Process for converting gaseous alkanes to olefins and liquid hydrocarbons
a technology of gaseous alkanes and liquid hydrocarbons, which is applied in the preparation of halogenated hydrocarbons, refining with non-metals, bromine, etc., can solve the problems of limited distance over which natural gas may be transported in gaseous form, high cost of lng process, and limited practical and economic limits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] As utilized throughout this description, the term “lower molecular weight alkanes” refers to methane, ethane, propane, butane, pentane or mixtures thereof. As also utilized throughout this description, “alkyl bromides” refers to mono, di, and tri brominated alkanes. Also, the feed gas in lines 11 and 111 in the embodiments of the process of the present invention as illustrated in FIGS. 2 and 3, respectively, is preferably natural gas which may be treated to remove sulfur compounds and carbon dioxide. In any event, it is important to note that small amounts of carbon dioxide, e.g. less than about 2 mol %, can be tolerated in the feed gas to the process of the present invention.
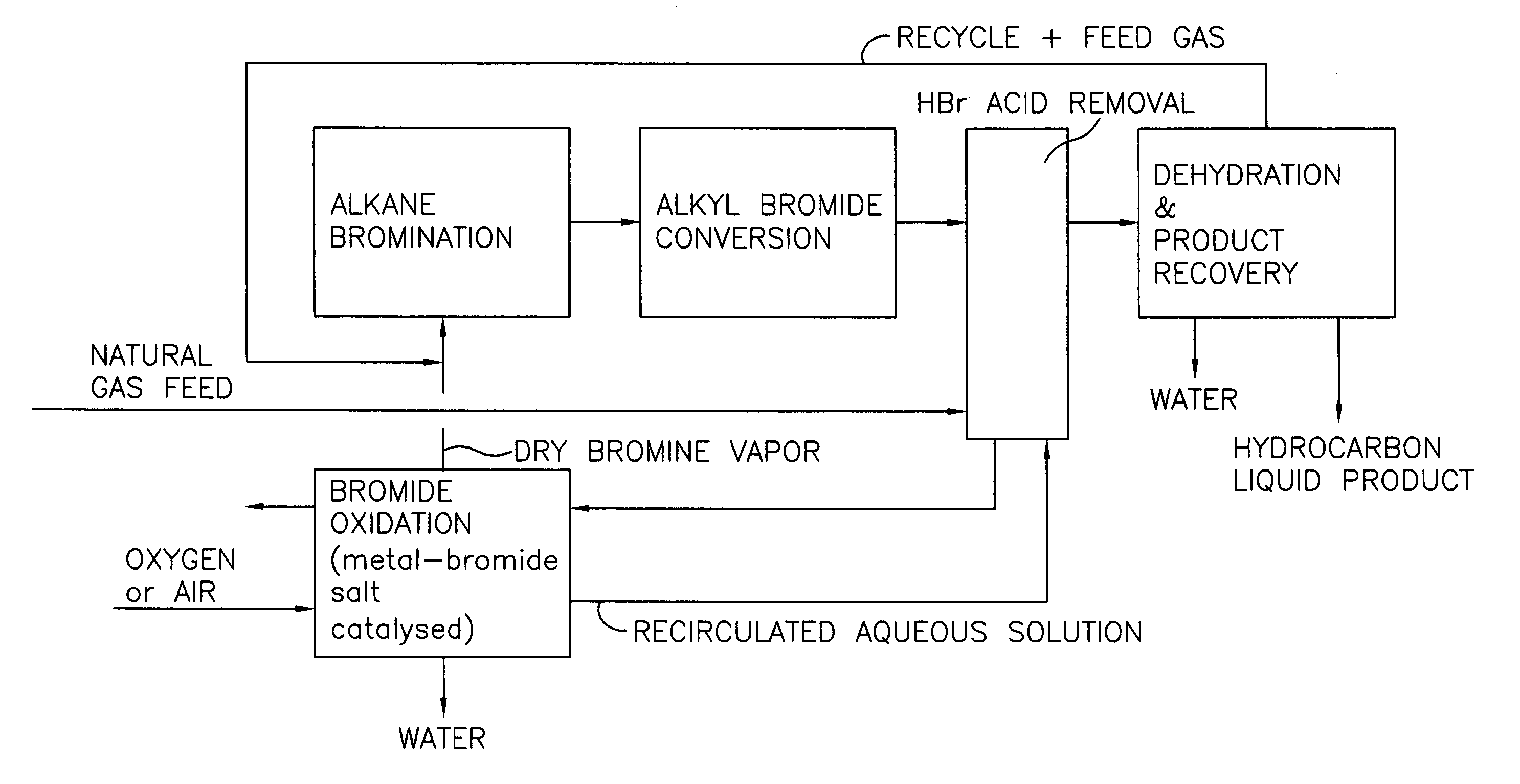
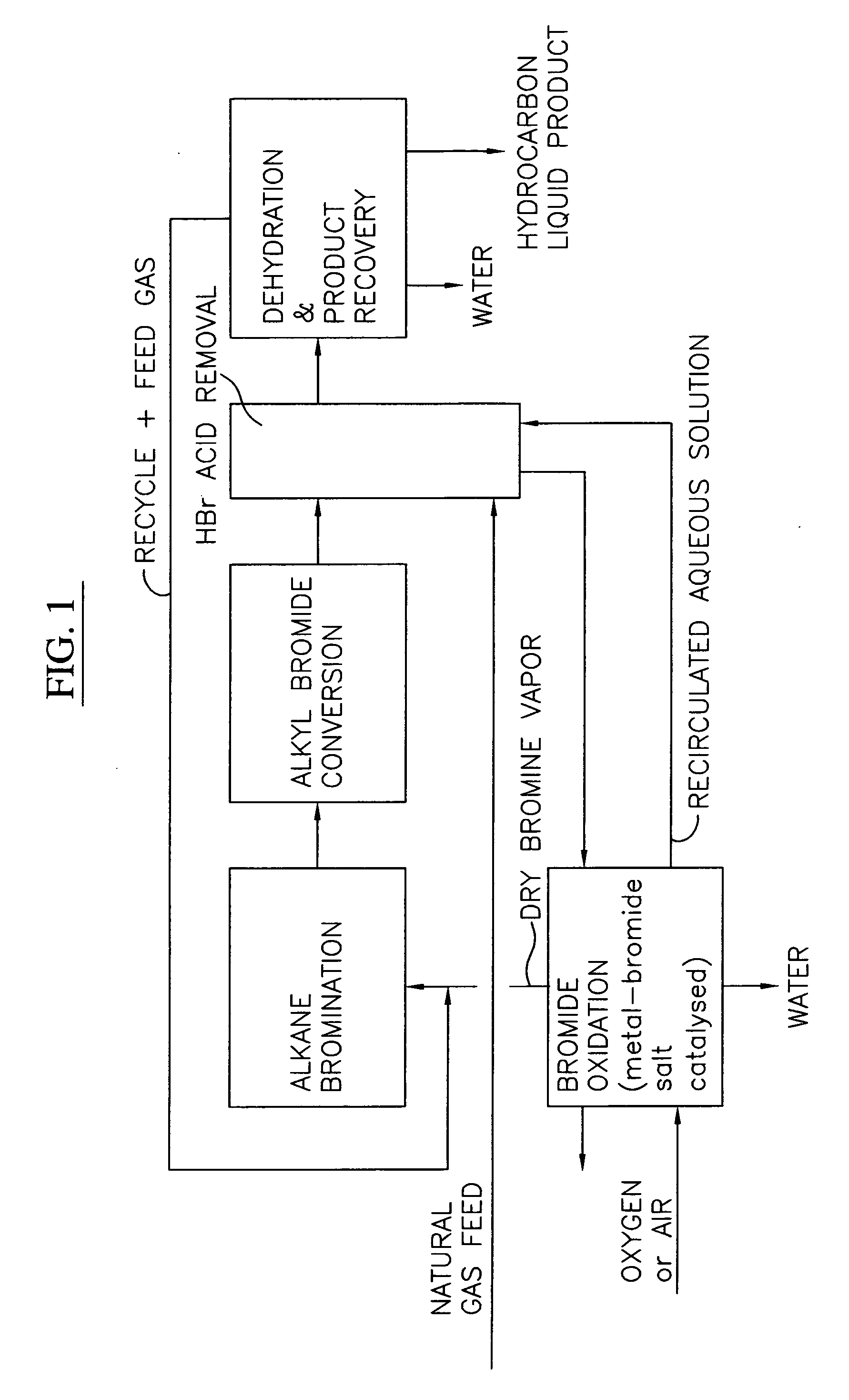
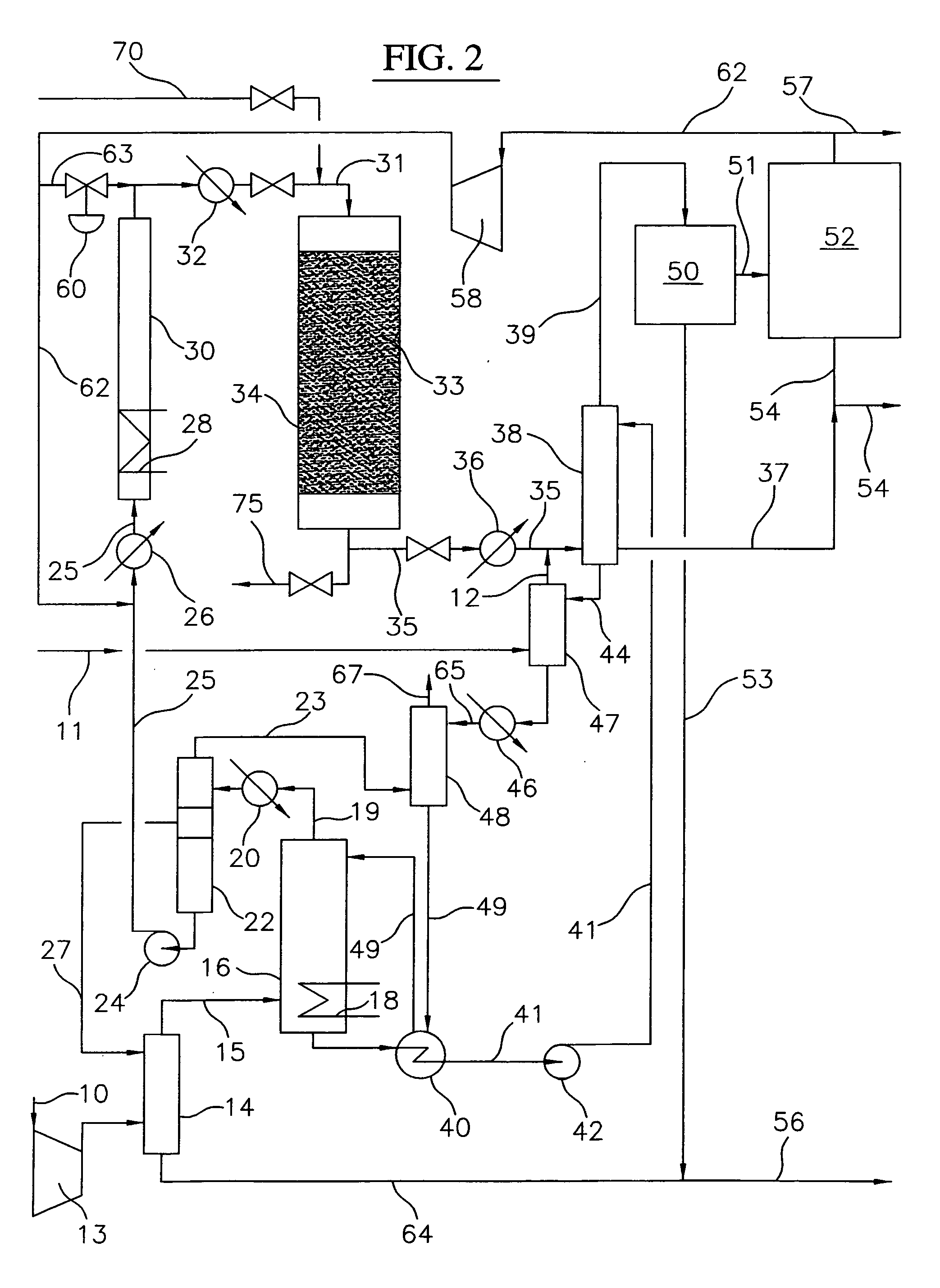
[0028] A block flow diagram generally depicting the process of the present invention is illustrated in FIG. 1, while specific embodiments of the process of the present invention are illustrated in FIGS. 2 and 3. Referring to FIG. 2, a gas stream containing lower molecular weight alkanes, comprised of a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com