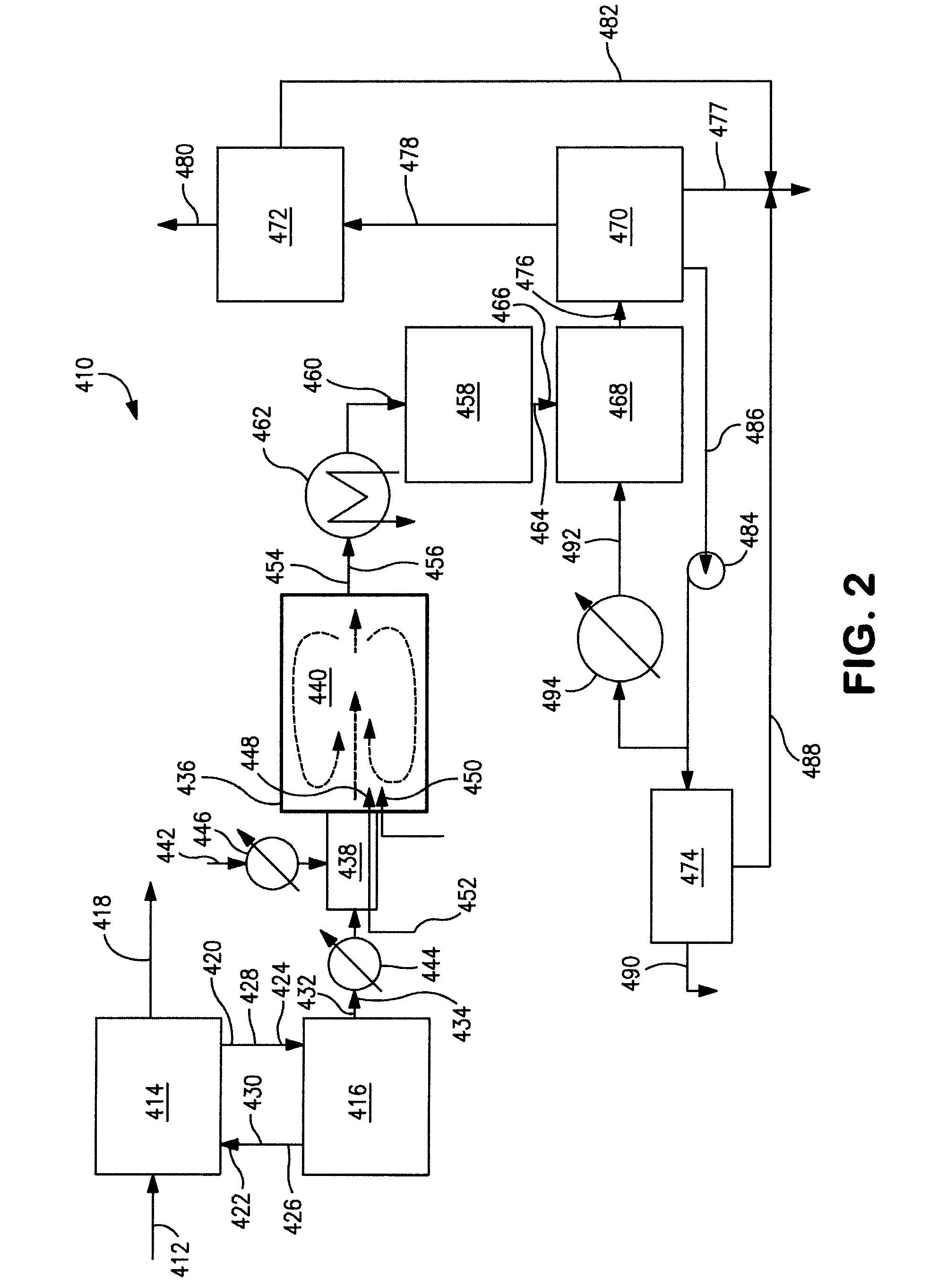
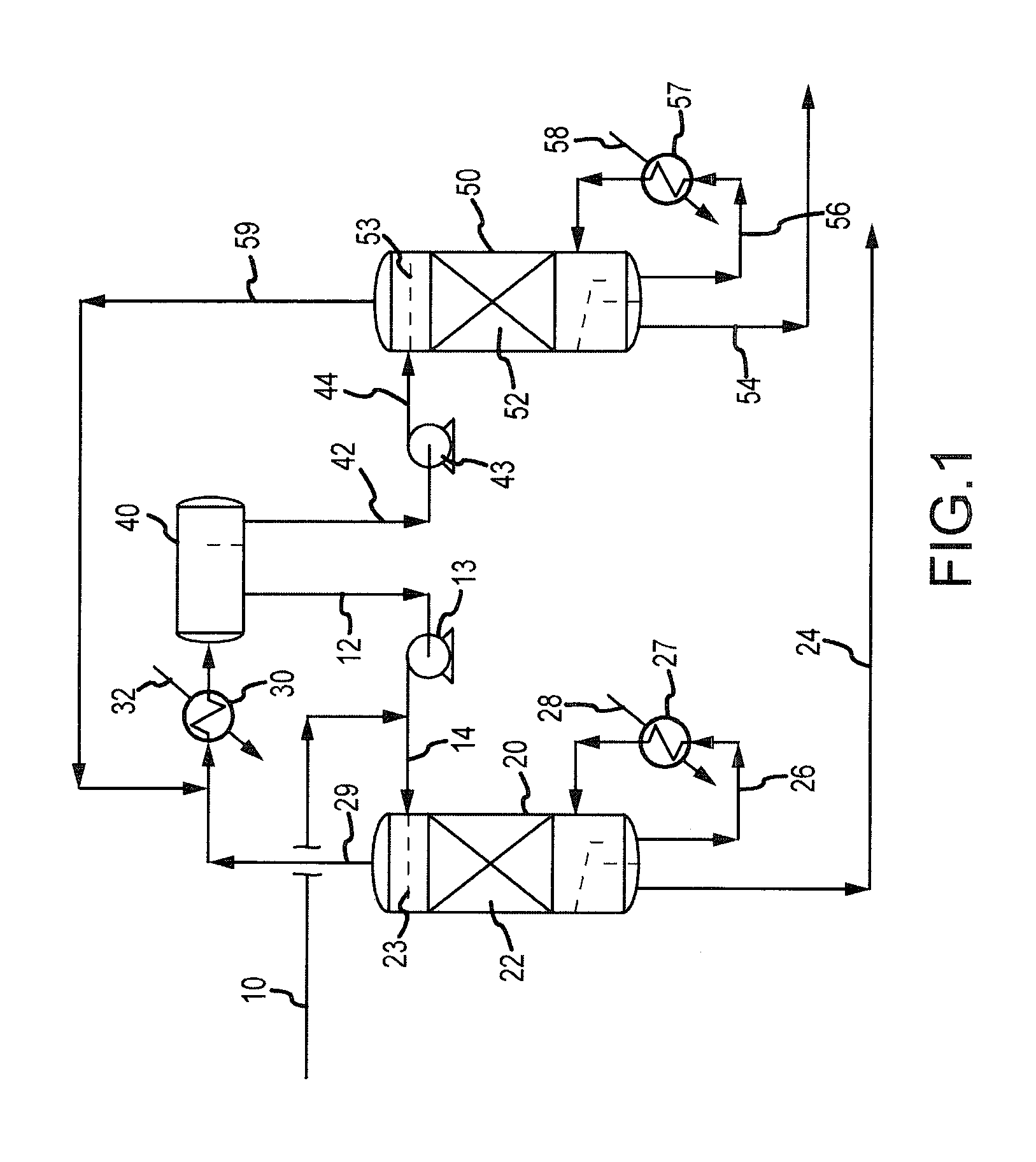
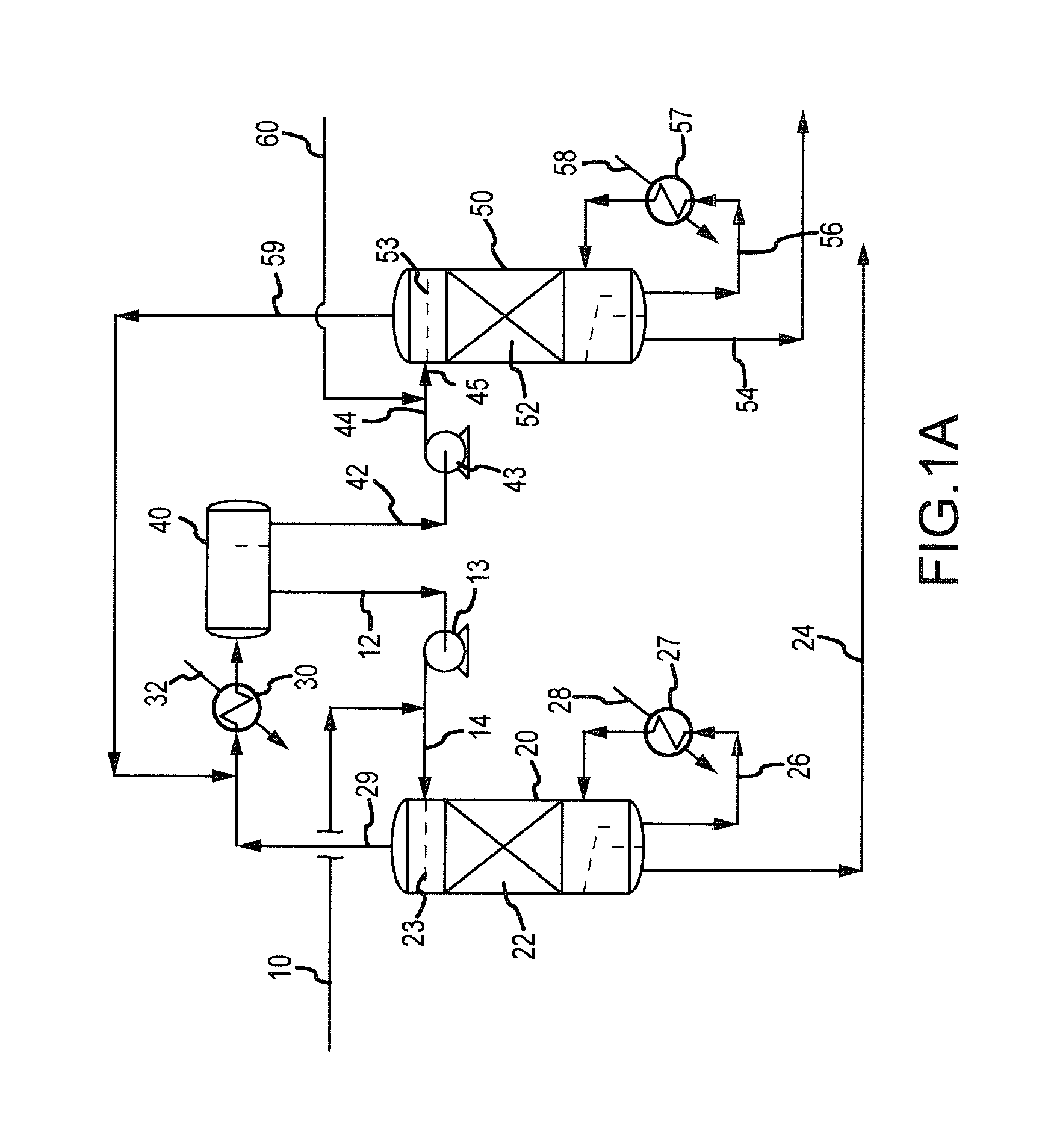
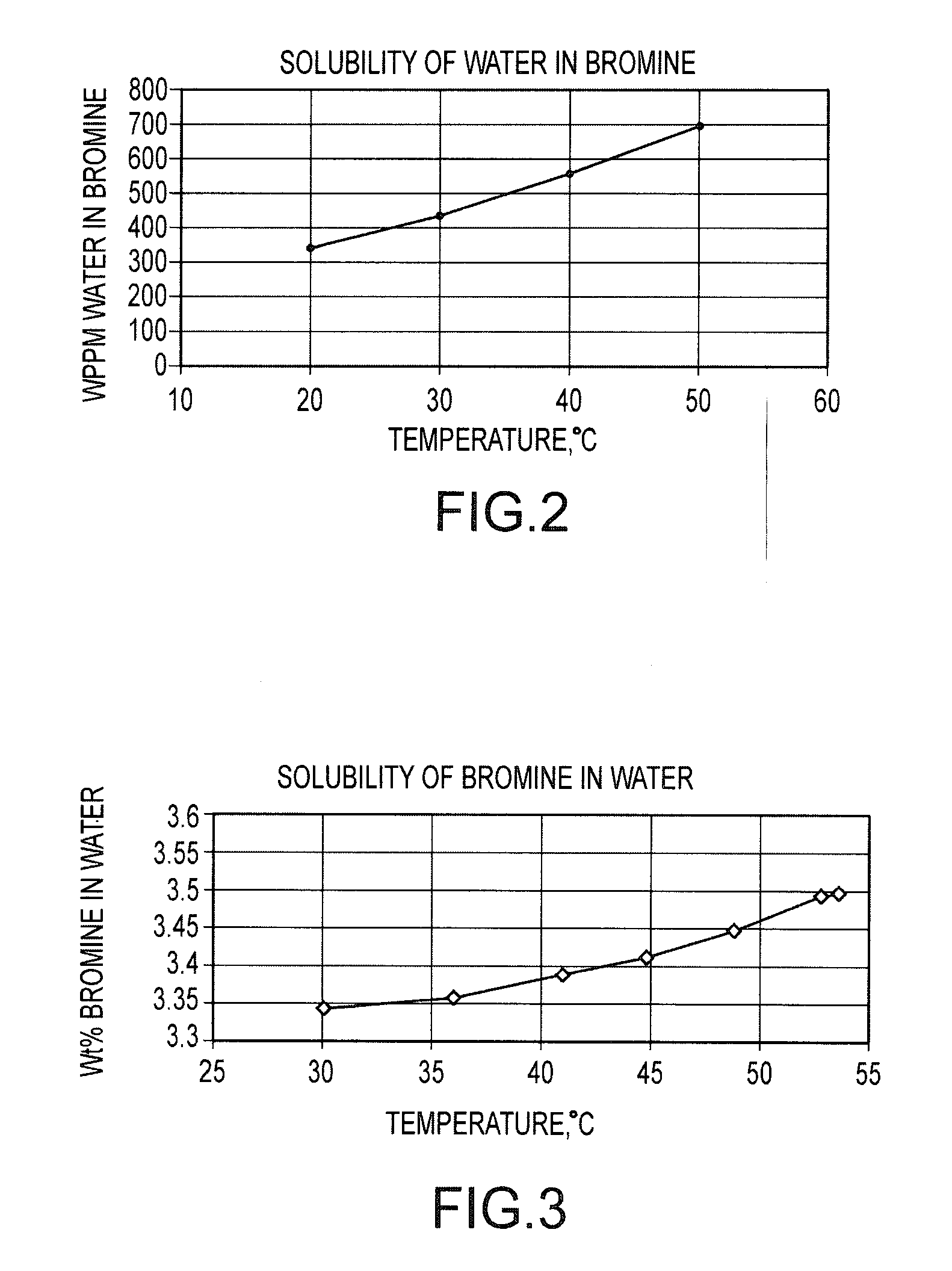
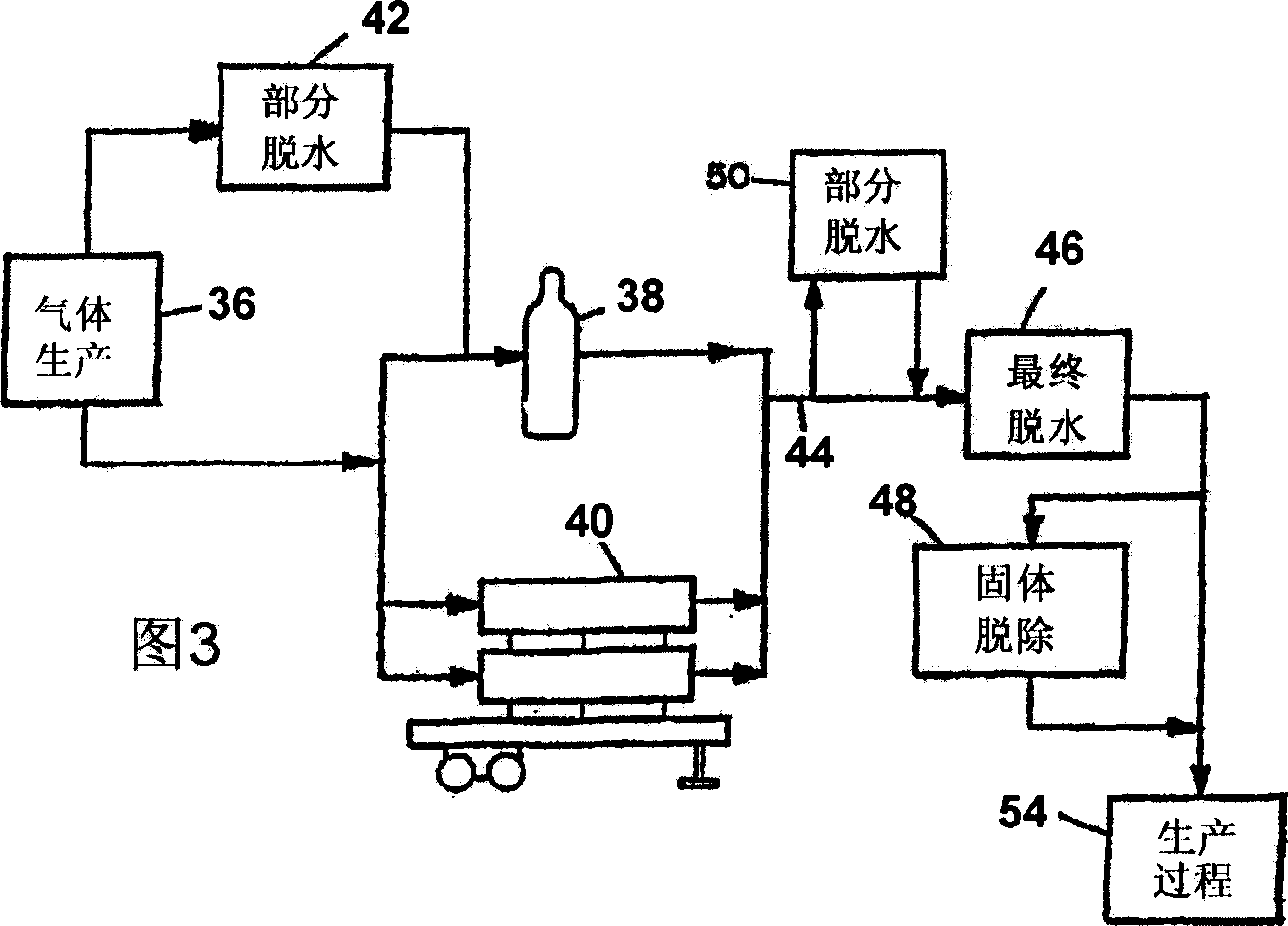
Processes and systems for drying liquid bromine
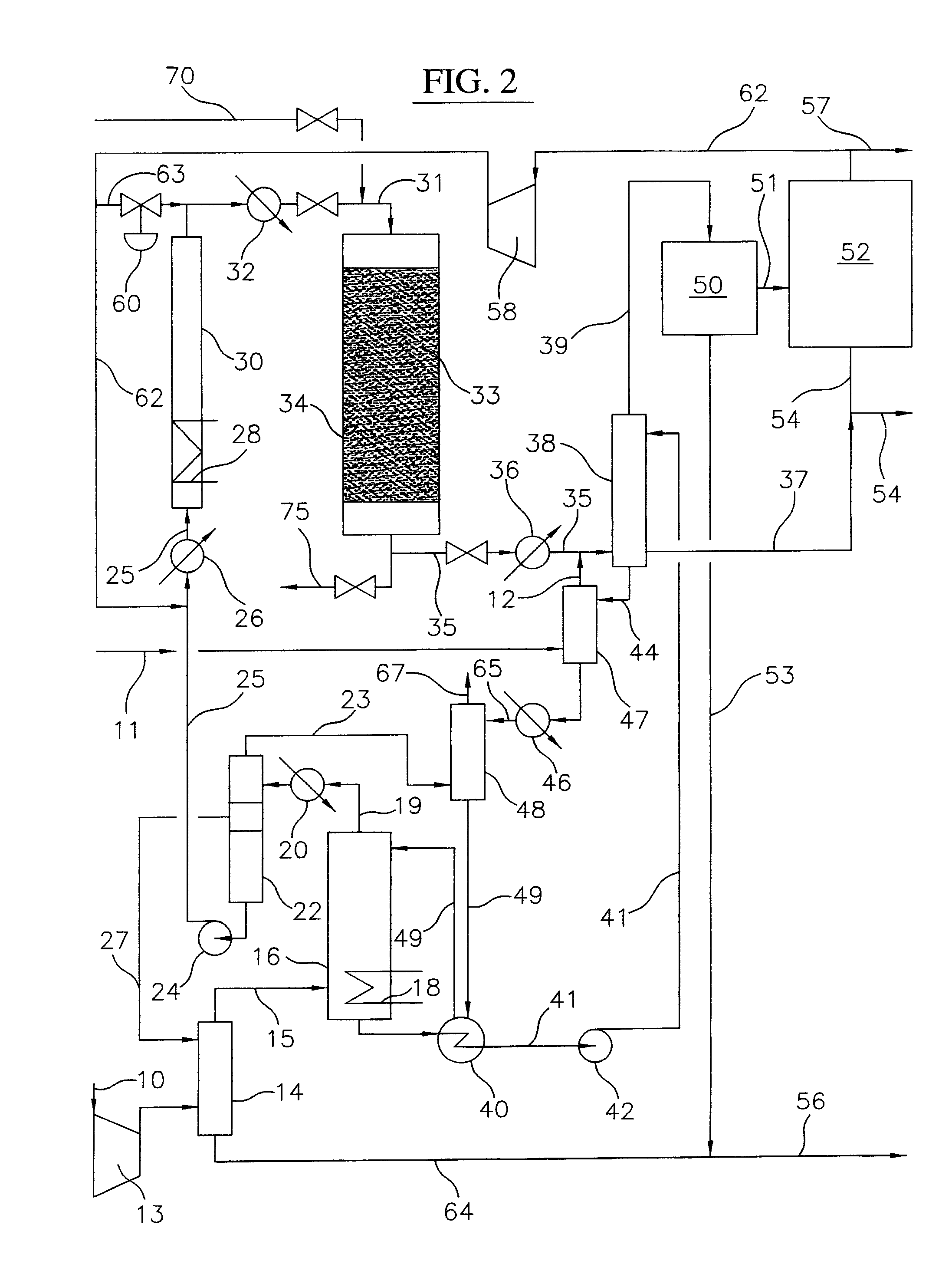
ActiveUS8815050B2Drying using combination processesDrying solid materials with heatBromineWater flow
Processes and systems for drying liquid bromine utilizing two fractionators to produce a substantially dry liquid bromine stream and a substantially bromine-free water stream. Wet bromine liquid may be conveyed to a first fractionator wherein a substantially dry bromine liquid is produced, while a vapor stream from the first fractionator may be condensed into a first liquid phase comprising bromine saturated with water and a second liquid phase comprising water saturated with bromine. The water saturated with bromine may be conveyed to a second fractionator to produce at least substantially bromine-free water.
Owner:SULZER MANAGEMENT AG
Continuous process for converting natural gas to liquid hydrocarbons
A method comprising: providing an alkyl halide stream; contacting at least some of the alkyl halides with a coupling catalyst to form a product stream comprising higher hydrocarbons and hydrogen halide; contacting the product stream with a solid reactant to remove at least a portion of the hydrogen halide from the product stream; and reacting the solid reactant with a source of oxygen to generate a corresponding halogen.
Owner:GRT INC
Processes and systems for drying liquid bromine
Processes and systems for drying liquid bromine utilizing two fractionators to produce a substantially dry liquid bromine stream and a substantially bromine-free water stream. Wet bromine liquid may be conveyed to a first fractionator wherein a substantially dry bromine liquid is produced, while a vapor stream from the first fractionator may be condensed into a first liquid phase comprising bromine saturated with water and a second liquid phase comprising water saturated with bromine. The water saturated with bromine may be conveyed to a second fractionator to produce at least substantially bromine-free water.
Owner:SULZER MANAGEMENT AG
Method for extracting bromine from bromine-containing solution
The invention discloses a method for extracting bromine from a bromine-containing solution. The method comprises the following steps: 1) acidifying the bromine-containing solution, and oxidizing; 2) carrying out extraction operation on the oxidized mixed solution in the step 1); 3) carrying out back extraction operation on the bromine-containing extraction organic phase obtained in the step 2); and 4) acidifying the back extraction solution obtained in the step 3) to precipitate bromine, thereby obtaining the simple substance bromine. By adopting the oxidization-extraction process, the method has the advantages of high extraction rate, simple equipment, low energy consumption and the like; the bromine extraction rate reaches 98% or above; and the extractant can be recovered.
Owner:HARBIN INST OF TECH AT WEIHAI
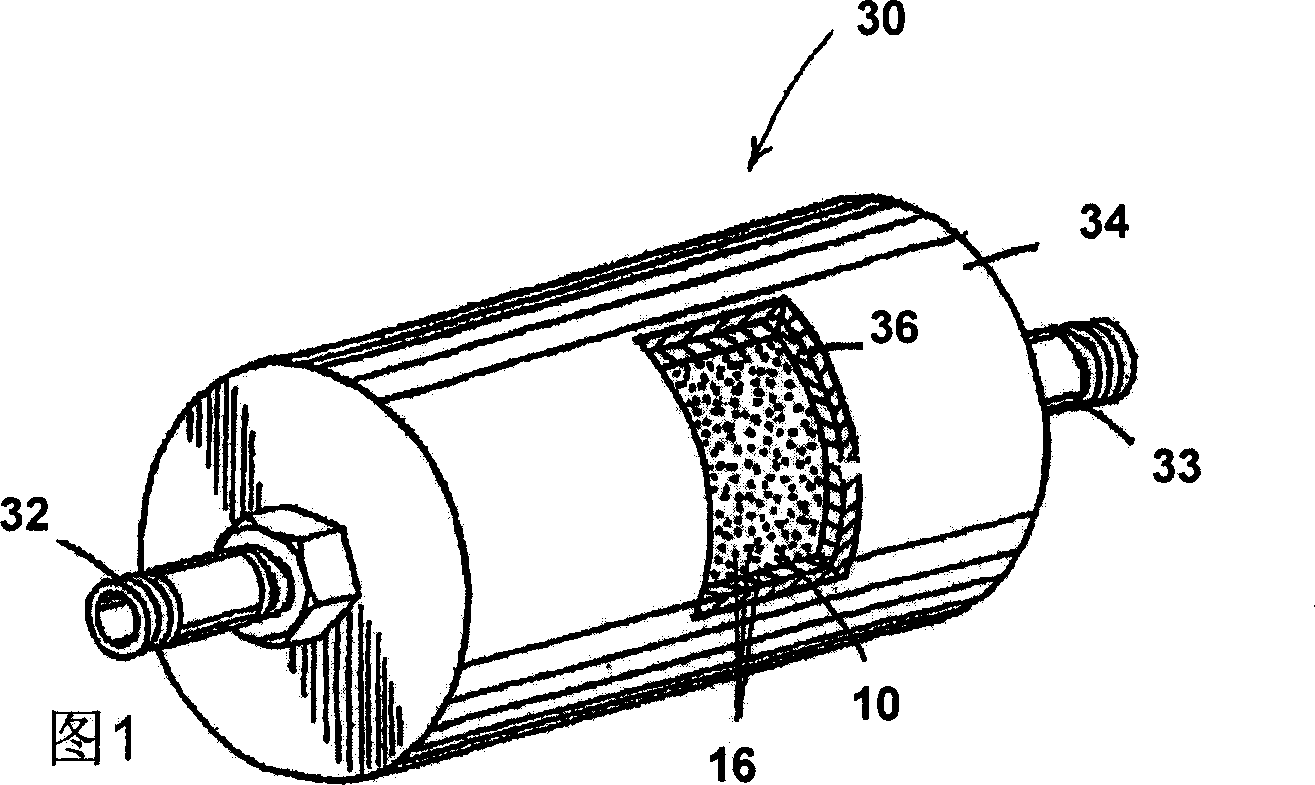
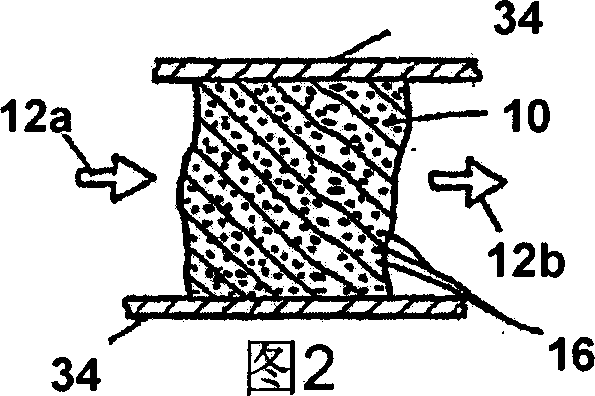
Apparatus and method for purification of corrosive gas streams
InactiveUS20070031321A1Reduce moistureWater contentMagnesium fluoridesHydrogen bromideParticulatesHalogen
A process, composition and apparatus for the removal of impurities from corrosive gases, particularly halogen-containing gases, down to about 100 ppb concentration are described. The critical component is zirconia (ZrO2), which in a variety of physical forms is capable of dehydrating such gases. The zirconia can be in the form of a coating on a substrate, as a granular bulk material, or deposited within the pores of a porous body. The zirconia is retained in a simple container which is easily installed in a gas supply line, such as to a gas- or vapor-deposition manufacturing unit. The purification process can be operated for long periods of time in the presence of these gases. The invention provides final purification to gas streams intended for gas- or vapor-deposition formation of high purity electronic, prosthetic or similar products, and can be used in combination with a preliminary dehydration process or a solid particulate removal unit upstream.
Owner:MYKROLIS CORP +1
Method for extracting bromine from brine
The invention belongs to the technical field of bromine production processes, and provides a method for extracting bromine from brine. The brine is sequentially subjected to an acidizing and oxidizing step, a blowout step, an enriching step, a distilling step and a separating step to extract bromine. The method is simple and convenient to operate, the acidizing efficiency is improved, energy saving and emission reducing are achieved, the requirement for environment protection is met, and cost is reduced. The utilization rate of chlorine is increased, chlorine is saved, and raw material consumption is reduced.
Owner:SHANDONG CHANGYI AOLI SALINIZATION CO LTD
Apparatus and method for purification of corrosive gas streams
InactiveCN1809412ALess susceptible to corrosionReduce moisture contentHydrogen bromideBromineHalogenPhysical chemistry
A process, composition and apparatus for the removal of impurities from corrosive gases, particularly halogen-containing gases, down to about 100 ppb concentration are described. The critical component is zirconia (ZrO2), which in a variety of physical forms is capable of dehydrating such gases. The zirconia can be in the form of a coating on a substrate, as a granular bulk material, or deposited within the pores of a porous body. The zirconia is retained in a simple container which is easily installed in a gas supply line, such as to a gas- or vapor-deposition manufacturing unit. The purification process can be operated for long periods of time in the presence of these gases. The invention provides final purification to gas streams intended for gas- or vapor-deposition formation of high purity electronic, prosthetic or similar products, and can be used in combination with a preliminary dehydration process or a solid particulate removal unit upstream.
Owner:ENTEGRIS INC
Processes for converting hydrogen sulfide to carbon disulfide
Processes for forming carbon disulfide from a gas stream containing hydrogen sulfide. A gaseous stream comprising lower molecular weight alkanes and hydrogen sulfide may be contacted with sufficient bromine at a temperature of from about 250° C. to about 530° C. to convert substantially all of said hydrogen sulfide to carbon disulfide. The gaseous stream may contain from about 0.001 to about 20 mol % hydrogen sulfide. The molar ratio of bromine to hydrogen sulfide may be about 2:1.
Owner:GTC TECHNOLOGY US LLC
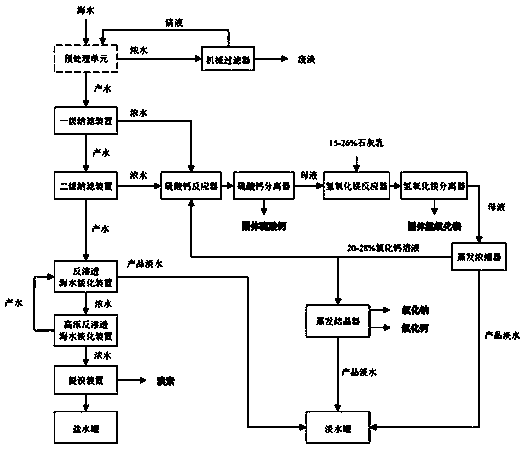
Efficient seawater desalination and comprehensive utilization method
ActiveCN110734166AEfficient removalHigh bromine contentGeneral water supply conservationCalcium/strontium/barium chloridesSeawaterUltrafiltration
The invention discloses an efficient seawater desalination and comprehensive utilization method. The efficient seawater desalination and comprehensive utilization method comprise following steps: carrying out pretreatment on raw seawater, filtering using a microfiltration and / or ultrafiltration device to obtain pretreated seawater; adding a scale inhibitor into the pretreated seawater; pressurizing and conveying the pretreated seawater into a primary nanofiltration device; adjusting the pH value of obtained primary nanofiltration produced water to 3-7, pressurizing and feeding into a secondarynanofiltration device; mixing obtained primary nanofiltration concentrated water with obtained secondary nanofiltration concentrated water; adding a precipitant to react with SO4<2-> in an obtained concentrated water mixed solution to generate calcium sulfate precipitate; continuously adding lime emulsion into an obtained reacted mother liquor to react with Mg<2+> in the mother liquor to generatemagnesium hydroxide precipitate, adjusting the pH value of the residual mother liquor to 7-8, producing sodium chloride and calcium chloride through multi-effect evaporation, adjusting the pH value of obtained secondary nanofiltration produced water to 6.5-7.8, and pressurizing and introducing the secondary nanofiltration produced water into a reverse osmosis seawater desalination device. According to the efficient seawater desalination and comprehensive utilization method, seawater grading pretreatment process is adopted, the component difference of various concentrated water is fully utilized, seawater desalination and the chemical technology are combined, and the fresh water yield and the seawater utilization rate are increased.
Owner:CHINA NAT OFFSHORE OIL CORP +3
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com