Catalyst for the synthesis of CF3I and CF3CF2I
一种催化剂、混合物的技术,应用在碳化合物催化剂、催化剂、催化剂活化/制备等方向,能够解决快速失活等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Catalyst preparation - oxides or salts of lanthanum supported on carbon (La 2 o 3 / C) preparation of the catalyst: the specified amount of La(NO 3 ) 3 Dissolve in deionized water (the amount of water is calculated from the pore volume of the support). La(NO 3 ) 3 After complete dissolution, slowly pour the specified amount of activated carbon (pre-dried at 100-120°C for 12 hours) into the solution, or vice versa. The batter was stirred continuously to achieve uniform impregnation, then left in a hood overnight for full impregnation to occur. Subsequently, the impregnated samples were dried in an oven at 100-120° C. for 12 hours and calcined at 450-550° C. for 4 hours in a nitrogen flow.
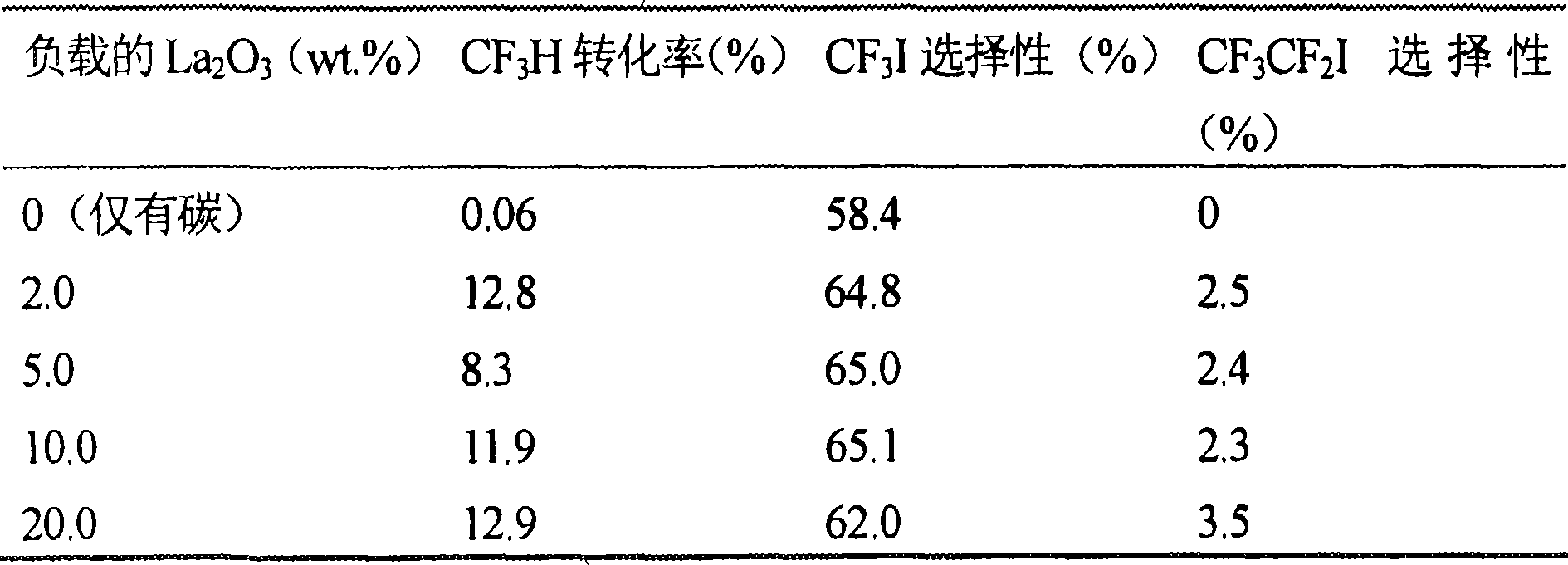
[0023] Promoted La 2 o 3 / C Catalyst Preparation: A specified amount of La(NO 3 ) 3 And the specified amount of accelerator precursor is dissolved in the required amount of deionized water. After all the salts are completely dissolved, slowly pour the specified am...
Embodiment 2
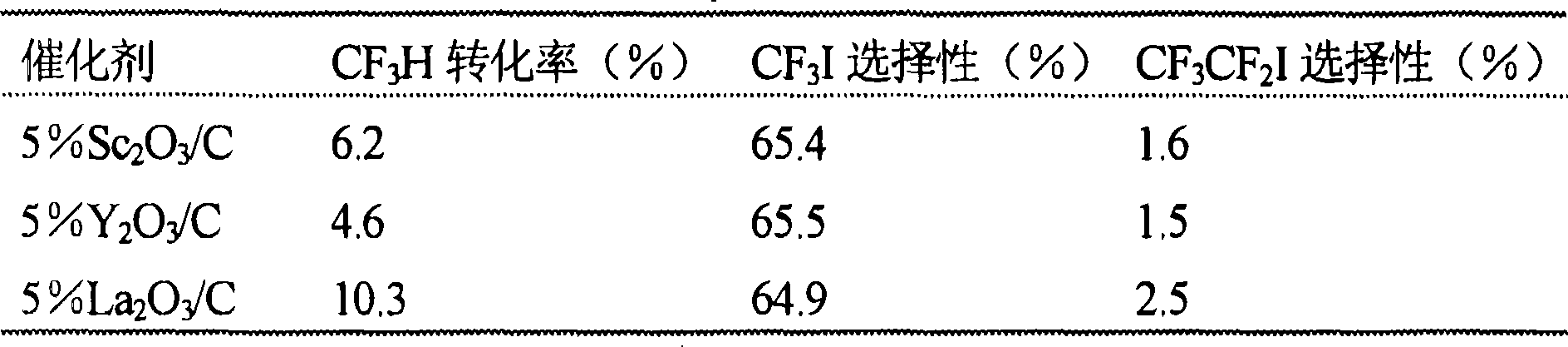
[0025] Example 2: Carbon supported containing with d 1 the s 1 Reactivity of Catalysts with Configuration Elements - Table 1 lists some activated carbon-supported catalysts containing d 1 the s 1 Activity and selectivity of catalysts with configuration elements such as scandium (Sc), yttrium (Y) and lanthanum (La) after 20 hours on stream at 500 °C. For 5% Sc 2 o 3 / C, began to show activity 7 hours after being put into production. 5% Sc 2 o 3 / C CF 3 Conversion of H and CF 3 The selectivities to I were 6.3% and 65.5%, respectively, after 10 hours on stream. The catalyst was stable after 20 hours on stream with a CF of 6.2% 3 H conversion, 65.4% CF 3 I selectivity and 1.6% CF 3 CF 2 I optional. 5% Y 2 o 3 / The induction period was 12 hours and it showed a CF of 4.6% after 20 hours of reaction 3 H conversion and 65.5% CF 3 I optional. against 5% La 2 o 3 / C, the induction period is 8 hours. In 10 hours of reaction time, it showed 8.3% CF 3 H conversion a...
Embodiment 3
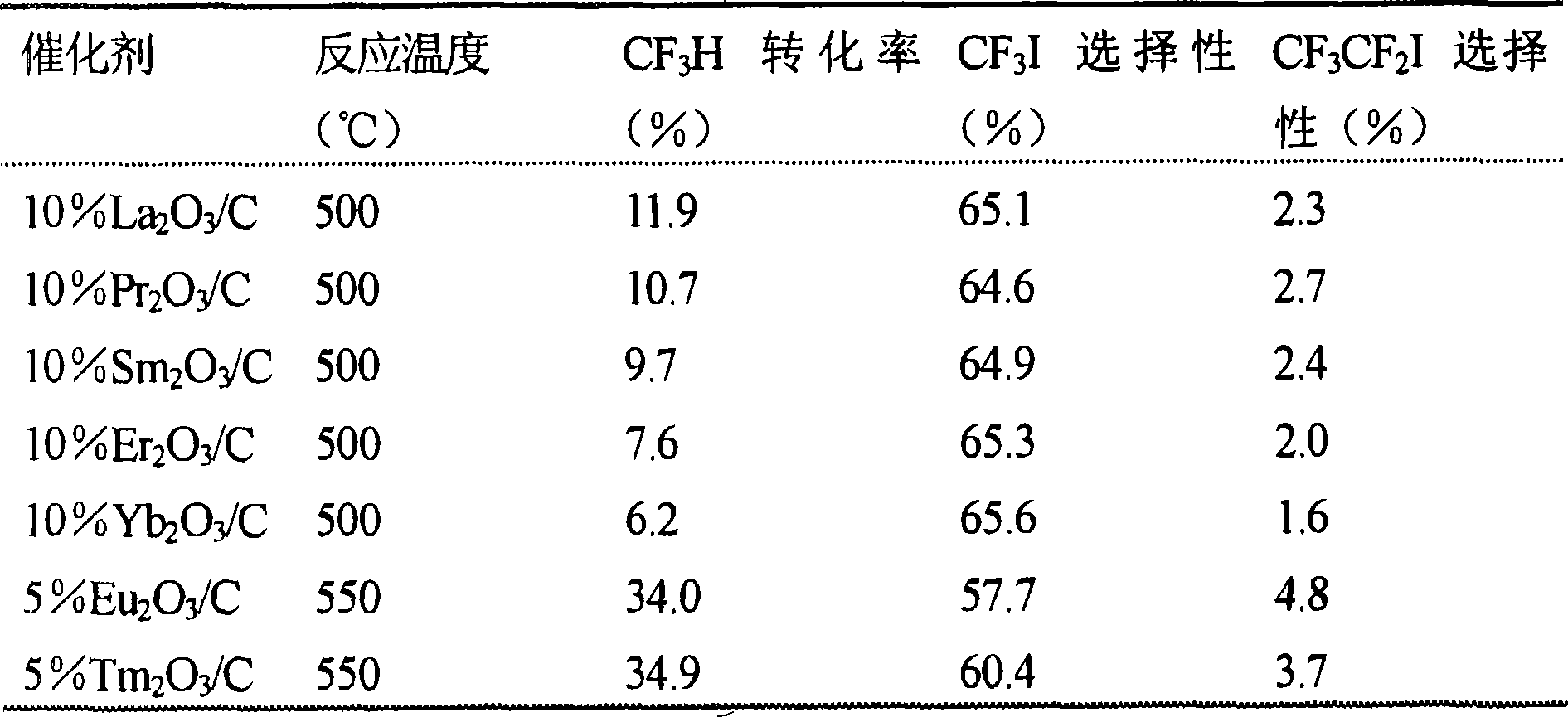
[0028] Example 3: Reactivity of Carbon-Supported Lanthanide (Rare Earth) Element-Containing Catalysts. The reactivity of the carbon supported catalysts containing various lanthanide (rare earth) elements is listed in Table 2. The data presented here were obtained after 10 hours in production. Catalysts containing 10 wt.% rare earth metal oxides were tested at 500 °C, which showed about 6-12% CF 3 H conversion, about 65% CF 3 I selectivity and about 2-3% CF 3 CF 2 I optional. 10%La 2 o 3 / C shows 11.9% CF 3 H conversion, slightly higher than other catalysts. To reduce the induction period, 5% Eu 2 o 3 / C and 5% Tm 2 o 3 / C Tested at 550°C. 5% Eu 2 o 3 / C CF 3 H conversion, CF 3 I selectivity and CF 3 CF 2 I selectivities were 34.0%, 57.7% and 4.8%, respectively, and a Tm of 5% 2 o 3 These figures for / C are 34.9%, 60.4% and 3.7%, respectively.
[0029] Table 2: Reactivity of carbon-supported lanthanide (rare earth)-containing catalysts. Reaction condition...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com